Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(3):497-506. doi:10.7150/jca.17444 This issue Cite
Research Paper
Identification and characterization of CD133+CD44+ cancer stem cells from human laryngeal squamous cell carcinoma cell lines
1. Department of Otolaryngology, Head & Neck Surgery, The First Hospital Affiliated with Shanxi Medical University, Taiyuan, Shanxi, China;
2. Shanxi Key Laboratory of Otolaryngology Head & Neck Cancer, Taiyuan, Shanxi, China;
3. Department of Pathology, Shanxi Cancer Hospital, Taiyuan, Shanxi, China.
*Equal contribution.
Received 2016-9-2; Accepted 2016-11-10; Published 2017-2-11
Abstract
Background: Laryngeal squamous cell carcinoma ranks second among head and neck squamous-cell carcinomas. Cancer stem cells can support cancer growth and malignant behavior. Therefore, cancer stem cells isolated from laryngeal squamous cell carcinoma tissue could be used to investigate the initiation, progression, and treatment strategies of this cancer. Methods: We isolated CD133-CD44-, CD133-CD44+, CD133+CD44- and CD133+CD44+ cell populations from laryngeal squamous-cell carcinoma cell lines Hep2 and TU-177 by magnetic-activated cell sorting. Sphere formation, cell proliferation, migration, invasion, colony formation, resistance to radio- and chemotherapy, and in vivo tumorigenicity of these populations were evaluated. Moreover, we investigated the expression of the stem-cell markers (sex determining region Y)-box 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) in CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ cell populations and parental Hep2 and TU-177 cells. Results: As compared with CD133-CD44-, CD133-CD44+, CD133+CD44- populations and parental cells, CD133+CD44+ cells showed higher cell viability, migration and invasive capability and colony formation ability as well as stronger resistance to cisplatin and irradiation. Moreover, levels of SOX2 and OCT4 and tumorigenicity in nude mice were greater in CD133+CD44+ Hep2 and TU-177 cells than other cell populations and parental cells.
Conclusion: The CD133+CD44+ population of laryngeal squamous-cell carcinoma Hep2 and TU-177 cells have stem cell properties and showed more malignant features than CD133+CD44- and CD133-CD44+ cell populations. CD133+CD44+ cancer stem cells may be a promising target for developing anticancer drugs and treatment strategies for laryngeal squamous cell carcinoma.
Keywords: Laryngeal squamous cell carcinoma, Magnetic activated cell sorting, CD133, CD44, cancer stem cell.
Introduction
Laryngeal squamous-cell carcinoma (LSCC) is the second most common malignant tumor of head and neck SCC; it represents about 2.4% of all cancer cases and 2.1% of all cancer deaths worldwide[1, 2]. In recent years, treatment such as functional laryngeal surgery, radiotherapy, chemotherapy, concurrent chemo-radiotherapy and gene therapy has resulted in better 5-year survival for patients. However, 30% to 40% of patients still die of tumor recurrence or metastasis [3, 4]. Therefore, understanding the origin, invasion, and metastasis of LSCC is important to develop new treatment methods. However, to date, the mechanism of LSCC initiation and progression remains unclear.
Cancer stem cells (CSCs) are a small subpopulation of cancer cells with capabilities of self-renewal, differentiation, and tumorigenicity[5]. CSCs are considered the major driving force in tumor progression and metastasis after conventional therapy because of their increased resistance to radio- and chemotherapy [6, 7]. Accumulating evidence has suggested that CSCs are present in LSCC [8-10], so isolating CSCs and investigating their properties could help elucidate the LSCC pathogenesis and the development of therapy.
The identification of CSCs from different kinds of cancer has suggested that a universal CSC marker is unlikely. Although studies have identified CSC markers across a variety of solid cancers, few of these markers have been studied in LSCC. Aldehyde dehydrogenase-1, CD24, CD44, and CD133 are among the most common CSC markers used for distinct solid tumor types[11, 12]. CD133 is a transmembrane glycoprotein commonly expressed in hematopoietic stem cells, endothelial progenitor cells, and various normal tissue stem cells. CD133 was used as a marker of CSC in several tumor types[11] and recently defined as a CSC marker in LSCC [7]. Moreover, upregulation of CD133 in colorectal cancer is strongly associated with liver metastasis and poor prognosis [13]. CD44 plays a role in facilitating cell-to-cell and cell-matrix interactions and is involved in cell adhesion and the assembly of growth factors on the cell surface. CD44+ cells have a more primitive, poorly differentiated morphology and increased expression of known stem-cell markers[14]. CD44 expression was also associated with poor prognosis in head and neck SCC, including LSCC[15, 16].
In the present study, we isolated the CD133+CD44+ subpopulation from LSCC Hep2 and TU-177 cell lines and analyzed whether these CD133+CD44+ populations possessed properties of CSCs by evaluating sphere and colony formation, self-renewal, resistance to radiotherapy and chemotherapy, migration, invasion, and expression of stem cell markers. In addition, we compared CD133+CD44+ CSCs and CD133+CD44-, CD133-CD44+, CD133-CD44- cell populations and parental Hep2 and TU-177 cells for proliferation capability, resistance to radiotherapy and chemotherapy, migration and invasion capability, and expression of stem cell markers.
Materials and methods
Cell line and culture
The human LSCC cell line Hep2 was obtained from the China Center for Type Culture Collection (Wuhan). The TU-177 cell line was obtained from Bioleaf Biotech Corp. (Shanghai). Hep2 cells were cultured in DMEM supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT). TU-177 cells were cultured in MEM supplemented with 10% fetal bovine serum (Hyclone). All cells were maintained in an incubator at 37 °C with 5% CO2 and 100% humidity.
Magnetic-activated cell sorting (MACS)
Cells positive for CD44 and CD133 surface markers were isolated from cultured Hep2 and TU-177 cells by MACS by using CD44 MicroBeads or CD133-PE antibody (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. Briefly, 107 cells in 100 μL buffer were incubated with 10 μL CD133-PE antibody. Cells were washed with 1 mL buffer and centrifuged at 300×g for 10 min, then the cell pellet was resuspended with 90 μL buffer, and 10 μL anti-PE Multisort MicroBeads (Miltenyi Biotec) was added and incubated for 15 min in the dark at 4℃. Cells were washed and underwent magnetic sorting, then CD133-enriched (CD133+) and CD133-depleted (CD133-) cell populations were collected. For 107 cells of CD133+ or CD133-, 20 μL CD44 MicroBeads was added and incubated for 15 min in the dark at 4 ℃, followed by washing and magnetic sorting, then CD133+CD44+, CD133+CD44-, CD133-CD44+, and CD133-CD44- cells were obtained. After isolation, cells with the 4 types of surface markers were cultured for further analysis, and some of these cells were used to evaluate the efficiency of magnetic separation by flow cytometry (Becton Dickinson).
Cell proliferation assay
CD133+CD44+, CD133+CD44-, CD133-CD44+, CD133-CD44+Hep2 or TU-177 cells and parental cells (1×104) were seeded into 96-well plates. An amount of 10 μL CCK-8 was added to each well at 24, 48, 72, 96, and 120 h and incubated for 1 h. An automatic microplate reader was used to determine optical density at 450 nm (absorbance value). The growth curves were graphed by growth days and absorbance, with the mean from three replicates calculated.
Transwell chamber assay
Cells were rinsed with PBS and resuspended with culture medium containing 1% fetal bovine serum (HyClone). An amount of 200 μL cell suspension was added into the Transwell chamber (Corning, NY, USA) at 5×105 cells/mL. Then 600 μL medium containing 30% FBS was added into the chamber below the 24-well plate for 48 h, and cells were rinsed with PBS and fixed in 0.5% methanol for 30 min, stained with 0.1% crystal violet for 20 min, and rinsed with PBS. Cells penetrating the membrane were observed by microscopy, then dissolved with glacial acetic acid. The optical density at 570 nm that reflects migration ability was measured by using an automatic microplate reader. Invasion ability was determined by coating the 24-well plates with Matrigel.
Colony-formation assay
Cells were serially diluted and inoculated in a Petri dish containing 1 mL medium with 100 cells/dish. After 2 weeks, clone spheres were formed in the dishes. Cells were rinsed with PBS, fixed with 5 mL 4% paraformaldehyde for 15 min, then stained with 0.1% crystal violet, and samples were rinsed with flow water, then air-dried. Images were taken under an inverted microscope (Leica, Wetzlar, Germany). Samples were dissolved with glacial acetic acid, and optical density at 570 nm was determined by using an automatic microplate reader.
Cell adhesion assay
Thawed Matrigel was diluted with culture medium at 1:100 proportion. An amount of 50 μL Matrigel was added to each well of 96-well plates, and 1×105 cells of CD133+CD44+, CD133+CD44-, CD133-CD44+, CD133-CD44- populations and parental Hep2 and TU-177 cells were seeded in each well. After 1-, 2- and 4-h incubation, cells were rinsed with PBS, then fixed with 5 mL 4% paraformaldehyde for 15 min, stained with 0.1% crystal violet, and samples were rinsed with flow water and air-dried; images were taken under an inverted microscope (Leica, Wetzlar, Germany). Samples were dissolved with glacial acetic acid, and the optical density at 570 nm was determined by using an automatic microplate reader.
Chemotherapy resistance assay
Cells were seeded in 96-well plates at 1×104 cells/well. Amounts of 1, 5, 10, 15, 20, and 25 μg/mL cisplatin were added to cells for 48 h, then 10 μL CCK-8 was added to each well and incubated for 1 h. An automatic microplate reader was used to determine the optical density at 450 nm (absorbance value), the mean of three replicates was calculated.
Radiotherapy resistance assay
Cells were incubated with serum-free culture, and 1×104 cells were seeded into 96-well plates, then irradiated with a linear accelerator dose of 10 Gy, then cultured for 24 h under normal conditions. An amount of 10 μL CCK-8 was added to each well and incubated for 1 h. An automatic microplate reader was used to determine the optical density at 450 nm (absorbance value), the mean of three replicates was calculated.
Spheroid-formation assay
CD133+CD44+ cells were cultured under stem-cell suspension culture conditions consisting of serum-free DMEM/MEM culture medium supplemented with B27 (1:50, Engreen Biosystem Co, China), epidermal growth factor (EGF; 20 ng/ml), and basic fibroblast growth factor (bFGF; 20 ng/ml) in an ultra-low-attachment culture flask. Spheroid formation was observed and counted under an inverted microscope (Leica, Wetzlar, Germany).
RNA extraction, reverse transcription, and real-time quantitative PCR (qPCR)
Total RNA was extracted by using Trizol reagent (Invitrogen) according to the manufacturer's instructions. First-strand cDNA synthesis involved use of the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). qPCR was performed using ChamQ SYBR qPCR Master Mix (Vazyme) on a ABI 7500 FAST real-time PCR system (Applied Biosystems, Foster City, CA). qPCR was run at 95°C for 30 sec, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The specificity of the primer amplicons was examined by melting curve analysis. The comparative Ct method was used for quantifying target mRNA expression normalized to that of β-Actin and relative to the calibrator. The primers used were for β-Actin: forward 5'-CATGTACGTTGCTATCCAGGC-3', reverse 5'-CTCCTTAATGTCACGCACGAT-3'; SOX2: forward 5'-TGGACAGTTACGCGCACAT-3', reverse 5'-CGAGTAGGACATGCTGTAGGT-3'; and OCT4: forward 5'-CTTGAATCCCGAATGGAAAGGG-3', reverse 5'- CCTTCCCAAATAGAACCCCCA-3'.
Nude mouse xenograft model
All experimental procedures and protocols involving animals were reviewed and approved by the Research Ethics Committee of Shanxi Medical University. The animal protocol was designed to minimize pain or discomfort to the animals. BALB/C nude mice (SPF grade, female, 4-6 weeks old) were purchased from Vital River (Beijing). Mice were divided into five groups for each cell line (3 mice per group): CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ populations and unsorted (parental) Hep2 or TU-177 cells inoculated subcutaneously on the left and right backs of mice (1×106 in 200 μL PBS). After 6 weeks, all mice were euthanized with excess CO2, and tumor formation and tumor weight were assessed.
Statistical analysis
The one-way ANOVA was used to evaluate the differences among five groups. An independent two-sample t test was used to assess the differences between two independent groups. Statistical significance was defined at p < 0.05. Statistical analysis involved use of SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
CD133+CD44+ LSCC enrichment and sphere‑forming assay
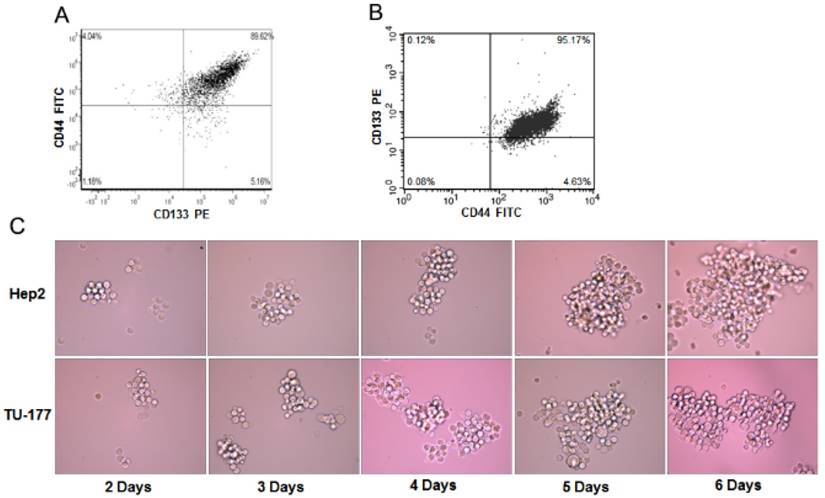
To determine the enrichment efficiency of CD133+CD44+ populations from Hep2 and TU-177 cells by MACS, we analyzed the CD133+CD44+ cell proportion after isolation by flow cytometry. The CD133+CD44+ fraction of Hep2 and TU-177 cells was 89.62% (Fig. 1A) and 95.17% (Fig. 1B), respectively, suggesting that MACS enriched CD133+CD44+ cells efficiently.
Sphere formation has been used to evaluate the properties of CSCs. To understand whether CD133+CD44+ cells have sphere-forming ability, CD133+CD44+ Hep2 and TU-177 cells obtained by MACS were cultured in serum-free medium with bFGF and EGF. After 2 days of culture, numerous individual cells in the CD133+CD44+ suspension culture survived and proliferated. Moreover, after 6 days' culture, these cells gradually formed spherical colonies of various sizes and irregular shapes (Fig. 1C). Therefore, the CD133+CD44+ subpopulations of Hep2 and TU-177 cells could form spheres and could be CSCs.
CD133+CD44+ LSCC cells were highly resistant to chemo- and radiotherapy
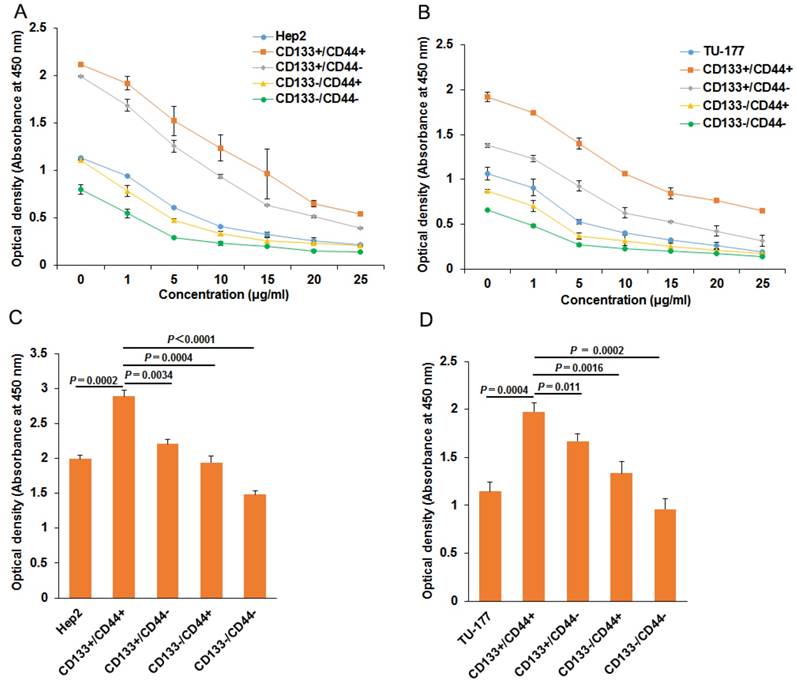
To evaluate the chemoresistance of CD133+CD44+, CD133-CD44-, CD133+CD44-, CD133-CD44+, CD133+CD44+ and parental cells were treated with cisplatin at 0, 1, 5, 10, 15, 20, 25 μg/ml for 48 h. CD133+CD44+ cells showed the highest viability at every concentration, followed by CD133+CD44- cells, parental cells, CD133-CD44+ cells, and CD133-CD44- cells (Fig. 2A and 2B). Moreover, CD133+CD44+ cells had stronger resistance to irradiation than other cell subpopulations and parental Hep2 or TU-177 cells (Fig. 2C and 2D).
The sphere formation of CD133+CD44+ Hep2 and TU-177 cells. (A) Magnetic-activated cell sorting of proportion of CD133+CD44+ Hep2 and TU-177 cells determined by flow cytometry with CD133-PE and CD44-FITC antibodies. (B) CD133+CD44+ Hep2 and TU-177 cells were cultivated under serum-free conditions with bFGF and EGF. Spheres were observed and recorded at the indicated times.
Chemotherapy and radiotherapy resistance assay of CD133+CD44+ Hep2 and TU-177 cells. (A) and (B) CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental cells were treated with cisplatin at the indicated concentration for 48 h, then cell viability was determined by use of a CCK-8 kit. (C) and (D) Cells were irradiated at 10 Gy with use of a linear accelerator, then cultured for 24 h, cell viability was determined by use of a CCK-8 kit. Data are expressed as mean ± SD of three independent experiments.
CD133+CD44+ LSCC cells exhibited high self-renewal potential
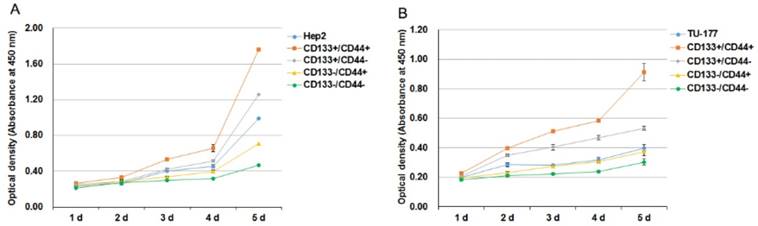
We analyzed the self-renewal potential of CD133+CD44+ LSCC cells by proliferative assay. CD133+CD44+, CD133-CD44-, CD133+CD44-, CD133-CD44+ cells and parental cells were passaged and plated onto 96-well plates. CD133+CD44+ Hep2 and TU-177 cells showed the highest proliferative ability as compared with other subpopulations and parental cells (Fig. 3A and B). Collectively, these results suggest that CD133+CD44+ LSCC cells have high self-renewal potential. The proliferative ability was slightly greater for parental Hep2 and TU-177 than CD133-CD44+ cells. Moreover, CD133+CD44- cells showed higher viability than CD133-CD44+ cells (Fig. 3A and 3B).
CD133+CD44+ LSCC cells show high colony-formation efficiency and adhesive ability
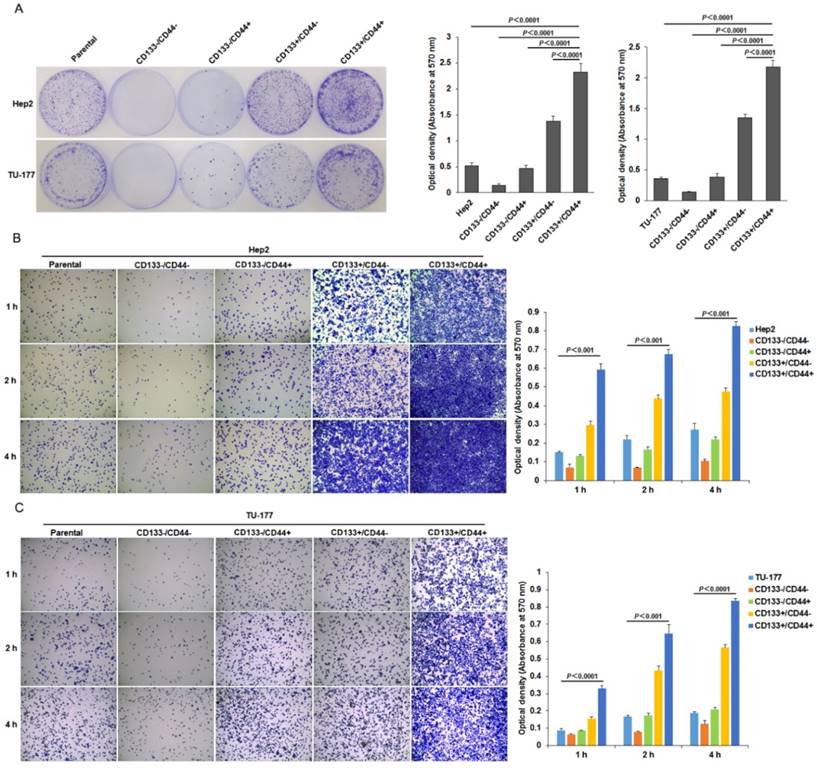
To further investigate the stem-cell potential of CD133+CD44+ LSCC cells, we evaluated their colony-formation efficiency. The colony-formation ability was higher for CD133+CD44+ Hep2 and TU-177 than CD133-CD44-, CD133+CD44-, CD133-CD44+ and parental cells (Fig. 4A). Moreover, adhesive ability was higher for both CD133+CD44+ Hep2 and TU-177 cells than other subpopulations and parental cells (Fig. 4B and 4C). As well, CD133+CD44- cells showed higher colony-formation efficiency and adhesive ability than CD133-CD44+ cells.
Cell proliferation of CD133+CD44+ Hep2 and TU-177 cells. (A,B) CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental Hep2 (A) and TU-177 (B) cells were seeded onto 96-well plates, and cell counts were determined by CCK-8 assay at the indicated times. Data are expressed as mean ± SD of three independent experiments.
Colony formation and adhesion ability of CD133+CD44+ Hep2 and TU-177 cells. (A) Colony formation of CD133+CD44+ Hep2 and TU-177 cells. CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental Hep2 and TU-177 cells were seeded in 35-mm dishes at 100 cells/well for 2 weeks, then colonies were stained with crystal violet and counted under a dissection microscope. Optical density at 570 nm of dissolved crystal violet was used to evaluate cell number. (B) and (C) Adhesive assay of CD133+CD44+ Hep2 and TU-177 cells. CD133+CD44+, CD133+CD44-, CD133-CD44+, CD133-CD44- and parental Hep2 or TU-177 cells were seeded in 96-well plates for 1, 2 and 4 h, then cells were fixed and stained with crystal violet. Optical density at 570 nm of dissolved crystal violet was used to evaluate cell number. Data are expressed as mean ± SD of three independent experiments.
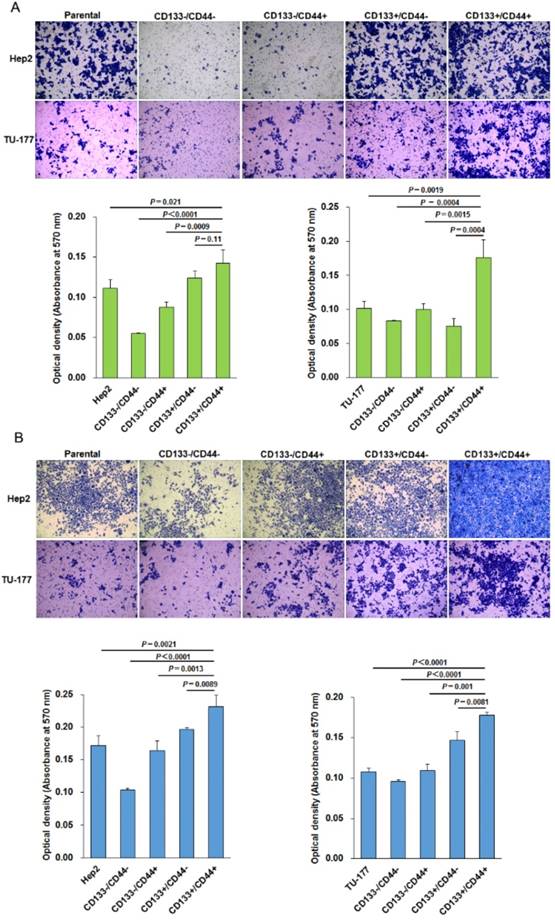
CD133+CD44+ LSCC cells have higher migration and invasive capacity
The migration ability tested by Transwell assay was significantly higher for CD133+CD44+ Hep2 and TU-177 cells than other cell subpopulations and parental cells (Fig. 5A). Moreover, the invasive capacity tested after incubation for 48 h showed that the number of cells penetrating the Transwell membrane was greater for CD133+CD44+ Hep2 and TU-177 cells than other cell subpopulations or parental cells (Fig. 5B).
Migration and invasion assays of CD133+CD44+ Hep2 and TU-177 cells. Migration ability (A) and invasive ability (B) of CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental Hep2 and TU-177 cells determined by Transwell chamber assay. Data are expressed as mean ± SD of three independent experiments.
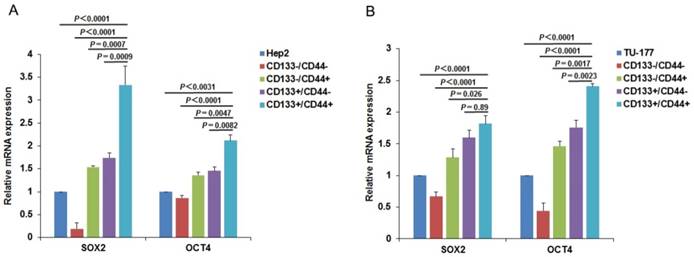
CD133+CD44+ cells express high levels of stem cell markers
To further confirm the CSC characteristics of the CD133+CD44+ LSCC cells, we examined the expression of CSC markers SOX2 and OCT4 in subpopulations and parental Hep2 and TU-177 cells by qPCR. As expected, the expression of SOX2 and OCT4 was higher in CD133+CD44+ than other subpopulations and parental cells (Fig. 6A and 6B) and was higher in CD133+CD44- than CD133-CD44+ cells. Collectively, these results confirm that the CD133+CD44+ cells isolated from Hep2 and TU-177 cells by MACS were CSCs.
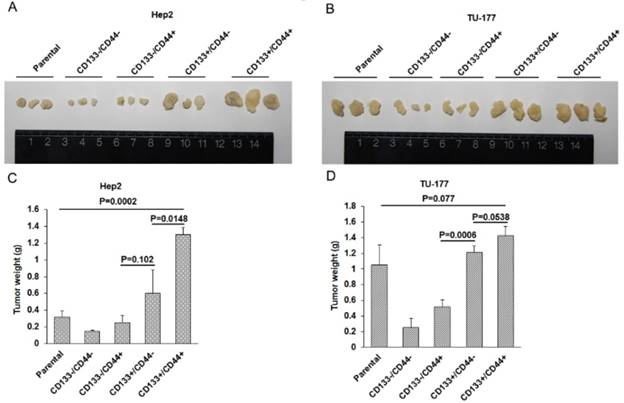
In vivo tumorigenicity of CD133+CD44+ CSCs
To evaluate the in vivo tumor formation potential of CD133+CD44+ CSCs, nude mice were injected with Hep2, TU-177 cells and MACS-enriched populations at 1×106, respectively. Tumorigenicity was monitored 6 weeks after injection. The size of tumors was larger in mice injected with CD133+CD44+ CSCs than other cell subpopulations and parental cells (Fig. 7A and 7B), and the mean weight of tumors was greater with injection of CD133+CD44+ CSCs than other cell subpopulations and parental cells (Fig. 7C and 7D). Particularly, the size and weight of tumors was larger in mice injected with CD133+CD44- than CD133-CD44+ CSCs (Fig. 7A -D). These results suggest that CD133+CD44+ CSCs had stronger ability to form tumors in vivo as compared to CD133+CD44-, CD133-CD44+, and CD133-CD44- cells and parental cells. In addition, tumorigenicity was stronger with CD133+ than CD44+ CSCs.
Expression of stem-cell markers in CD133+CD44+ cancer stem cells from Hep2 and TU-177 cells. (A) Total RNA was extracted from CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental (A) Hep2 and (B) TU-177 cells, then expression of stem-cell markers SOX2 and OCT4 was determined by qPCR. The expression of SOX2 and OCT4 was normalized to that of β-Actin and was relative to parental Hep2 or TU-177 cell expression. Data are expressed as mean ± SD of three independent experiments.
In vivo tumorigenicity of CD133+CD44+ cancer stem cells from Hep2 and TU-177 cells. (A) and (B) Morphology and size of tumors in nude mice injected with CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental Hep2 or TU-177 cells. (B) Weight of tumors in nude mice injected with CD133-CD44-, CD133-CD44+, CD133+CD44-, CD133+CD44+ and parental Hep2 or TU-177 cells. Data are expressed as mean ± SD of three independent experiments.
Discussions
The major properties of LSCC of local invasion, regional lymph node metastases, and resistance to conventional chemotherapy results in unfavourable prognosis. Therefore, despite improved experience in surgical technology and adjuvant therapies in the past decades, the overall prognosis of LSCC remains unimproved[17, 18]. Since CSCs play essential roles in cancer recurrence and metastatic spread, the identification and targeted elimination of CSCs has been considered a new method for LSCC treatment. In the present study, we isolated CD133 and CD44 double-positive subpopulation cells from SCC Hep2 and TU-177 cells by MACS and compared the properties of CD133+CD44+ cells with CD133+CD44-, CD133-CD44+, CD133-CD44- cell subpopulations and parental cells. Cell viability, migration and invasive capability and colony-formation ability was higher and resistance to cisplatin and irradiation was stronger for CD133+CD44+ than CD133-CD44-, CD133-CD44+, CD133+CD44- subpopulations and parental cells. Moreover, levels of SOX2 and OCT4 and tumorigenicity in nude mice were greater in CD133+CD44+ Hep2 and TU-177 cells than other cell subpopulations and parental cells. These CD133+CD44+ CSCs may be a promising target for developing anticancer drugs and treatment strategies for LSCC.
MACS is a highly specific cell separation technology commonly used in cancer research, immunology, neuroscience, and stem cell research[19-21]. Compared with fluorescent-activated cell sorting and other separation technologies, MACS is a comparatively simple and cost-effective approach and is able to retain better cell viability[22]. Previous study showed that CSC-enriched cell populations in head and neck SCC express a high level of the stem-cell markers CD133 and CD44[23, 24]. In the present study, we isolated CD133 and CD44 double-positive subpopulation cells from SCC Hep2 and TU-177 cells by MACS and compared the properties of CD133+CD44+ cells with CD133+CD44-, CD133-CD44+, CD133-CD44- cell subpopulations and parental cells. The proportion of separated cells was determined by flow cytometry and confirmed that MACS-enriched cells had the specified surface markers. Furthermore, the separated cells were easily maintained in vitro.
CSCs may be the driving force of tumorigenesis and metastases; therefore, these cells may be ideal targets of cancer therapy. Over the past years, several CSC markers were identified in a wide range of solid and haematopoietic malignancies[11]. CD133 and CD44 are well-known markers in isolating and identifying CSCs[25, 26]. Moreover, populations of cancer cells highly expressing CD133 or CD44 are invasive in vitro and are associated with poor prognosis[27-30]. Recently, cancer stem-like cells were isolated from primary laryngeal cancer cells and LSCC cell lines, and CD133 or CD44 was used as a screening marker in isolating CSCs from laryngeal cancer cells[7, 31-33].
To understand the difference between CD133+ and CD44+ LSCC cells, we enriched CD133+CD44+ cells from Hep2 and TU-177 cells. CSCs possess certain features such as self-renewal, migration and invasion, and chemoresistance[34]. Interestingly, we observed that Self-renewal potential, colony-formation efficiency and adhesive ability and migration and invasive capacity were higher for CD133+CD44+ Hep2 and TU-177 cells than CD133+ or CD44+ cells alone. These results were consistent with the functions of CD44 and CD133 in cell adhesion and promoting tumor progression[12]. Moreover, resistance to chemotherapy and radiotherapy was greater for CD133+CD44+ Hep2 and TU-177 cells than CD133+ or CD44+ cells alone. The ability to form spheres and expression of stem-cell markers are considered hallmarks of CSCs[34]. Consistently, the CD133+CD44+ Hep2 and TU-177 cells were able to form spheres under serum-free condition. Most importantly, levels of SOX2 and OCT4 were greater in CD133+CD44+ Hep2 and TU-177 cells than CD133+ or CD44+ cells alone.
Collectively, these data confirm that we isolated CD133+CD44+ CSCs from Hep2 and TU-177 cells, and the stemness of CD133+CD44+ CSCs was stronger than CD133+ and CD44+ cells alone. Self-renewal potential, colony-formation efficiency and adhesive ability, migration and invasive capacity and expression of the stem-cell markers SOX2 and OCT4 was higher in CD133+CD44- than CD133-CD44+ cells, so CD133+ cells may contribute more to the malignancy of LSCC.
Since CD133+CD44+ CSCs from Hep2 and TU-177 cells showed high capacity for self-renewal, high migration and invasion as well as colony formation and adhesion, these CD133+CD44+ CSCs may have high tumorigenicity. Consistently, CD133+CD44+ CSCs had stronger tumor-formation ability in vivo in nude mice than did parental Hep2/TU-177, CD133-CD44-, CD133-CD44+ and CD133+CD44- cells. Moreover, tumorigenicity was stronger in CD133+ Hep2/TU-177 than CD44+ Hep2/TU-177 cells. Investigating new drugs that specifically kill CD133+CD44+ LSCC cells would be helpful for targeting therapy in laryngeal carcinoma.
In conclusion, we isolated CD133+CD44+ CSCs from the LSCC cell lines Hep2 and TU-177 by MACS and characterized the CSC identities of these cell subpopulations. Moreover, we analyzed self-renewal, colony formation, adhesion, migration, invasion, resistance to chemotherapy and radiotherapy, sphere formation, expression of stem cell markers, and in vivo tumorigenicity of CD133+CD44+, CD133+CD44-, CD133-CD44+, CD133-CD44-, and parental Hep2 and TU-177 cells. CD133+CD44+ CSCs from Hep2 and TU-177 cells had stronger stemness, malignancy and tumorigenicity than CD133+/CD44-, CD133-/CD44+ and parental cells. In addition, CD133 may be a more important maker than CD44 for sorting CSCs from LSCC cells. These findings provide new insights into the study of laryngeal CSCs and generate valuable cell resources for development of new treatment strategies for laryngeal cancer.
Acknowledgements
This work was supported by Shanxi Scholarship Council of China (2016-118), the National Natural Science Foundation of China (Grant No. 81572670, 81402256, 81602394), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (STIP, 2016-92), the Research Project of Shanxi Province Health and Family Planning Commission (201301073, 2014028), the Excellent talent science and technology innovation project of Shanxi Province (201605D211029), and China Postdoctoral Science Foundation (2016M591412).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rudolph E, Dyckhoff G, Becher H, Dietz A, Ramroth H. Effects of tumour stage, comorbidity and therapy on survival of laryngeal cancer patients: a systematic review and a meta-analysis. Eur Arch Otorhinolaryngol. 2011;268(2):165-79
2. Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work-up. Otolaryngol Clin North Am. 2008;41(4):673-95 v
3. Shah PK, Shah KK, Karakousis GC, Reinke CE, Kelz RR, Fraker DL. Regional recurrence after lymphadenectomy for clinically evident lymph node metastases from papillary thyroid cancer: a cohort study. Ann Surg Oncol. 2012;19(5):1453-9
4. Baek SK, Jung KY, Kang SM. et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20(2):147-52
5. Xia P. Surface markers of cancer stem cells in solid tumors. Curr Stem Cell Res Ther. 2014;9(2):102-11
6. Odoux C, Fohrer H, Hoppo T. et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68(17):6932-41
7. Karatas OF, Suer I, Yuceturk B. et al. The role of miR-145 in stem cell characteristics of human laryngeal squamous cell carcinoma Hep-2 cells. Tumour Biol. 2016;37(3):4183-92
8. Suer I, Karatas OF, Yuceturk B. et al. Characterization of stem-like cells directly isolated from freshly resected laryngeal squamous cell carcinoma specimens. Curr Stem Cell Res Ther. 2014;9(4):347-53
9. Wu CP, Zhou L, Xie M. et al. Identification of cancer stem-like side population cells in purified primary cultured human laryngeal squamous cell carcinoma epithelia. PLoS One. 2013;8(6):e65750
10. Yu L, Zhou L, Wu S. et al. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J Surg Oncol. 2014;12:118
11. Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15(4):338-44
12. Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlöw B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One. 2014;9(4):e94621
13. Horst D, Scheel SK, Liebmann S. et al. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009;219(4):427-34
14. Prince ME, Sivanandan R, Kaczorowski A. et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973-8
15. Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. 2014;14:15
16. Kokko LL, Hurme S, Maula SM. et al. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral Oncol. 2011;47(6):510-6
17. Timmermans AJ, de Gooijer CJ, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. T3-T4 laryngeal cancer in The Netherlands Cancer Institute; 10-year results of the consistent application of an organ-preserving/-sacrificing protocol. Head Neck. 2015;37(10):1495-503
18. Gourin CG, Dy SM, Herbert RJ. et al. Treatment, survival, and costs of laryngeal cancer care in the elderly. Laryngoscope. 2014;124(8):1827-35
19. Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod. 2007;22(3):733-42
20. Valli H, Sukhwani M, Dovey SL. et al. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102(2):566-580.e7
21. Di MD, Toso A, Chen JJ. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515(7525):134-7
22. Welzel G, Seitz D, Schuster S. Magnetic-activated cell sorting (MACS) can be used as a large-scale method for establishing zebrafish neuronal cell cultures. Sci Rep. 2015;5:7959
23. Pozzi V, Sartini D, Rocchetti R. et al. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell Physiol Biochem. 2015;36(2):784-98
24. Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173
25. Vincent Z, Urakami K, Maruyama K, Yamaguchi K, Kusuhara M. CD133-positive cancer stem cells from Colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile. Genes Cancer. 2014;5(7-8):250-60
26. Noto Z, Yoshida T, Okabe M. et al. CD44 and SSEA-4 positive cells in an oral cancer cell line HSC-4 possess cancer stem-like cell characteristics. Oral Oncol. 2013;49(8):787-95
27. Xu R, Cai MY, Luo RZ, Tian X, Han JD, Chen MK. The Expression Status and Prognostic Value of Cancer Stem Cell Biomarker CD133 in Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2016;152(3):305-11
28. Bi Y, Meng Y, Wu H, Cui Q, Luo Y, Xue X. Expression of the potential cancer stem cell markers CD133 and CD44 in medullary thyroid carcinoma: A ten-year follow-up and prognostic analysis. J Surg Oncol. 2016;113(2):144-51
29. Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH, Kim HR. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. 2015;46(4):1582-8
30. Zhong C, Wu JD, Fang MM, Pu LY. Clinicopathological significance and prognostic value of the expression of the cancer stem cell marker CD133 in hepatocellular carcinoma: a meta-analysis. Tumour Biol. 2015;36(10):7623-30
31. Sim MW, Grogan PT, Subramanian C. et al. Effects of peritumoral nanoconjugated cisplatin on laryngeal cancer stem cells. Laryngoscope. 2016;126(5):E184-90
32. Huang CX, Zhu Y, Duan GL. et al. Screening for MiRNAs related to laryngeal squamous carcinoma stem cell radiation. Asian Pac J Cancer Prev. 2013;14(8):4533-7
33. Chen XH, Bao YY, Zhou SH, Wang QY, Wei Y, Fan J. Glucose transporter-1 expression in CD133+ laryngeal carcinoma Hep-2 cells. Mol Med Rep. 2013;8(6):1695-700
34. Wang L, Guo H, Lin C, Yang L, Wang X. Enrichment and characterization of cancer stem-like cells from a cervical cancer cell line. Mol Med Rep. 2014;9(6):2117-23
Author contact
![]() Corresponding authors: Prof. Binquan Wang (wbq_xyorg) and Prof. Shuxin Wen (wensxsxcom), Department of Otolaryngology, Head & Neck Surgery, The First Hospital Affiliated with Shanxi Medical University, 85 Jiefang Road South, Taiyuan, China, Tel: (+86-0351)4639218.
Corresponding authors: Prof. Binquan Wang (wbq_xyorg) and Prof. Shuxin Wen (wensxsxcom), Department of Otolaryngology, Head & Neck Surgery, The First Hospital Affiliated with Shanxi Medical University, 85 Jiefang Road South, Taiyuan, China, Tel: (+86-0351)4639218.

 Global reach, higher impact
Global reach, higher impact