3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(6):976-982. doi:10.7150/jca.18124 This issue Cite
Research Paper
Is pretreatment Epstein-Barr virus DNA still associated with 6-year survival outcomes in locoregionally advanced nasopharyngeal carcinoma?
1. Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, People's Republic of China;
2. Department of Radiation Oncology, the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai 519001, Guangdong Province, China;
3. Department of Medical Statistics and Epidemiology & Health Information Research Center & Guangdong Key Laboratory of Medicine, School of Public Health, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
* These authors contributed equally to this manuscript.
Received 2016-10-28; Accepted 2016-12-23; Published 2017-3-12
Abstract
Purpose: The objective of this study was to confirm the association between pretreatment Epstein-Barr virus (EBV) DNA (pre-DNA) load and survival outcomes after long-term follow-up in patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Materials and Methods: Between November 2009 and February 2012, a total of 1036 patients with LA-NPC were enrolled. There were 762 patients in stage III and 274 in stage IVA-B. All patients were treated with radical radiotherapy with or without chemotherapy, and pre-DNA concentrations were quantified by a polymerase chain reaction assay. Patient outcomes were evaluated.
Results: The 5-year overall survival (OS), distant metastasis-free surviva (DMFS), locoregional relapse-free survival (LRFS), and progression-free survival (PFS) rates were 84.7%, 87.0%, 90.2%, and 77.1%, respectively. By using previously defined pre-DNA cutoff value (1500 copies/ml pretreatment), pre-DNA was an independent prognostic predictor for OS, DMFS, and PFS using log-rank test. Multivariate Cox analysis also confirmed these results. Subgroup analysis indicated that the 5-year OS, DMFS, and PFS rates in patients staged IVA-B with pre-DNA < 1500 copies/ml were similar to those patients staged III with pre-DNA ≥ 1500 copies/ml, whereas patients staged IVA-B patients with pre-DNA ≥ 1500 copies/ml predicted worse outcome.
Conclusions: In this expanded study, the prognostic significance of pre-DNA was confirmed using predefined cutoff value in an independent patient group, and pre-DNA was identified as an independent prognostic marker for the risk stratification in LA-NPC.
Keywords: Nasopharyngeal carcinoma, Epstein-Barr virus DNA, Survival, Overall stage, Prognostic value.
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in South China and Southeast Asia, with an annual incidence of 15-50 cases per 100,000 [1]. As a result of anatomic constraints and its high degree of radiosensitivity, radiotherapy is the mainstay treatment modality for NPC. Recent advances in radiation technology and imaging techniques, the outcome for patients with early-stage disease is usually favorable; however, the outcome for locoregionally advanced NPC (LA-NPC, defined as an overall stage of III/IVA-B) is still unsatisfactory [2-3].
Currently, the extent of disease, as embodied by the TMN staging system, is the most important prognostic factor for NPC [4]. However, TNM staging system may not be precise enough to predict prognosis of NPC because patients with the same TNM stage and histological classification often have different prognoses. The limited power of TNM staging in determining individual patient outcomes highlights the need for better prognostic indicators for NPC.
Numerous attempts have been made to establish useful systems to predict the survival of NPC patients [5-6]. The circulating of pretreatment Epstein-Barr virus (EBV) DNA (pre-DNA) has been identified as a useful prognostic factor of individual tumor characteristics, facilitating the evaluation of disease development and treatment effectiveness [7-8]. On the basis of premise, we hypothesized that the combination of pre-DNA and TNM staging could improve the prognostic value of treatment outcomes for LA-NPC. To test this hypothesis, we replicated our prognostic analysis of pre-DNA in an independent cohort of 1036 patients with LA-NPC and further investigated the relation between pre-DNA levels and treatment outcomes in these patients after long-term follow-up.
Materials and Methods
Study population
We retrospectively reviewed the records of all NPC patients who were treated with intensity-modulated radiotherapy (IMRT) at the Cancer Center of Sun Yat-sen University (Guangzhou, People's Republic of China) between November 2009 and February 2012. The eligibility criterias were as follows: pathologically diagnosed non-keratinizing or undifferentiated carcinoma of the nasopharynx (World Health Organization [WHO] type II or III); age of 18-70 years; stage III-IVA-B disease according to the American Joint Committee on Cancer staging system (7th edition) [9]; no evidence of distant metastasis; Karnofsky performance score ≥70; receiving radical IMRT with or without chemotherapy as per the standard guidelines.
All patients underwent a pretreatment examination that included a complete medical history, physical examination, hematology and biochemistry profiles, magnetic resonance imaging (MRI) of the neck and nasopharynx, chest radiography, abdominal ultrasonography, and a whole body bone scan or positron emission tomography (PET)/CT. The scans were independently evaluated by two radiologists specializing in head and neck cancers and any disagreements were resolved by consensus. The ethic committee of Sun Yat-Sen University Cancer Center approved this study.
Treatment
All patients received a planned total dose of 68-72 Gy in 2-2.27 Gy fractions at 5 fractions/week suing IMRT. Combination chemoradiotherapy was used when indicated. Details of the radiotherapy techniques and rationale of using chemotherapy have been published previously [10]. In all, 972 out of 1036 (93.8%) patients received chemotherapy, and 64 out of 1036 (6.2%) patients received radiotherapy alone. The chemotherapy regimens included concurrent chemotherapy alone, concurrent chemotherapy combined with neoadjuvant chemotherapy (NACT), in conjunction with a platinum-based therapeutic clinical trial. Deviations from the institutional guidelines were due to organ dysfunction, suggesting intolerance to the chemotherapy, and patient refusal.
Quantification of plasma Epstein-Barr Virus DNA
Peripheral venous blood (3 mL) was collected before treatment from each patient into EDTA-containing tubes and centrifuged at 3000 rpm for 5min. Plasma DNA was extracted and subjected to a real-time quantitative PCR assay as described previously [11]. A cutoff level of 1,500 copies/ml was chosen to define low and high EBV DNA levels because this threshold has previously been shown to be prognostic in previous NPC studies using the same measurement system [8, 11].
Follow-up and Statistical analysis
After treatment, patients were followed every 3 months for the first 3 years and every 6 months thereafter. The end points included distant metastasis-free survival (DMFS), defined as the period from the date of treatment to the date of distant relapse or patient censoring at the date of last follow-up; local-regional relapse-free survival (LRFS), defined as the period from the first day of treatment to the date of locoregional relapse or patient censoring; progression-free survival (PFS), defined as the time to first failure at any site development of second primary cancer or death of any cause or patient censoring; and overall survival (OS), defined as the date of treatment to the date of death or patient censoring.
All analyses were performed using R3.1.2. Chi-square test or Fisher's exact test was used to compare the basic characteristics of the patients between two groups. Actuarial rates were calculated using the Kaplan-Meier method and differences were compared using the log-rank test. Univariate stratified survival analyses were performed with odds ratio (OR) and 95% confidence interval (CI). Cox proportional hazards models were used to assess the impact of independent prognostic factors in multivariate analyses. We then evaluated the combined association of pre-DNA and overall stage with patients' survival by dividing participants into different groups. Two-tailed P values <0.05 were considered statistically significant.
Results
Patient Characteristics
At total of 1036 LA-NPC patients who met all of the criteria were analyzed. Among these patients, 762 patients were stage III and 274 patients were stage IVA-B. There were 785 males and 251 females, with a sex ratio of 3.1:1. The median patient age was 45 years (range, 18-70 years). Chemotherapy was administered to 972 (94%) patients, and 64 (6%) patients received IMRT alone. Concurrent chemoradiotherapy (CCRT) was delivered to 630 (61%) patients, and NACT + CCRT was delivered to 342 (33%) patients. Overall, 396 patients (38%) had pre-DNA < 1500 copies/ml, 640 patients (62%) had pre-DNA ≥ 1500 copies/ml. In patients with pre-DNA < 1500 copies/ml, there were 298 (75%) patients diagnosed with stage III and 98 (25%) with stage IVA-B, respectively. In patients with pre-DNA ≥ 1500 copies/ml, there were 464 patients (73%) diagnosed with stage III and 176 (28%) with stage IVA-B, respectively. The baseline clinicopathological features of the two groups are listed in Table 1. Compared with pre-DNA < 1500 copies/ml, patients with pre-DNA ≥ 1500 copies/ml were more likely to present with stage IVA-B, but the correlation did not reach statistical significance (P = 0.071). The pre-DNA level was higher in patients staged IVA-B than in patients with staged III (P < 0.001), with median values of 1390 copies/ml (quartile range: 0-9,300 copies/ml) in patients with staged III and 5160 copies/ml (quartile range: 0-40,200 copies/ml) in patients with staged IVA-B, respectively.
Treatment outcomes
All of the patients were followed up to April 30, 2016. The median follow-up was 60.1 months (range: 1.3-79.5 months). Two hundred and thirty-seven patients had treatment failure, with 102 of them experiencing local-regional relapse, and 135 developing distant metastases, and 22 patients suffering both failure types. Of the 135 patients who had distant metastasis, liver is the most common metastasis site, about 53 patients finally developed liver metastasis, followed by bone (48 patients), lung (39 patients), and paraaortic lymph nodes (28 patients). Thirty patients had developed multi-organ metastasis.
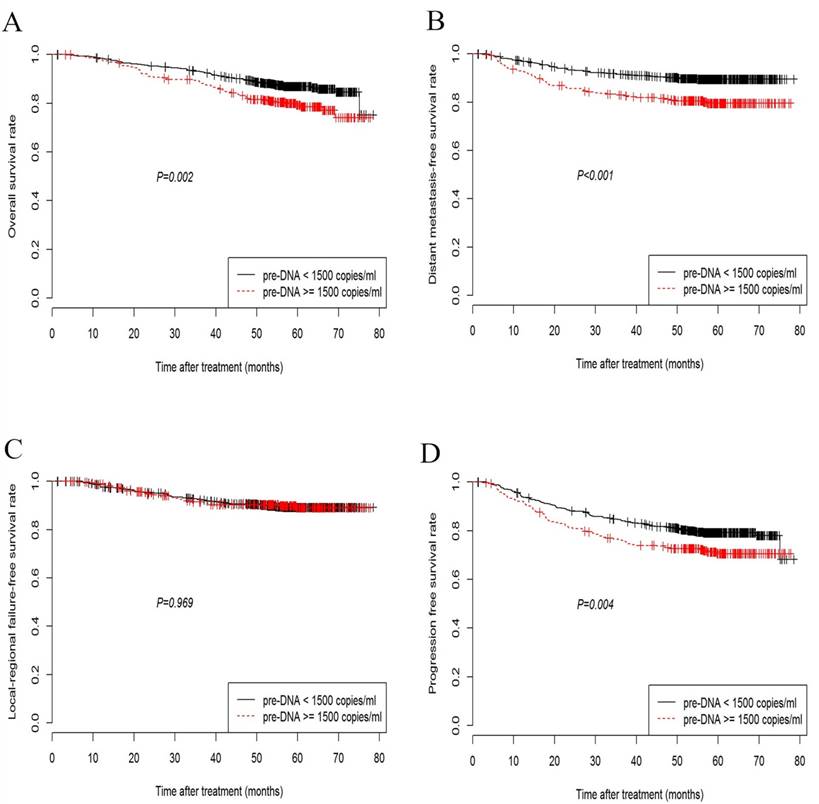
Overall, the 5-year OS, DMFS, LRFS, and PFS rates were 84.7%, 87.0%, 90.2%, and 77.1%, respectively. The 5-year OS rate among the patients with low pre-DNA levels was significantly higher than the rate in patients with high pre-DNA levels (86.9% vs. 79.4%, P = 0.002; Fig. 1A). Significant differences between patients with low and high pre-DNA levels were also detected in the case of the 5-year DMFS (89.9% and 80.4%, P < 0.001; Fig. 1B) and PFS (79.4% vs. 71.8%, P = 0.004; Fig. 1D). However, the 5-year LRFS was the same regardless of the pre-DNA value at diagnosis (90.0% vs. 90.5%, P = 0.969; Fig. 1C).
Patient characteristics
| Characteristic | n (%) | Pre-DNA, copies/mL | P | |
|---|---|---|---|---|
| < 1500 | ≥ 1500 | |||
| Age (years) | 0.892 | |||
| < 60 | 912 (88.0) | 346 | 566 | |
| ≥ 60 | 124 (12.0) | 51 | 73 | |
| Gender | 0.051 | |||
| Male | 785 (75.8) | 287 | 498 | |
| Female | 251 (24.2) | 109 | 142 | |
| Histology | 0.943 | |||
| WHO II | 59 (5.7) | 21 | 38 | |
| WHO III | 977 (94.3) | 375 | 602 | |
| T stagea) | 0.069 | |||
| T1 | 42 (4.1) | 20 | 22 | |
| T2 | 56 (5.4) | 18 | 38 | |
| T3 | 736 (71.0) | 289 | 447 | |
| T4 | 202 (19.5) | 69 | 133 | |
| N stagea) | 0.162 | |||
| N0 | 122 (11.8) | 60 | 62 | |
| N1 | 580 (56.0) | 228 | 352 | |
| N2 | 222 (21.4) | 72 | 150 | |
| N3 | 112 (10.8) | 36 | 76 | |
| Clinical stagea) | 0.071 | |||
| III | 762 (73.6) | 298 | 464 | |
| IVA-B | 274 (26.4) | 98 | 176 | |
| Treatment method | 0.357 | |||
| IMRT alone | 64 (6.2) | 33 | 31 | |
| CCRT | 630 (60.8) | 248 | 382 | |
| NACT + CCRT | 342 (33.0) | 115 | 227 | |
Abbreviations: WHO, World Health Organization; IMRT, intensity-modulated radiotherapy; CCRT, concomitant radiochemotherapy; NACT, neoadjuvant chemotherapy.
a) According to the 7th American Joint Committee on Cancer/International Union Against Cancer staging system;
Kaplan-Meier curves showing overall survival (A), distant metastasis-free survival (B), loco-regional failure-free survival (C), and progression-free survival (D) in nasopharyngeal carcinoma patients with pre-DNA < 1500 or ≥ 1500 copies/ml.
Univariate & Multivariate analysis
Univariate analysis by log-rank test revealed that overall stage and pre-DNA were prognostic factors for OS, DMFS and PFS. And histology was prognostic factor for LRFS (Supplementary table 1). Multivariate analysis was performed to adjust for various prognostic factors. The following parameters were included in a Cox proportional hazards model: sex, age (≥ 60 years vs. < 60 years), histology, treatment (RT alone vs. CCRT vs. NACT + CCRT), T category, N category, overall stage, and pre-DNA (≥ 1500 copies/ml vs. < 1500 copies/ml). The age, pre-DNA and overall stage were found to be independent prognostic factors for OS (OR = 1.78, 95%CI: 1.25-2.52; OR = 1.65, 95%CI: 1.10-2.47; OR = 1.93, 95%CI: 1.31-2.86, respectively). The multivariate analysis also revealed that the age, pre-DNA and overall stage were significant predictors of PFS. N category (OR = 1.55, 95%CI: 0.64-0.97), pre-DNA (OR = 2.99, 95%CI: 1.84-4.87) and overall stage (OR = 1.61, 95%CI: 1.07-2.44) were independent prognostic factors for DMFS. However, only the histological type (OR = 2.49, 95%CI: 0.21-0.75) was an independent indicator of LRFS (Table 2).
Multivariate analysis of prognostic factors for NPC patients
| Endpoint | Characteristics | OR | 95% CI |
|---|---|---|---|
| OS | |||
| Age | 1.78 | 1.25-2.52 | |
| Pre-DNA | 1.65 | 1.10-2.47 | |
| N stage | 1.26 | 0.79-2.01 | |
| Overall stage | 1.93 | 1.31-2.86 | |
| DMFS | |||
| Pre-DNA | 2.99 | 1.84-4.87 | |
| N stage | 1.55 | 0.64-0.97 | |
| Overall stage | 1.61 | 1.07-2.44 | |
| LRFS | |||
| Histology | 2.49 | 0.21-0.75 | |
| Pre-DNA | 1.13 | 0.79-1.96 | |
| Overall stage | 1.50 | 0.99-2.28 | |
| PFS | |||
| Age | 1.37 | 1.05-1.79 | |
| Pre-DNA | 1.68 | 1.23-2.29 | |
| N stage | 1.22 | 0.83-1.80 | |
| Overall stage | 1.57 | 1.15-2.14 |
Abbreviations: CI, confident interval; OR, odds ratio; DMFS, distant metastasis free survival; LRFS, locoregional relapse free survival; PFS, progression-free survival; OS, overall survival.
Combination of the pre-DNA and overall stage
The incidence of DMFS was evaluated for patients stratified by both overall stage and pre-DNA. The highest rate of 5-year DMFS was observed in patients with stage III & low pre-DNA (97.4%). The 5-year DMFS rate was 86.2% in patients with stage III & high pre-DNA, which was similar to patients staged IVA-B & low pre-DNA (86.2% vs. 86.8%; P=0.341), suggesting these patients have a similar risk of developing distant metastasis. The lowest rate of 5-year DMFS was observed in patients with stage IVA-B & high pre-DNA, down to 79.1%. According to the pre-DNA level and overall stage, patients were split into the following categories: group 1, stage III & low pre-DNA (510 patients); group 2, stage III & high pre-DNA and stage IVA-B & low pre-DNA (368 patients); group 3, stage IVA-B & high pre-DNA (158 patients).
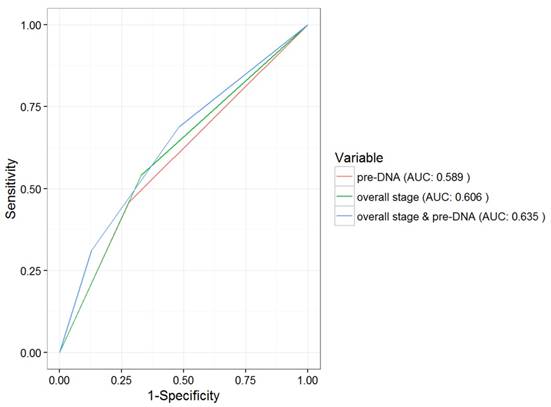
The above patient groups were also significantly correlated with OS and PFS. The 5-year OS rates in groups 1-3 were 95.1%, 86.1% and 79.1%, respectively (P = 0.012). Significant differences between the three subgroups were also seen in the case of the 5-year PFS (89.5%, 77.5% and 69.8%, respectively, P < 0.001). However, the 5-year LRFS did not differ among the three groups (93.1%, 91.6% and 88.8%, respectively, P = 0.618). In addition, the AUC for pre-DNA overall stage, and the combination of overall stage and pre-DNA with respect to DMFS were 0.589, 0.606 and 0.635, respectively (Fig. 2).
Discussion
Nowadays, TNM staging at initial presentation not only is important for reporting the extent of disease but also is crucial in determining the proper management strategy. However, according to recent studies, the TNM staging system is not satisfactorily accurate in terms of risk segregation and survival prediction [12]. Thus, it is necessary to integrate new biomarkers and diagnostic imaging assays to determine the risk segregation and survival prediction. Recent advances in cancer biology have helped develop many clinically useful biomarkers that can aid in grouping patients according to risk for those who have similar stage and can guide clinicians toward the optimal treatment for each patient. The goal of our study was to use pre-DNA and overall stage as outcome predictors to stratify the LA-NPC patients in improving outcome prediction.
ROC curve analysis in comparing the prognostic value of pre-DNA, overall stage and the combination of pre-DNA and overall stage. Abbreviations: ROC = Receiver operating characteristic; AUC = area under curve.
Consistent with previous studies, we found that the level of pre-DNA is strongly associated with DMFS, PFS, and OS in LA-NPC [13-15]. According to our findings, patients who had pre-DNA < 1500 copies/ml significantly reduced risks of disease progression and distant metastasis compared with those with pre-DNA ≥ 1500 copies/ml. Furthermore, high levels of pre-DNA leads to poorer survival outcomes in patients with stage IV. Meanwhile, patients who diagnosed with stage III & high levels of pre-DNA had similar risks of disease progression and locoregional relapse with those who diagnosed with stage IV & low levels of pre-DNA.
It is inspiring to see that pre-DNA and overall stage are two complementary factors for the stratification of patients with high risk for treatment failure. Based on the TNM stage and the level of pre-DNA, we identified groups at low, medium, and high risk of distant metastasis. Interestingly, patients with high levels of pre-DNA & stage III had a similar incidence of distant metastasis with patients diagnosed with stage IV NPC & low pre-DNA, suggesting that including pre-DNA levels as another parameter for evaluating tumor stage may help identify patients at higher risk for distant metastasis than would be suggested by their TNM stage alone. While the mechanistic link between high pre-DNA and distant metastasis is not clear, it may be inferred that higher levels of pre-DNA reflect more malignant cells in NPC.
Early identification of risk stratification may be critical for the choice of treatment strategy and will be useful in improving outcomes. Apparently, this analysis indicates that patients diagnosed with stage IV & high levels of pre-DNA most frequently experienced distant failure; therefore, this subgroup requires more intense treatment. Several studies [16-17] revealed a positive impact on survival of NACT combined with CCRT for patients with LA-NPC. It is possible that NACT followed by CCRT is not effective enough to eradicate micrometastases in this group of patients, therefore, the inclusion of adjuvant chemotherapy may be necessary. Secondly, the EBV viral antigens expressed by NPC tumor cells have become attractive targets for immunotherapy as another form of targeted therapy in this era of personalized management [18-19]. Adoptive immunotherapy is a potential avenue for patients in the high-risk group. Thirdly, the administration of an additional target agent, such as bevacizumab, is a known chimeric monoclonal antibody that targets vascular endothelial growth factor [20].
Lin et al [11] and Leung et al [21] reported that pre-DNA was a significant prognostic factor for LRFS, but we did not observe a relationship between pre-DNA and posttreatment relapse. The difference in the results may be reflect differences in the radiation techniques utilized in the different studies. For example, Peng et al [22] reported that the 5-year local-regional control rate was only 75% for patients treated with 2D-CRT. However, more recently Lin et al [23] and Xiao et al [24] reported that the 5-year local control rate exceeds 90% in patients with LA-NPC treated with IMRT. Additionally, the stronger benefit of chemotherapy in locoregional control and overall survival by enhancing the local effect of radiotherapy has been proven [25-26]. In our study, more than 90% of the patients received concurrent chemotherapy. The prognostic value of pre-DNA in LRFS may be diluted and narrowed due to the good locoregional control of concurrent chemotherapy. Thirdly, local relapse is also associated with gross tumor volume [27] and this study does not rule out the impact of gross tumor volume on local relapse. It is possible that high pre-DNA levels may place patients at higher risk of relapse than patients with similar a gross tumor volume and low pre-DNA levels.
Although this study has many clinical implications, we should be clear that it is a retrospective study with its own limitations. A major limitation of our study is that the plasma pre-DNA measurements are from a single center and the techniques for measuring plasma EBV DNA are not yet standardized across centers. Second, we lacked the date of posttreatment EBV DNA, future study need to continue to evaluate the prognostic value of posttreatment EBV DNA in the IMRT era.
Conclusion
In summary, we concluded that pre-DNA can be used to predict subsequent failure and patient survival based on the current analysis of our entire group of patients with long-term follow-up. Both pre-DNA and overall stage have significant prognostic impact in patients with LA-NPC. Several studies that included fewer patients also have produced similar results [28-29]. It may be inferred from our study that pre-DNA could be used in conjunction with traditional TNM staging before treatment to perform further risk stratification and early treatment modification.
Supplementary Material
Supplementary table 1.
Acknowledgements
This work was supported by grants from the Sun Yat-sen University Clinical Research 5010 Program (No.2012011), the National Natural Science Foundation of China (No.81372409), and the National Natural Science Foundation of China (No. 81402532). The funders had no role in study design, data collection and analysis, decisions regarding publication, or preparation of the manuscript.
Authors' contributions
JYN, YJJ and ZF conducted data collection and drafted the manuscript. ZWJ and ZGQ helped to perform the statistical analysis. WSY and QZY participated in the design of the study. SY conceived of the study, and participated in its design. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90
2. Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IW, Lu JJ. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer. 2013;119(23):4111-8
3. Qiu WZ, Huang PY, Shi JL, Xia HQ, Zhao C, Cao KJ. Neoadjuvant chemotherapy plus intensity-modulated radiotherapy versus concurrent chemoradiotherapy plus adjuvant chemotherapy for the treatment of locoregionally advanced nasopharyngeal carcinoma: a retrospective controlled study. Chin J Cancer. 2016;35:2
4. Xu L, Pan J, Wu J. et al. Factors associated with overall survival in 1706 patients with nasopharyngeal carcinoma: significance of intensive neoadjuvant chemotherapy and radiation break. Radiother Oncol. 2010;96(1):94-9
5. Tang LQ, Li CF, Li J. et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst. 2015:108 (1)
6. Tang LQ, Chen QY, Guo SS. et al. The impact of plasma Epstein-Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer. 2014;111(6):1102-11
7. Chan KC. Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):598-603
8. Wang WY, Twu CW, Chen HH. et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer. 2013;119(5):963-70
9. Edge SB, Byrd DR, Compton CC. American Joint Committee on Cancer manual for staging of cancer [M]. 7th ed. Philadelphia: JB Lippincot. 2009
10. Lai SZ, Li WF, Chen L. et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661-8
11. Lin JC, Wang WY, Chen KY. et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461-70
12. Lee AW, Ng WT, Chan LK. et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012;48(10):1007-13
13. Chen WH, Tang LQ, Guo SS. et al. Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin-Based Concurrent Chemoradiotherapy in Intensity- Modulated Radiotherapy Era. Medicine. 2016;95(5):e2642
14. Shao JY, Zhang Y, Li YH. et al. Comparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. 2004;24(6):4059-66
15. Hou X, Zhao C, Guo Y. et al. Different clinical significance of pre- and post-treatment plasma Epstein-Barr virus DNA load in nasopharyngeal carcinoma treated with radiotherapy. Clin Oncol. 2011;23(2):128-33
16. Boscolo-Rizzo P, Tirelli G, Mantovani M. et al. Non-endemic locoregionally advanced nasopharyngeal carcinoma: long-term outcome after induction plus concurrent chemoradiotherapy in everyday clinical practice. Eur Arch Otorhinolaryngol. 2015;272(11):3491-8
17. Chen YP, Guo R, Liu N. et al. Efficacy of the Additional Neoadjuvant Chemotherapy to Concurrent Chemoradiotherapy for Patients with Locoregionally Advanced Nasopharyngeal Carcinoma: a Bayesian Network Meta-analysis of Randomized Controlled Trials. J Cancer. 2015;6(9):883-92
18. Masmoudi A, Toumi N, Khanfir A. et al. Epstein-Barr virus-targeted immunotherapy for nasopharyngeal carcinoma. Cancer Treat Rev. 2007;33(6):499-505
19. Gottschalk S, Heslop HE, Rooney CM. Adoptive immunotherapy for EBV-associated malignancies. Leuk Lymphoma. 2005;46(1):1-10
20. Lee NY, Zhang Q, Pfister DG. et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172-80
21. Leung SF, Lo YM, Chan AT. et al. Disparity of sensitivities in detection of radiation-naïve and postirradiation recurrent nasopharyngeal carcinoma of the undifferentiated type by quantitative analysis of circulating Epstein-Barr virus DNA1,2. Clin Cancer Res. 2014;9(9):3431-4
22. Peng G, Wang T, Yang KY. et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286-293
23. Lin S, Pan J, Han L. et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2012;110(3):385-9
24. Xiao WW, Huang SM, Han F. et al. Local control, survival, and late toxicities of locoregionally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874-83
25. Lee AW, Lau WH, Tung SY. et al. Hong Kong Nasopharyngeal Cancer Study Group. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23(28):6966-75
26. Baujat B, Audry H, Bourhis J. et al. Chemotherapy in locoregionally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64(1):47-56
27. Guo R, Sun Y, Yu XL. et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol. 2012;104(3):294-9
28. Chan AT, Lo YM, Zee B. et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94(21):1614-9
29. Lin JC, Wang WY, Liang WM. et al. Long-term prognostic effects of plasma epstein-barr virus DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(5):1342-8
Author contact
![]() Corresponding authors: Ying Sun, Ph.D., Department of Radiation Oncology, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel: +86-20-87342253; Fax: +86-20-87343295; E-mail: sunyingorg.cn Zhen-Yu Qi, M.D., Department of Radiation Oncology, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel: +86-20-87342590; Fax: +86-20-87342590; E-mail: qizhyorg.cn
Corresponding authors: Ying Sun, Ph.D., Department of Radiation Oncology, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel: +86-20-87342253; Fax: +86-20-87343295; E-mail: sunyingorg.cn Zhen-Yu Qi, M.D., Department of Radiation Oncology, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel: +86-20-87342590; Fax: +86-20-87342590; E-mail: qizhyorg.cn

 Global reach, higher impact
Global reach, higher impact