Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(10):1786-1794. doi:10.7150/jca.17859 This issue Cite
Research Paper
Bone Marrow Derived Mesenchymal Stem Cells Involve in the Lymphangiogenesis of Lung Cancer and Jinfukang Inhibits the Involvement In Vivo
1. Department of Respiratory Medicine, Jiangsu Province Hospital of Traditional Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, PR China;
2. State Key Laboratory of Analytical Chemistry for Life Science and Collaborative Innovation Center of Chemistry for Life Sciences, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023, PR China;
3. Tumor Institute of Traditional Chinese Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, PR China
Received 2016-10-9; Accepted 2017-4-8; Published 2017-7-1
Abstract
Lymphangiogenesis plays an important role in cancer metastasis. Bone marrow-derived mesenchymal stem cells (BMMSCs) migrate to the site of tumorigenesis and in turn promote the metastasis. However, whether BMMSCs involve in the lymphangiogenesis of lung cancer is unclear. Jinfukang has clinically been used for the treatment of non small cell lung cancer (NSCLC) in China. In this study, to investigate the involvement of BMMSCs in lymphangiogenesis in lung cancer, and evaluate the inhibitory effect of Jinfukang on the lymphangiogenesis, chimeric mice were prepared by transplanting bone marrow from green fluorescent protein (GFP) transgenic mice (C57BL/6-EGFP) into irradiated C57BL/6 mice. Then, the chimeric mice were injected subcutaneously with freshly prepared Lewis lung carcinoma cell suspension to make lung tumor model, and the model mice were further orally administrated with Jinfukang once per day for 3 weeks. Four weeks after the bone marrow transplantation, GFP-positive cells primarily existed in bone marrow of acceptor mice, and three more weeks after, Lewis lung carcinoma cells formed a tumor mass in chimeric mice. Observation of GFP-positive cells revealed that BMMSCs transferred into the lung tumor. Immunofluorescent analyses of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), a lymphatic endothelium marker, demonstrated a part of lymphatic endothelial cells in lung cancer were derived from BMMSCs, and those lymphatic endothelial cells contributed to the lung tumor lymphangiogenesis. Furthermore, Jinfukang treatment resulted in a significant reduction of the average weight of the tumor mass in chimeric mice, and displayed a significant lower number of LYVE-1 positive cells. The present results suggest that BMMSCs transfer to tumor, differentiate into lymphatic endothelial cells, and involve in the lymphangiogenesis in lung cancer of mice. Jinfukang inhibits the lung tumor mass via suppression of the BMMSCs transformation and lung tumor lymphangiogenesis. Our findings might provide the potential for the cancer therapies.
Keywords: bone marrow-derived mesenchymal stem cells, chimeric mice, lung cancer, lymphangiogenesis, Jinfukang
Introduction
Lung cancer, as the leading cause of cancer death worldwide, is the second most common cancer among both men and women that carries tremendous social and economic burden [1, 2]. The 5-year survival rate for lung cancer remains low at 16.8% and even < 5% for patients with metastatic diseases [2]. The metastasis of tumor cells to distant organs is a characteristic of most tumor types, and the primary fatal reason of cancer patients and over 90% of cancer death is associated with metastatic spread [3]. Generally, most metastases occur following the invasion and dissemination through the circulatory systems, blood and lymphatic vessels. Although it is well known that solid tumor spreads via the blood vessels, increasing evidences suggest that the most common pathway of initial metastasis is via the lymphatic vessels, and metastatic spread of cancer through the lymphatic system affects hundreds of thousands of patients yearly [4, 5]. The detection of tumor cells in the tumor-draining lymph node is one of the most important predictors of both patient prognosis and therapeutic strategy [5]. Furthermore, the meaningful thing is that growth of new lymphatic vessels, named as lymphangiogenesis, is activated in cancer, but is largely inactive in normal physiology. Therefore, inhibition of lymphangiogenesis provides the great therapeutic potential for cancers [6].
Bone marrow-derived mesenchymal stem cells (BMMSCs), as key components of the bone marrow microenvironment, are hierarchical postnatal stem/progenitor cells capable of self-renewing and differentiating into a limited number of cell types, supporting hematopoietic cells, and regulating immune activity [7]. BMMSCs have been widely applied for tissue engineering and cell-based therapies in all fields ranging from orthopedic to cardiovascular medicine [8]. BMMSCs support hematopoietic cells and regulate immune activity, while, their immunophenotypic features are still in dispute [9]. Studies demonstrate that BMMSCs have possessed great potential as a novel therapeutic strategy for a variety of lung diseases, including emphysema, bronchopulmonary dysplasia, fibrosis, and acute respiratory distress syndrome [10]. However, increasing evidences demonstrate that activated by cancer cells, BMMSCs migrate to the site of tumorigenesis and in turn promote metastasis, such as breast, bone cancers [11]. Human BMMSCs from a physiologic bone environment can home to orthotopically implanted primary human breast tumors [12], and promote breast cancer metastasis when mixed with human breast carcinoma cells [13]. Thus, BMMSCs stimulate lymphangiogenesis in physiological and pathological (malignant tumor) conditions [14].
As described above, lymphangiogenesis plays an important role in the cancer metastasis, lymph vessels are an important component in lymph node metastasis. Therefore, understanding of the association between BMMSCs and lymph vessels is extremely important. Tawada et al revealed that transplantation of green fluorescent protein-positive bone marrow (BM) in nude mice that implanted human gastric cancer cells (MKN45) represented recruitment and incorporation of BM-derived lymphatic endothelial progenitor cells (LEPCs) into gastric lymphatics [15]. It was also reported that co-injection of BMMSCs and lung cancer cells (Lewis lung carcinoma) in mice increased intratumoral lymphatic vessel density and the tumor growth in vivo [16]. However, whether BMMSCs can transform into lymphatic endothelial cells and then involve in the lymphangiogenesis in lung cancer is still unclear.
Jinfukang is an oral liquid formulation that consists of 12 Chinese herbal medicines (Table S1). The main chemical components of Jinfukang include icarrin and astragaloside A, which were used for quality control [17, 18] (Figure S1). Jinfukang was developed in China, approved by the China Food and Drug Administration (CFDA), and has clinically been used for the treatment of non small cell lung cancer (NSCLC). It was also reported that Jinfukang reduced side effects and improved the response rates when combined with chemotherapy in NSCLC patients [19, 20]. However, the effect of Jinfukang on lymphangiogenesis of lung cancer is unknown.
In the present study, aiming at clarifying direct correlation between BMMSCs and lymphangiogenesis in vivo and exploring the effect of Jinfukang on the lymphangiogenesis in lung cancer, we investigated the transformation of BMMSCs into lymphatic endothelial cells and their involvement in lymphangiogenesis of Lewis lung cancer using chimeric mice by transplanting bone marrow from green fluorescent protein (GFP) transgenic mice (C57BL/6-EGFP) into irradiated C57BL/6 mice. The inhibitory effect of Jinfukang on the lymphangiogenesis was also incorporated.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee of Jiangsu Province Hospital of Traditional Chinese Medicine, Jiangsu (Permit Number: 2015-011). All operations were performed by skillful experimenters under ethyl ether anaesthesia, and all efforts were made to minimize suffering. Sacrificed animals were euthanized by CO2.
Animals were housed in a climate-controlled and specified pathogen free (SPF) room, 12 h light/dark photoperiod. All the animals had free access to food and water. To adapt to the new environment, the mice were held for 1 week before experiments.
Production of bone marrow chimeras
Green fluorescent protein (GFP) (C57BL/6-EGFP) transgenic mice that ubiquitously express GFP were purchased from laboratory animal center of Academy of Military Medical Science of China (Beijing, China). C57BL/6 mice were obtained from Changzhou Cavens Lab Animal Co. Ltd. (Changzhou, China). Four- to six-week-old female GFP-transgenic mice (n = 10) were used as bone marrow (BM) donors, and eight-week-old male C57BL/6 mice were used as recipients.
The generation of bone morrow chimeric mice was referred to the reported method [21]. Briefly, as depicted in Fig 1, under general anesthesia with pentobarbital (Sigma, USA), bone marrow cells (BMCs) were obtained by flushing the femora and tibiae bones of twelve C57BL/6-EGFP donor mice with RPMI-1640 culture medium (HyClone, USA). After centrifugation (1500 rpm), the cell viability of BMCs was checked with trypan blue assay (Sigma, USA) and the viability is greater than 98%. Then, the cells were suspended in sterile Dulbecco's phosphate-buffered saline (PBS) (Sigma, USA) at 5×107 cell/mL. Thirty nine recipient mice were administered a single 8 Gy dose of whole-body irradiation (2 Gy/min). After 2 h of irradiation, 0.1 mL (5×106 cell/mouse) of the BMCs suspension was injected into the tail vein of each irradiated recipient mouse under ethyl ether anaesthesia. As a negative control, 4 mice were received the same amount of the sterile phosphate-buffered saline (PBS) without the cells through the tail vein. During the 4-week experiment period, the mice were surveyed with a daily observation and the number of dead mice. Four weeks after bone marrow transplantation, to determine the transplantation efficiency of GFP-positive BM, eye peripheral blood (10 μL) from recipient mice was collected under ethyl ether anaesthesia, and GFP-positive cells were observed by fluorescent microscopy on cover slides, and also analyzed with flow cytometry (Backman FC500, USA). Furthermore, the BM was taken from 5 mice that euthanized by CO2, and the ratio of GFP+ and total leukocytes in BM with more than 90% indicated the success of the transplantation. Finally, at the end of the experiment, BM was taken from all mice, and the ratio of GFP+ and total leukocytes was calculated.
Lewis lung carcinoma cell culture
Lewis lung carcinoma cells (3LL, called also LLC) were purchased from Nanjing KeyGen Biotech Inc. (Nanjing, China). The cells were cultured in RPMI-1640 culture medium (HyClone, USA) with 10% heat inactivated fetal bovine serum (HyClone, USA) in a CO2 incubator under moist condition of 5% CO2 in air at 37 oC. The cells were harvested from exponentially growing cultures, and resuspended in Dulbecco's phosphate-buffered saline (PBS) (Sigma, USA), 1×108 viable cells/mL for animal model preparation. Viability of cells was determined by trypan blue (Sigma, USA).
Mice tumor model
Thirty chimeric mice were used for the following experiments. The mice were injected subcutaneously with 0.1 mL of the freshly prepared Lewis lung carcinoma cells suspension (1×107 cells/mouse) into armpit of the mice under ethyl ether anaesthesia, and then the mice were maintained under daily survey in standard laboratory conditions.
During the entire experiment period, the bidirectional diameters of tumors in mice were measured with caliper every other day. Tumor volumes were calculated using the formula: V = 0.5ab2, where V as tumor volume, a as the larger diameter, and b as the smaller diameter [22].
An overview of strategies used in the bone marrow chimeric mice and Lewis lung tumor model generation. Bone marrow cells were collected from green fluorescence protein (GFP) transgenic mice donor (C57BL/6-EGFP). Irradiation (8 Gy, 2 Gy/min) killed all bone marrow cells in the host mice (C57BL/6). GFP-tagged bone marrow cell transplantation was performed, and 4 weeks later, GFP-positive bone marrow cells in host mouse were analyzed to confirm chimerism. Lewis lung carcinoma cells suspension (1×107 cell/mouse) was injected subcutaneously into armpit of the chimeric mice to prepare Lewis lung tumor model, and Jinfukang was orally administrated to the model mice.
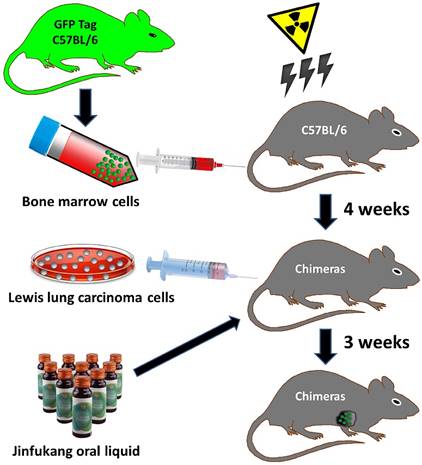
Mice grouping and intervention procedures
Jinfukang oral liquid formulation was kindly provided by Jilin Jinfukang Pharmaceutical Company (Jilin, China) and contains 3 g of raw herbal medicines per milliliter.
Two days after Lewis lung carcinoma cells injection, 30 mice were randomized into five groups, each group 6 mice: control group; Jinfukang groups at doses of 15, 30, 60 g/kg (equivalent to raw herbal medicines), respectively; positive control group cyclophosphamide at dose of 30 mg/kg. Jinfukang was diluted to the designed concentrations and mice were orally administrated with 0.4 mL of Jinfukang once per day. The mice in control group were given with same volume of saline. Cyclophosphamide was intraperitoneally injected at dose of 30 mg/kg once every two days for 14 days in positive control group. The experiment period was 3 weeks.
The overall workflow used in the present study is summarized in Figure 1.
Observations and measurements
During the whole experiment period, the tumor volumes and mice body weights were recorded every other day. At the end of the experiment, the mice were euthanized by CO2, and the xenografts were excised, weighted immediately, and then the tumors were kept in liquid nitrogen for further assays.
The tumor inhibition rate was calculated with following formula: tumor inhibition rate (%) = (mean weight of tumors in the control group - mean weight of tumors in the treated group)/mean weight of tumors in the control group × 100%.
Immunofluorescent analyses
The fresh tumor tissues were cut and frozen in OTC (Sakura Finetek USA Inc., Torrance, CA), and the blocks were fastened to the cryomicrotome (CM1950, Leica, Germany). Then, the samples were cut at 8 μm thickness, and mounted on a glass slide.
As lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) is the specific marker of lymphatic vessel endothelial cells, the sections were immunostained with the primary antibody, rabbit anti-mouse LYVE-1 (1:100; Santa Cruz Biotechnology, Inc., USA), rabbit anti-mouse secondary antibody FITC/TRITC that emits red fluorescence (1:200; Jackson ImmunoResearch, USA), and 40,6-diamidino-2-phenylindole (DAPI, Nanjing KeyGen Biotech Inc., Nanjing, China). Images were recorded using a laser scanning confocal microscope (TCS SP5, Leica, Germany), in which the lymphatic vessel endothelial shows red fluorescence. The images of the tumor sections were analyzed with Image pro-Plus 6.0 software (Media Cybernetics, Inc., Maryland, USA). The numbers of BM sourced cells with green fluorescence (GFP+), lymphatic endothelial cells with red fluorescence (LYVE-1+), and BM-derived lymphatic endothelial cells co-expressed green and red fluorescence (GFP+/LYVE-1+) were counted separately. The ratio of BM-derived lymphatic endothelial cells was calculated as GFP+/LYVE-1+ cells to total LYVE-1+ cells.
Statistical analyses
All data were expressed as mean ± standard error. Statistical analyses were conducted by analysis of variance (ANOVA). All analyses were performed using the SPSS 18.0 software package, and probability values of 0.05 or less were considered to be statistically significant.
Results
Evaluation of chimeric mice
In order to prepare the chimeric mice, we transplanted whole bone marrow cells from the mice that expressed green fluorescent protein into lethally irradiated mice. During 16th to 21th day period after bone marrow transplantation, 4 mice without bone marrow transplantation showed a great activity decrease, and a tremendous lack of responsiveness to manual stimulation. Therefore, 4 mice were separately euthanized by CO2, and eye peripheral blood and bone marrow were collected. As usual, no GFP-positive cells were observed in eye peripheral blood, and further, almost no cells were observed in BM (data not shown).
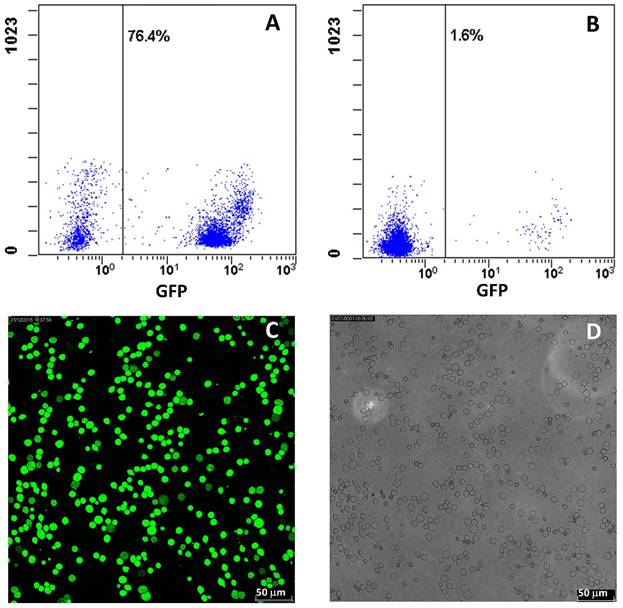
Four weeks after bone marrow transplantation, the transplanted GFP-positive cells were checked. All of the mice transplanted BM displayed GFP-positive cells in the peripheral circulation under fluorescent microscopy, while the cell number displayed a quite big variation. To get more reliable data, the GFP-positive cells were analyzed with flow cytometry, and the result exhibited that the average GFP-positive cell number was about 33.6% ranged from 1.6% to 76.4% (Figure 2A, 2B). Thus, to clarify the success of chimerism, we further randomly selected 5 mice and confirmed the GFP-positive cells in BM. The results demonstrated that GFP-positive cells primarily existed in BM of all 5 mice, and the ratio of GFP-positive cells and total leukocytes from 5 mice was 95 ± 5.3% (Figure 2C, 2D). After the whole experiment, GFP-positive cells in BMs from all mice were checked and the ratio was in all mice more than 90%.
Assessment of GFP-positive cells from chimeric mice. A, B: Typical flow cytometry analysis for GFP-positive cells in eye peripheral blood from chimeric mice. C: Representative fluorescent images of GFP-positive cells from bone marrow of chimeric mice. D: Total leukocytes from bone marrow of chimeric mice. The ratio of GFP-positive cells to total leukocytes was counted from C and D.
Bone marrow derived mesenchymal stem cells (BMMSCs) and lymphangiogenesis in lung tumor
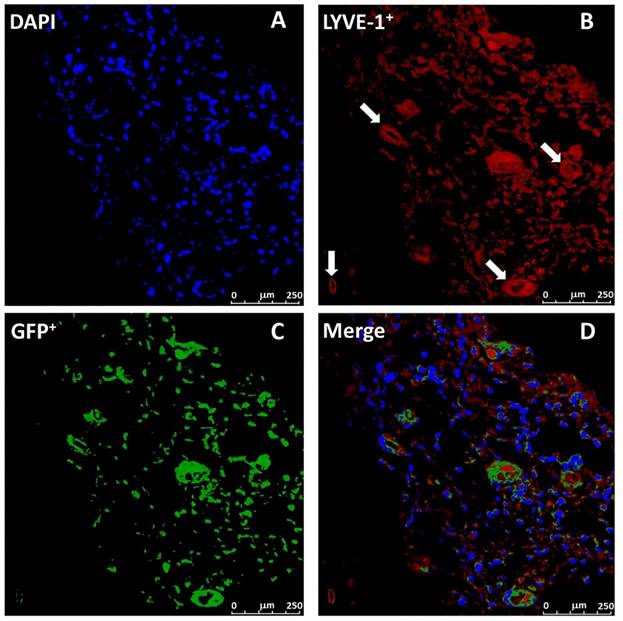
As Lewis lung carcinoma cells were subcutaneously implanted in the mice receiving GFP-positive BM transplantation, three weeks later, Lewis lung carcinoma cells formed a tumor mass, and the tumor size reached to the 3339.001 ± 570.482 mm3. In order to verify the lymphangiogenesis, a histological analysis of lung tumor was performed. The sections of tumor tissue were immunestained with antibody of LYVE-1, a lymphatic marker expressed by lymphatic endothelium [23]. As shown in Figure 3B, LYVE-1-positive cells formed lymphatic vessels and revealed a clear lung tumor lymphangiogenesis. To study whether the BMMSCs sourced cells contribute to tumor lymphangiogenesis, GFP-positive cells in the tumor were further monitored.
Confocal microscopic images revealed that GFP-positive cells from BMMSCs clearly appeared in the lung tumor (Figure 3C), which revealed that BMMSCs transferred into the tumor. Detailed observation showed that a majority of GFP-positive cells were consistent with the locations of tumor lymphatics, and 85.4 ± 3.3% of LYVE-1-positive lymphatic endothelial cells co-expressed GFP (Figure 3D). The results demonstrated that a part of the lymphatic endothelial cells in lung cancer were derived from BMMSCs, and those lymphatic endothelial cells greatly contributed to the lung tumor lymphangiogenesis.
Effects of Jinfukang on kinetics and growth of lung tumor
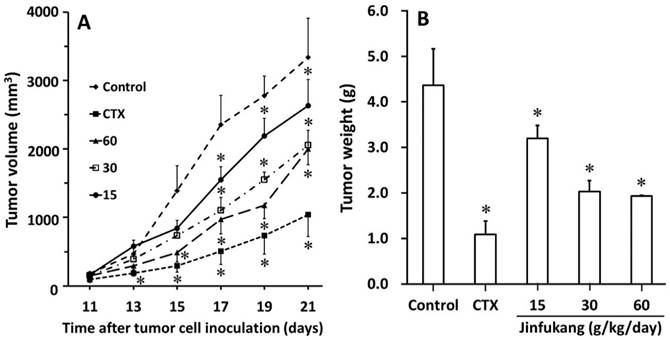
In order to understand the direct growth inhibition of Jinfukang on Lewis lung tumor in vivo, we investigated the inhibitory effects on kinetics and growth of tumor in chimeric mice. The progress of tumor formation in each chimeric mouse was carefully examined during the period of 3 weeks. As can be seen in Figure 4A, there were no significant differences in tumoral volumes until day 11, while on day 13, Jinfukang at dose of 60 g/kg/day inhibited the tumor growth significantly (470.9 ± 92.4 mm3 of control group vs 292.3 ± 54.0 mm3 of Jinfukang group, p < 0.01). From day 15 to the end of the experiment, Jinfukang at all doses presented statistically lower volumes compared with control group in a dose dependent manner.
At the end of the experiment, the in vivo tumor growth was also analyzed with measuring the weight of tumor mass and the results were displayed in Figure 4B. The average weights of the tumor mass formed in the chimeric mice of all Jinfukang treated groups exhibited a significant lower number compared with that of control group. The results revealed that the administration of Jinfukang, not only slowed the onset of the tumor development, but also inhibited the increase of tumor growth.
Jinfukang Inhibited BMMSCs transformation and lymphangiogenesis
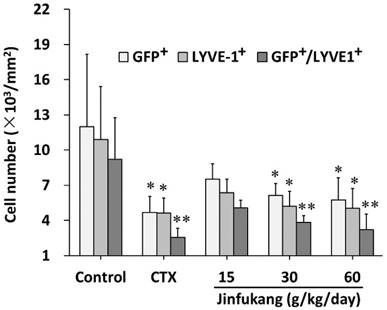
To understand whether Jinfukang has effect on BMMSCs transferring into the tumor, the GFP-positive cells in the tumor were also monitored. As shown in Figure 5, although Jinfukang at dose of 15 mg/kg showed an inhibitory tendency, the number of the GFP-positive cells was significant smaller in Jinfukang treated groups at doses of 30, 60 mg/kg (6144 ± 984 and 5760 ± 1312, respectively), which suggested that Jinfukang inhibited the BMMSCs transferring.
As LYVE-1 is a lymphatic marker, to verify whether Jinfukang affects the lymphangiogenesis of lung cancer, the LYVE-1+ lymphatic endothelial cells in lung tumor sections were counted. As can be seen in Figure 5, Jinfukang treated groups displayed a significant lower number of LYVE-1+ compared with control group in a dose dependent manner. The result demonstrated that Jinfukang suppressed the lymphangiogenesis of lung cancer. Furthermore, the cell number co-expressed GFP and LYVE-1 was also analyzed. The data clearly demonstrated that Jinfukang suppressed the BMMSCs differentiated into lymphatic endothelial cells.
Representative images of tumor lymphatics and BM-derived lymphatic endothelial cells in C57BL/6 chimeric mice. Images stained with DAPI (A, blue), LYVE-1 (B, red), GFP (C, green), and D is a merge of A, B and C. BM-derived lymphatic endothelial cells were detected as double positive for LYVE-1 and GFP. White arrow indicated the lymph vessel.
Effect of Jinfukang on the growth of Lewis lung cancer in C57BL/6 chimeric mice. A: Tumor growth kinetics. B: Tumor weight. Control group received vehicle. CTX group intraperitoneally injected with cyclophosphamide at dose of 30 mg/kg, once every two days. Groups 15, 30, 60 treated with Jinfukang oral liquid at doses of 15, 30, 60 g/kg/day (equivalent to raw herbal drugs), respectively. Data are expressed as mean ± SD, n = 6. *p < 0.01 compared with untreated group (Control).
Effect of Jinfukang on BMMSCs transformation and lymphangiogenesis in lung tumor. GFP+, GFP-positive cells; LYVE-1+, LYVE-1-positive cells; GFP+/LYVE-1+, the cells co-expressed GPF and LYVE-1. Control group received vehicle. CTX group intraperitoneally injected with cyclophosphamide at dose of 30 mg/kg, once every two days. Groups 15, 30, 60 treated with Jinfukang oral liquid at doses of 15, 30, 60 g/kg/day (equivalent to raw herbal drugs), respectively. Data are expressed as mean ± SD, n = 6. *p < 0.05, **p < 0.01 compared with untreated group (Control).
Discussion
It is well accepted that BMMSCs contribute to tumor outgrowth and metastasis through different mechanisms [11-13], however, their involvement during lymphangiogenesis in lung cancer is poorly described. In the present study, we firstly transplanted Lewis lung cancer cells into chimeric mice that prepared via transplantation of whole bone marrow cells from GFP-positive transgenic mice into irradiated mice. The results demonstrated that BMMSCs transferred into lung tumor, differentiated to lymphatic endothelial cells, involved in lymphatic vessels formation and contributed to the lung tumor lymphangiogenesis in vivo. Furthermore, a Chinese traditional prescription, Jinfukang was investigated on the Lewis lung tumor, and the data revealed that Jinfukang inhibited not only the Lewis lung tumor mass, but also lymphangiogenesis of the lung tumor.
Mesenchymal stem cells (MSCs) are a population of pluripotent progenitor cells that originate from bone marrow or other tissues, such as adipose tissue and cord blood, and play a crucial role in lung cancer cell growth, metastasis [14]. Over the past decade, MSCs are increasingly being used in tissue engineering and cell-based therapies in all fields, including novel therapeutic strategy for a variety of lung diseases, in which BMMSCs are the most studied cell therapy [24, 25]. However, as co-cultures and co-injections of MSCs and tumor cells were used in the most researches, probably due to the different ratios, effects of MSCs on lung cancer growth and progression were controversial [24]. As BMMSCs from GFP transgenic mice (C57BL/6-EGFP) expressed GFP, and enabled the distinction of host from other cells with single-cell resolution [26], to understand the correlations between MSCs and lung cancer, we collected BMMSCs from the transgenic mice, and prepared chimeric mice.
As a proper conditioning of the recipient mice is a key factor in successful BMCs transplantation [27], to deplete the bone marrow from the recipient mice while minimize the damage, a single 8 Gy dose of whole-body irradiation was conducted. The result demonstrated that there were almost no cells in bone marrow from the mice received the irradiation but did not receive the transplantation, which suggested the success of the irradiation. To confirm the success of the chimeric mice preparation, we firstly checked the BMCs in peripheric blood and the results demonstrated that the GFP positive cells displayed a big variation, in some of mice the cells were occasionally observed, which was consistent with the reported data [15]. Therefore, to confirm the success of the transplantation, we checked the GFP-positive cells in bone marrow. The results demonstrated that the GFP-positive cells clearly existed in the bone marrow with more than 90% of total leukocytes from 4 weeks after the transplantation to the end of the current study, suggesting the BMCs from donor mice were successfully engrafted in recipient ones.
Although BMMSCs reside predominantly in the bone marrow, increasing evidence revealed that BMMSCs have the ability to migrate and home to primary and metastatic tumors [28]. In the present study, the GFP-positive cells clearly presented in the lung tumor, which revealed that the BMCs traveled into the tumor from bone marrow.
Recently, growing evidences suggest that lymphangiogenesis is an important initial event in solid tumor growth and spread, tumor-induced lymphangiogenesis has been correlatively and functionally associated with metastasis formation [3, 29]. Lymphatic endothelial cells (LECs) compose the lymphatic vasculature, and control lymphocyte migration into and out of lymph nodes [30]. It is well accepted that BMMSCs have the potential to differentiate into mesenchymal tissues such as osteocytes, chondrocytes, and adipocytes myocytes and neurons [31], while, little information is available on their potential to differentiate into lymphatic endothelial cells in lung tumor. LYVE-1 is a cell surface receptor on LECs that can be used as a LECs marker [32]. In the current study, the cells co-expressed GFP and LYVE-1 were distinctly presented in the lung tumor and engaged into lymph vessel formation. These results demonstrated that BMMSCs transferred to lung tumor, differentiated into LECs, and involved in the lymphangiogenesis. It was also reported that MSCs contained in NSCLC patients showed accelerated tumor growth kinetics and might crucially contribute to lung cancer progression [33], our data showed that MSCs definitely involved in the lymphangiogenesis of lung cancer at least in vivo.
As mentioned above, lymphangiogenesis is an extremely important process in cancer, inhibition of lymphangiogenesis provides a great therapeutic potential to restrict the cancer progression [34]. Jinfukang, a traditional Chinese herbal medicine, slows tumor growth and progression, and inhibits tumor angiogenesis in NSCLC patients. The possible mechanism might be via inhibition of the tumor cells to secrete vascular endothelial growth factor (VEGF) [35]. The present data revealed that Jinfukang inhibited kinetics and growth of lung tumor at least partially via the suppression the transfer of BMMSCs r to lung tumor site, differentiation into LECs, and the lymph vessel formation.
Lymphangiogenesis is a complex cellular event, including proliferation, sprouting, migration and tube formation. LECs have an active role in the interactions of tumor cells with lymphatic vessels, and various factors, such as VEGF-C/VEGF receptor 3 (VEGFR3), VEGFD, neuropilin 2 (NRP2) et al. are involved in their survival, proliferation and migration [3, 35]. In the current study, which signaling pathways are key factors engaged in the process are unclear. Furthermore, which chemical components responsible for the inhibitory effect of Jinfukang on lymphangiogenesis are warranted to be further clarified.
In summary, our findings demonstrate for the first time that BMMSCs transfer to lung tumor site, differentiate into LECs, and further involve in lymphangiogenesis of lung tumor in vivo. Furthermore, we affirmed that Jinfukang inhibits lung tumor mass at least via suppression of BMMSCs related lymphangiogenesis. Our results provide the potential for the cancer therapies. Further research is required to clarify the molecular mechanism between BMMSCs and lymphangiogenesis process, and determine the active components of Jinfukang for developing anti-lymphangiogenesis agents.
Supplementary Material
Figure S1 and Table S1.
Abbreviation
BMSCs: Bone marrow-derived mesenchymal stem cells (BMMSCs); MSCs: Mesenchymal stem cells; NSCLC: non small cell lung cancer; GFP: green fluorescent protein; LYVE-1: lymphatic vessel endothelial hyaluronan receptor-1; BM: bone marrow; LEPCs: lymphatic endothelial progenitor cells; BMCs: bone marrow cells; LECs: lymphatic endothelial cells
Acknowledgements
This work was supported by Research Project for Practice Development of National TCM Clinical Research Bases (JDZX2012117), and the Project of Key Discipline for TCM Construction of Jiangsu Province, China (No. JS1302).
Competing Interests
The authors declare that they have no competing interests to disclose.
References
1. Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer—Recent advances and future perspectives. Int J Cancer. 2016;138:2549-61
2. Kanodra NM, Silvestri GA, Tanner NT. Screening and early detection efforts in lung cancer. Cancer. 2015;121:1347-56
3. Li S, Li Q. Cancer stem cells, lymphangiogenesis, and lymphatic metastasis. Cancer Lett. 2015;357:438-47
4. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159-72
5. Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene. 2014:1-10
6. Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancerprogression. Oncogene. 2012;31:4499-508
7. Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y. et al. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012;3:40
8. Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise Review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713-23
9. Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37-47
10. Antunes MA, Laffey JG, Pelosi P, Rocco PRM. Mesenchymal Stem Cell Trials for Pulmonary Diseases. J Cell Biochem. 2014;115:1023-32
11. El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM. et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci USA. 2012;109:17460-65
12. Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2012;70:10044-50
13. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-63
14. Maertens L, Erpicum C, Detry B, Blacher S, Lenoir B, Carnet O. et al. Bone marrow-derived mesenchymal stem cells drive lymphangiogenesis. PLoS ONE. 2014;9:e106976
15. Tawada M, Hayashi S, Osada S, Nakashima S, Yoshida K. Human gastric cancer organizes neighboring lymphatic vessels via recruitment of bone marrow-derived lymphatic endothelial progenitor cells. J Gastroenterol. 2012;47:1057-60
16. Buttler K, Badar M, Seiffart S, Laggies S, Gross G, Wilting J. et al. De novo hem- and lymphangiogenesis by endothelial progenitor and mesenchymal stem cells in immunocompetent mice. Cell Mol Life Sci. 2014;71:1513-27
17. Ning JS, Yang JO. Analysis of icariin in Jinfukang oral solution with HPLC. Chinese J Pharmaceut Anal. 2002;22:328-9
18. Luo YH, Zhou GP, Long XH, Yang JO, Ning JS. Study on quality standards for Jifukang oral solution. Chinese Tradi Patent Med. 2001;23:180-2
19. Liu J, Shi Z, Li H, Xu Z, Zhu Y, Zhao L. et al. Clinical observation on 271 cases of non-small lung cancer treated with Yifei Kangliu Yin (Jin-Fu-Kang). Chin J Integr Tradit West Med. 2001;7:247-50
20. Liu J, Pan M, Li Y, Ye D, Guo Y, Li Y. Clinical study of oral liquid Jin Fu Kang for the treatment of primary non-small cell lung cancer. Tumor (Shanghai). 2001;21:463-5
21. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Reinhard Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25-33
22. Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229-34
23. Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, McCarthy JB. Hyaluronan, inflammation, and breast cancer progression. Front Immunol. 2015;6:236
24. Liu R, Wei S, Chen J, Xu S. Mesenchymal stem cells in lung cancer tumor microenvironment: their biological properties, influence on tumor growth and therapeutic implications. Cancer Lett. 2014;353:145-52
25. Antunes MA, Laffey JG, Pelosi P, Rocco PRM. Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem. 2014;115:1023-32
26. Hoffman RM. Application of GFP imaging in cancer. Lab Invest. 2015;95:432-52
27. Aparicio-Vergara M, Shiri-Sverdlov R, de Haan G, Hofker MH. Bone marrow transplantation in mice as a tool for studying the role of hematopoietic cells in metabolic and cardiovascular diseases. Atherosclerosis. 2013;213:335-44
28. Hong IS, Lee HY, Kang KS. Mesenchymal stem cells and cancer: friends or enemies? Mutat Res-Fund Mol M. 2014;768:98-106
29. Martinez-Corral I, Makinen T. Regulation of lymphatic vascular morphogenesis: implications for pathological (tumor) lymphangiogenesis. Exp Cell Res. 2013;319:1618-25
30. Tewalt EF, Cohen JN, Rouhani SJ, Engelhard VH. Lymphatic endothelial cells - key players in regulation of tolerance and immunity. Front Immunol. 2012;3:305
31. Ikhapoh IA, Pelham CJ, Agrawal DK. Sry-type HMG box 18 contributes to the differentiation of bone marrow-derived mesenchymal stem cells to endothelial cells. Differentiation. 2015;89:87-96
32. Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13:1-7
33. Gottschling S, Granzow M, Kuner R, Jauch A, Herpel E, Xu EC. et al. Mesenchymal stem cells in non-small cell lung cancer - different from others? Insights from comparative molecular and functional analyses. Lung Cancer. 2013;80:19-29
34. Wang ZC, Zeng JL, Zhang HL, Li JW. Jin Fu Kang on tumor growth and metastasis and vascular endothelial growth factor of patients with advanced non-small cell lung cancer. Practical J Clin Med. 2011;4:148-9
35. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159-72
Author contact
![]() Corresponding author: Xian-Mei Zhou, E-mail: zhouxianmeijscom, phone: +86-25-86617141; Ling Xu, E-mail: xulq67com, phone: +86-21-65611782; Jian-Xin Li, E-mail: lijxnjuedu.cn, phone: +86-25-89686419
Corresponding author: Xian-Mei Zhou, E-mail: zhouxianmeijscom, phone: +86-25-86617141; Ling Xu, E-mail: xulq67com, phone: +86-21-65611782; Jian-Xin Li, E-mail: lijxnjuedu.cn, phone: +86-25-89686419

 Global reach, higher impact
Global reach, higher impact