Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(10):1801-1808. doi:10.7150/jca.17999 This issue Cite
Research Paper
Effect of pomalidomide on relapsed/refractory multiple myeloma: a systematic review and meta-analysis
Department of Hematology and Oncology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu Province, P.R. China
Received 2016-10-19; Accepted 2017-2-1; Published 2017-7-1
Abstract
In this work, we aim to further analyze the effect of pomalidomide for relapsed and/or refractory multiple myeloma (RRMM). A systematic literature search of PubMed, MEDLINE and EMBASE was conducted on September 20, 2016. Pooled effect size (ES) with corresponding 95% confidence intervals (CIs) were calculated using random-effects model. STATA software (version 12.0; Stata Corporation; College Station, TX, USA) was employed to do all statistical analyses. A total of 8 studies were included for analysis. The combined results demonstrated that the pooled proportion of overall response rate (ORR) was 0.35 (95% CI 0.27 to 0.43, P=0.000), and the pooled proportion of complete response rate (CRR) was 0.02 (95% CI 0.01 to 0.03, P=0.541). Pomalidomide was generally well tolerated by patients reported in the studies. Further studies would be required to conduct more prospective randomized controlled trials (RCTs) with larger samples to assess the proper place of pomalidomide as single agent or combined with other agents for RRMM.
Keywords: pomalidomide, multiple myeloma, meta-analysis
Introduction
Multiple myeloma (MM) is a hematologic disorder characterized by the proliferation of malignant plasma cell clones in the bone marrow or/and extramedually sites [1]. It is the second most common hematologic malignancy and accounts for as many as 20% of deaths from hematological malignancies and 2% of deaths from all cancers [2, 3]. MM is a heterogeneous disease, with its wide spectrum of aggression and treatment resistance and a diverse array of malignant cellular malfunctions, which drive individual clones [4, 5]. Although progresses have been made over the last few decades for the development of new and increasingly effective agents, the prognosis of MM still remains unfavorable and it is regarded as an incurable disease characterizing by rapid relapse and broad treatment refractoriness [6, 7]. To overcome this drug resistance, a number of therapeutic approaches have been developed in recent years [8]. The introduction of the immunomodulatory drugs (IMiDs) (eg. thalidomide and lenalidomide) and the proteasome inhibitors (eg. bortezomib and calfizomib), used either as single agent or combined with classic chemotherapy, have improved the outcome for patients with MM [9, 10]. However, even in patients who achieve stringent complete response (sCR), the disease will inevitably relapse, highlighting the necessity for the development of novel agents in treating newly diagnosed and relapsed/refractory MM (RRMM) [1, 4, 11-16].
Pomalidomide is one of the potent IMiDs and has been tested with very encouraging results for MM patients in early investigations, especially in those who have been refractory to both lenalidomide- and bortezomib-based therapies [17, 18]. It was approved by the Food and Drug Administration (FDA) in February 2013 and the European Medicines Agency (EMA) in August 2013 for use alone or in combination with dexamethasone for those patients with MM who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on their last therapy [13, 17]. Several clinical trials have shown that pomalidomide was effective for patients with RRMM [19]. However, the overall response rate (ORR) of pomalidomide varies in these studies, and these published reports consisted of the clinical trials with small sample sizes which have no enough power to determine the efficacy of pomalidomide for RRMM [20]. Also, there are no complete summary of the efficacy and toxicity of pomalidomide for updated published clinical trials. Here, we performed a systematic review and a meta-analysis of clinical trials to summarize the effect of pomalidomide for the treatment of patients with RRMM.
Methods
Study selection
We performed a literature search without language restrictions using the databases of PubMed, MEDLINE and EMBASE on September 20, 2016 according to the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) guidelines [21]. The search strategy included the phase “pomalidomiade” pairing independently with “multiple myeloma” or “MM”. The reference lists were screened of all of the identified studies in the field. Prospective trials (randomized controlled trials or single-armed observational trials) examining pomalidomiade as the treatment for RRMM were included. We included full texts and did not apply any restriction on age, gender or ethnicity. Retrospective studies, case reports, review articles and studies with less than 5 patients were excluded. When multiple publications reported on the same population, only the most recent study was included.
Data extraction
Data from each study were independently extracted by two reviewers using a standardized data-extraction form. Any disagreements were resolved by consensus or by consultation with a third reviewer. The following information was extracted from each study: (1) the first author's last name, (2) year of publication, (3) study design, (4) number and characteristics of subjects included, (5) mean age of subjects, (6) definition of RRMM, (7) dosage and procedure of pomalidomiade treatment, (8) response of the treatment, (9) patients' survival of the treatment and (10) effect size (95% confidential interval (CI)). Qualities of included non-comparative cohort studies using the Newcastle-Ottawa scale [22] and randomized controlled trials (RCTs) using the Cochrane tool for assessment of bias were assessed [23].
Statistical analysis
Considering some of the inter-study variation, the random-effects model was chosen to increase power and precision of this analysis regardless of heterogeneity for the entire study. All statistical analyses were conducted by the STATA software (version 12.0; Stata Corporation; College Station, TX, USA). Test results were considered to be statistically significant at p<0.05. We estimated relative risk (RR) with their 95% CI using the standardized mean difference (SMD). Heterogeneity was evaluated by I2 values, and we considered significant heterogeneity to be present when the I2 statistic was >50%, and moderate heterogeneity when the I2 statistic was >30%.
Results
Literature search
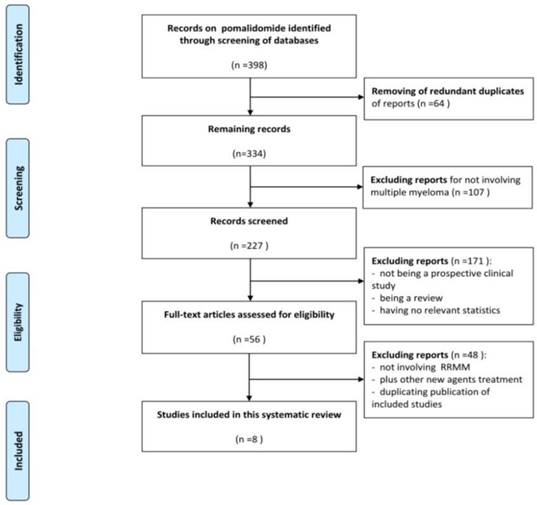
A total of 398 publications were identified during the initial search. After removing of redundant duplicates, 334 studies were included and considered as potentially relevant studies. After screening the title or abstract, 107 studies were excluded for not involving MM. Of the remaining 227 records, 171 reports were further excluded. Afterwards, 56 reports were retrieved and evaluated in detail. 48 of these studies met the exclusion criteria with 43 not involving RRMM, 2 combining carfilzomib (another novel agent) with pomalidomiade, and 3 duplicate publications of included studies. Eventually, 8 complete papers met the selection criteria and were included in this meta-analysis (Figure 1).
Study characteristics and qualities
The design features and characteristics of the included studies were presented in Table 1, including 4 non-comparative studies [24-27] and 4 RCTs [28-31]. A total of 891 evaluable patients were enrolled in the included eight prospective studies. The overall quality of the four single-arm pilot studies was moderate according to Newcastle-Ottawa scale, and the overall quality of the four RCTs were adequate according to Cochrane tool for assessment of bias (Table 2). Regimens and dosage of pomalidomiade in different studies were also different (Table 3).
Data flow chart of number of studies identified and included into the meta-analysis
Basic information and characteristics of included studies
| Study (year) | Country | Period | Design | No. of patients | Median age, range | Disease characteristics |
|---|---|---|---|---|---|---|
| Lacy et al. (2009)24 | US | November 2007 to August 2008 | Phase 2 | 60 | 66(35-88) | At least one but no more than three prior regimens (lenalidomide, thalidomide, or bortezomib) |
| Lacy et al. (2010)25 | US | November 2008 to April 2009 | Phase 2 | 34 | 62(39-77) | Previously treated, symptomatic, histologically confirmed MM refractory to lenalidomide therapy |
| Lacy et al. (2011)26 | US | May 2009 to November 2009 | Phase 2 | 35 (2mg) | 63(39-77) | Previously treated, symptomatic MM refractory to both lenalidomide and bortezomib therapy |
| November 2009 to April 2010 | 35(4mg) | 61(45-77) | ||||
| Leleu et al. (2013)28 | France | October 2009 to August 2010 | Randomized phase 2 | 43 (arm 21/28) | 60(45-81) | Relapsed MM after at least one prior regimen of myeloma treatment, nonresponders to at least two cycles of either the last line of lenalidomide or bortezomib |
| 41 (arm 28/28) | 60(42-83) | |||||
| San et al. (2013)29 | Australia, Canada, Europe, Russia and the US | March 2011 to Aug 2012 | Randomized phase 3 | 302* | 64(35-84) | Refractory or relapsed and refractory MM, and had failed at least two previous treatments of bortezomib and lenalidomide |
| Richardson et al. (2014)30 | US and Canada | December 2009 to April 1, 2011 | Randomized phase 2 | 113(POM+LoDEX) | 64(34-88) | Aged ≥18 years, had RRMM, and had measurable M-paraprotein levels in serum or urine. All patients had received ≥2 prior antimyeloma therapies, including ≥2 cycles of lenalidomide and ≥2 cycles of bortezomib, given separately or in combination |
| 108(POM alone) | 61(37-88) | |||||
| Leleu et al. (2015)27 | France | January 2012 to July 2013 | Phase 2 | 50 | 59(30-80) | RRMM following at least 1 prior regimen of myeloma treatment. All patients had loss of 17p (46%) and/or t(4;14) (64%) |
| Baz et al. (2016)31 | US | December 2011 to March 2014 | Randomized phase 2 | 36(PomDex) | 64(50-78) | RRMM received ≥2 prior lines of therapies to include a prior immunomodulatory drug, and patients were required to be refractory to lenalidomide |
| 34(PomCyDex) | 65(47-80) |
*Another 153 patients in the study were received high-dose dexamethasone (40 mg/day on days 1-4, 9-12, and 17-20, orally)
MM, multiple myeloma; POM, pomalidomide; PomCyDex, pomalidomide, dexamethasone and cyclophosphamide; PomDex, pomalidomide and low-dose dexamethasone; POM+LoDEX, pomalidomide plus low-dose dexamethasone; RRMM, relapsed/refractory multiple myeloma.
The quality of included studies
| Noncomparative studies | ||||||
|---|---|---|---|---|---|---|
| Study (year) | Representativeness of study sample | Ascertainment of exposure | Demonstration outcome was not present at start | Detection bias minimized | Attribution bias minimized | Follow-up time appropriate |
| Lacy et al. (2009)24 | Yes | Yes | Yes | Yes | Yes | Yes |
| Lacy et al. (2010)25 | Yes | Yes | Yes | Yes | Yes | Yes |
| Lacy et al. (2011)26 | Yes | Yes | Yes | Yes | Yes | Yes |
| Leleu et al. (2015)27 | Yes | Yes | Yes | Yes | Yes | Yes |
| Randomized controlled trials | ||||||
| Study | Random sequence generation | Allocation concealment | Performance bias | Detection bias | Attribution bias minimized | Reporting bias minimized |
| Leleu et al. (2013)28 | Yes | Unclear | Unclear | Unclear | Yes | Unclear |
| San et al. (2013)29 | Yes | Unclear | Unclear | Unclear | Yes | Unclear |
| Richardson et al. (2014)30 | Yes | Unclear | Unclear | Unclear | Yes | Unclear |
| Baz et al. (2016)31 | Yes | Unclear | Unclear | Unclear | Yes | Unclear |
Regimen and Dosage of the treatment
| Study (year) | Treatment |
|---|---|
| Lacy et al. (2009)24 | Pomalidomide was administered orally at a dose of 2 mg daily on days 1 through 28 of a 28-day cycle. Dexamethasone 40 mg daily was administered orally on days 1, 8, 15, and 22 of each cycle. |
| Lacy et al. (2010)25 | Pomalidomide was given orally at a dose of 2 mg daily on days 1-28 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15 and 22 of each cycle. |
| Lacy et al. (2011)26 | Pomalidomide was given orally at a dose of 2 or 4 mg daily on days 1-28 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15, and 22 of each cycle. |
| Leleu et al. (2013)28 | Pomalidomide 4 mg was given orally either daily on days 1 to 21 of each 28- day cycle (arm 21/28 days) or continuously of each 28-day cycle (arm 28/28 days). Dexamethasone 40 mg was given orally and once weekly to all patients. |
| San et al. (2013)29 | Patients assigned to the pomalidomide plus low-dose dexamethasone group were given 28 day cycles of pomalidomide (4 mg/day on days 1-21, orally) plus low-dose dexamethasone (40 mg/day on days 1, 8, 15, and 22, orally). Patients assigned to the high-dose dexamethasone group were given 28 day cycles of high-dose dexamethasone (40 mg/day on days 1-4, 9-12, and 17-20). Dexamethasone dose was reduced to 20 mg/day in all patients older than 75 years. Treatment was continued until progressive disease or unacceptable toxicity occurred. |
| Richardson et al. (2014)30 | Patients were randomized (1:1) to POM (4 mg/day on days 1-21 of each 28-day cycle) alone or with LoDEX (40 mg/week). Treatment continued until disease progression or unacceptable toxicity. |
| Leleu et al. (2015)27 | Pomalidomide 4 mg was given orally daily on days 1 to 21 of each 28-day cycle along with dexamethasone 40 mg, which was given orally to all patients on days 1, 8, 15, and 22 of each cycle. The treatment was given until progression. |
| Baz et al. (2016)31 | In the phase 1 (arm A) portion of the study, patients received pomalidomide at 4 mg orally on days 1 to 21 of a 28-day cycle, oral weekly cyclophosphamide (dose escalation 300-500 mg) on days 1, 8, and 15 (dose level 21 was cyclophosphamide 300 mg orally on days 1 and 8 only). Patients also received dexamethasone 40 mg orally on days 1 to 4 and 15 to 18 of a 28-day cycle for the first 4 cycles and subsequently 40 mg orally on days 1, 8, 15, and 22. The dose escalation used a standard “3+3” design. In the phase 2 portion of the study, patients were randomized to either arm B (pomalidomide and low-dose dexamethasone) or arm C (pomalidomide cyclophosphamide, and low-dose dexamethasone at the recommended phase 2 dose determined in arm A). Arm B patients received pomalidomide at 4 mg orally days 1 to 21 and dexamethasone 40 mg weekly and arm C patients received pomalidomide 4 mg days 1 to 21, dexamethasone 40 mg weekly, and oral cyclophosphamide 400 mg orally on days 1, 8, and 15 of a 28-day cycle. Patients who experienced progressive disease in arm B were allowed to crossover to arm D at the discretion of the treating physician, in which case oral weekly cyclophosphamide (400 mg orally on days 1, 8, and 15) was added to their tolerated dose of pomalidomide and dexamethasone. |
Response rate of pomalidomide treatment
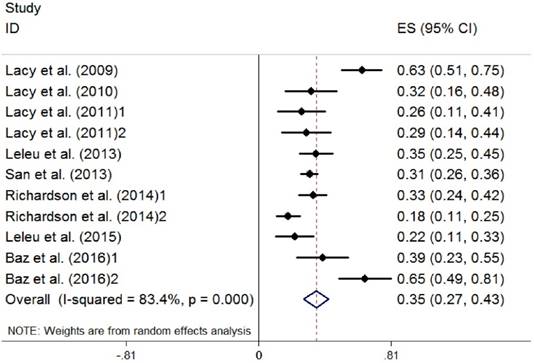
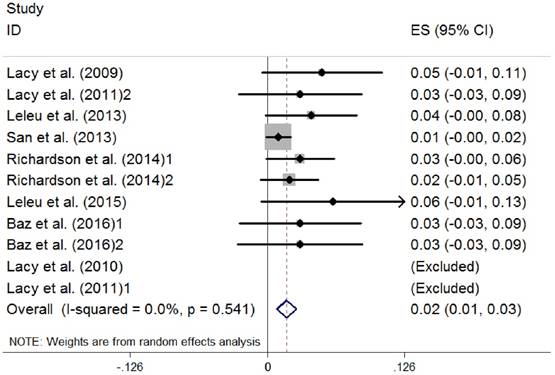
Because there were three studies using different regimens of pomalidomide, we divided them into two parts when analyzed [26, 30, 31]. Efficacy of the treatment was summarized in Table 4, including ORR, complete response (CR), very good partial response (VGPR), partial response (PR), median time-to-response (TOR), median overall survival (OS), median progression-free survival (PFS) and median duration of response (DOR). Data on the ORR (the rate of CR plus VGPR and PR) were extracted from the eight studies selected (891 patients). The random-effects model was chosen, and a high heterogeneity between studies (I2 =83.4%) was observed. The pooled proportion of ORR was 0.35 (95% CI 0.27 to 0.43, P=0.000) (Figure 2). Data on the complete response rate (CRR) were also extracted, and no heterogeneity existed (I2 =0.0%). The pooled proportion of CRR was 0.02 (95% CI 0.01 to 0.03, P=0.541) (Figure 3).
Potential side effects of pomalidomide treatment
The safety of pomalidomide was deemed good and no long-term complications were reported. In our included studies, common hematologic toxicities of the patients in different studies consisted of anemia, neutropenia and thrombocytopenia; nonhematologic toxicities included fatigue, dyspnea, bone pain, renal failure and pneumonia (Table 5). Mortality of patients seldom occurred in the studies and no deaths were attributed to pomalidomide.
Efficacy of the treatment
| Study (year) | Total no. | ORR(≥PR) | CR | VGPR | PR | Median TOR, months | Median OS, months | Median PFS, months | Median DOR, months |
|---|---|---|---|---|---|---|---|---|---|
| Lacy et al. (2009)24 | 60 | 38 (63%) | 3 (5%) | 17 (28%) | 18 (30%) | - | Not reached | 11.6 | Not reached |
| Lacy et al. (2010)25 | 34 | 11(32%) | 0 | 3(9%) | 8(24%) | 2 | 13.9 | 4.8 | 9.1 |
| Lacy et al. (2011)26 | 35(2mg) | 9(26%) | 0 | 5(14%) | 4(11%) | 1 | Not reached | 6.5 | Not reached |
| 35(4mg) | 10(29%) | 1(3%) | 3(9%) | 6(17%) | 1.7 | Not reached | 3.2 | 3.9 | |
| Leleu et al. (2013)28 | 84 | 29(35%) | 3(4%) | 2(2%) | 24 (29%) | 5.4 | 14.9 | 4. | 7.3 |
| San et al. (2013)29 | 302 | 95 (31%) | 3(1%) | 14(5%) | 78(26%) | - | 13.1 | 4.0 | 7.5 |
| Richardson et al. (2014)30 | 113(POM+LoDEX) | 37(33%) | 3(3%) | 0 | 34(30%) | 1.9 | 16.5 | 4.2 | 8.3 |
| 108(POM alone) | 19(18%) | 2(2%) | 0 | 17(16%) | 4.3 | 13.6 | 2.7 | 10.7 | |
| Leleu et al. (2015)27 | 50 | 11(22%) | 3(6%) | 0 | 8(16%) | 4.1 | 12 | 2.8 | 5.5 |
| Baz et al. (2016)31 | 36(PomDex) | 14(39%) | 1(3%) | 4(11%) | 9(25%) | - | 16.8 | 4.4 | - |
| 34(PomCyDex) | 22(65%) | 1(3%) | 3(9%) | 18(53%) | - | Not reached | 9.5 | - |
CR, complete response; DOR, duration of response; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; POM, pomalidomide; PomCyDex , pomalidomide, dexamethasone and cyclophosphamide; PomDex, pomalidomide and low-dose dexamethasone; POM+LoDEX, pomalidomide plus low-dose dexamethasone; TOR, time to response; VGPR, very good partial response
Overall response of pomalidomide treatment in patients with RRMM. (RRMM, relapsed/refractory multiple myeloma; ES, effect size)
Complete response of pomalidomide treatment in patients with RRMM. (RRMM, relapsed/refractory multiple myeloma; ES, effect size)
Adverse effects of pomalidomide treatment
| Study (year) | Treatment |
|---|---|
| Lacy et al. (2009)24 | Toxicity consisted primarily of myelosuppression. Grade 3 or 4 hematologic toxicity occurred in 23 patients (38%) and consisted of anemia (5%), thrombocytopenia (3%), and neutropenia (32%).The most common grade 3 or 4 nonhematologic toxicities consisted of fatigue (17%) and pneumonia (8%). |
| Lacy et al. (2010)25 | Toxicity consisted primarily of myelosuppression. Grade 3 or 4 hematologic toxicity occurred in 13 patients (38%) and consisted of anemia (12%), thrombocytopenia (9%) and neutropenia (29%). The most common grade 3/4 non-hematologic toxicity was fatigue (9%). |
| Lacy et al. (2011)26 | Toxicity consisted primarily of myelosuppression. Grade 3 or 4 hematologic toxicity regardless of attribution occurred in 83% (2-mg cohort) and 80% (4-mg cohort) and at least possibly attributed to the regimen occurred in 71% (2-mg cohort) and 74% (4-mg cohort). Grade 3 or 4 neutropenia (regardless of attribution) was seen in 51% (2-mg cohort) and 66% (4-mg cohort). Grade 3 or 4 nonhematologic toxicity regardless of attribution occurred in 69% (2-mg cohort) and 54% (4-mg cohort) and at least possibly attributed to the regimen was seen in 26% (2-mg cohort) and 26% (4-mg cohort). The most common nonhematologic toxicity was fatigue (2-mg cohort: 88%; 4-mg cohort: 91%) with grade 3/4 fatigue occurring in 9% of patients in both cohorts. |
| Leleu et al. (2013)28 | Grade 3 and 4 AEs that occurred in >10% of cases were neutropenia in 62%, anemia in 36%, thrombocytopenia in 27%, pneumonia in 13%, bone pain in 11%, renal failure in 11%, and dyspnea in 12%. |
| San et al. (2013)29 | The most common grade 3-4 hematological AEs in the POM+LoDEX and HiDEX groups were neutropenia (143 [48%] of 300 vs 24 [16%] of 150, respectively), anemia (99 [33%] vs 55 [37%], respectively), and thrombocytopenia (67 [22%] vs 39 [26%], respectively). Grade 3-4 non-hematological adverse events in the POM+LoDEX and HiDEX groups included pneumonia (38 [13%] vs 12 [8%], respectively), bone pain (21 [7%] vs seven [5%], respectively), and fatigue (16 [5%] vs nine [6%], respectively). |
| Richardson et al. (2014)30 | The most common grade 3-4 AE was neutropenia, which occurred in 41% of patients treated with POM+LoDEX and 48% of patients treated with POM alone. The incidence of grade 3-4 febrile neutropenia was low in the POM+LoDEX and POM alone groups (3% and 5%, respectively). The most common grade 3-4 nonhematologic AE was pneumonia (22% with POM+LoDEX and 15% with POM alone). In the POM1LoDEX group, 27% of the cases of any grade pneumonia were also associated with dyspnea (any grade). |
| Leleu et al. (2015)27 | The toxicity profile of the Pom-Dex combination consisted primarily of myelosuppression, as previously reported, and appeared manageable in these fragile RRMM patients. A total of 49 patients (98%) experienced an AE, of which 44 (88%) were treatment related. The incidence rate of grade 3 and 4 AEs was 45 (90%), including hematologic AEs, and 32 (64%) experienced a serious adverse event (SAE). |
| Baz et al. (2016)31 | Grade 3 and 4 anemia, neutropenia, and thrombocytopenia were noted in 11%, 31%, and 6% of arm B patients vs in 24%, 52%, and 15% of arm C patients, respectively. Gastrointestinal toxicity including nausea, vomiting, and diarrhea was also similar in the 2 treatment arms. |
AEs, adeverse effects; HiDEX, high-dose dexamethasone; LoDEX, low-dose dexamethasone; POM, pomalidomide
Discussion
Pomalidomide is a second generation IMiDs and has demonstrated effective even in MM patients who were refractory to lenalidomide and bortezomib [32]. The reason why it was approved by FDA is that in several clinical trials it shows sustained and significant effects and great antitumor activity in RRMM [29, 33-35]. In this meta-analysis, we summarized and evaluated the efficacy of pomalidomide in the treatment of RRMM. We identified eight studies, including four RCTs and four single-armed prospective studies with 891 patients. The qualities of the eight studies were adequate. The random-effects model was chosen, and a high heterogeneity between studies was observed.
Current treatment standards of RRMM include salvage chemotherapy, salvage autologous stem cell transplantation (auto-SCT), allogeneic stem cell transplantation (allo-SCT) and post-transplant consolidation/maintenance therapy [36-38]. For those patients who received salvage chemotherapy, thalidomide, lenalidomide and bortezomib could be the treatments of choice. However, if the patients are still refractory to bortezomib or lenalidomide, it seemed it would be no good options for them. As a novel agent for RRMM, pomalidomide showed to have encouraging result for RRMM, as our analysis showed that the pooled proportion of ORR was 0.35 and CRR was 0.02 after pomalidomide therapy. This might better guide us the further use this agent. Of noted, ORR of pomalidomide as single agent was only 18% in the study conducted by Richardson et al [30], but ORR became 33% once combining pomalidomide with dexamethasone for RRMM patients. The effect of combination of pomalidomide with dexamethasone or cyclophosphamide were better than that of single agent was also seen in other studies included, but the severe toxicities resulted from combination treatment also needs our attention. It seems that the higher dosage of pomalidomide (4mg) is not correlated with better ORR and survival of RRMM patients compared to that of lower dosage (2mg) in our analysis, but we need further confirmation in case that it is the coincidence because only a small number of patients were included in the analysis. Given these findings, we may conclude that combination treatment would be better than that of the single agent therapy for RRMM.
Several limitations associated with this meta-analysis were recognized. Firstly, most of the studies we included had different treatment regimens and dosage, and it is hard to be uniformed. Also, the precision of pooled ES can be affected by the small sample size of some studies; therefore, we chose the random-effects model for the entire study to increase power and precision regardless of heterogeneity. Moreover, the effect of pomalidomide might vary by different ethnicities around the world, and it is difficult to summarize them.
Further studies would be required to address the more concrete mechanisms of pomalidomide for MM. Though the pooled ORR and CR in our analysis demonstrated some advantages of pomalidomide for those patients even refractory to bortezomib and lenalidomide, the sample size is small, so the conduction of more prospective RCTs with larger samples to assess the proper place of pomalidomide for single agent or combined with other agents in RRMM is necessary, and toxicities of pomalidomide should also be carefully monitored. What's more, whether pomalidomide can be extended to newly diagnosed or more advanced MM [39-41] or other hematological malignancies require further studying [42].
Author Contributions
RC and BC had the idea and designed this meta-analysis. RC and CL identified reports of trials and extracted data. XZ, YW and CG provided statistical advice and RC did all statistical analyses. RC, CL, YW and BC checked for statistical inconsistency and interpreted data. RC drafted the report and all other authors critically reviewed and approved final article. BC is guarantor of this article.
Competing Interests
The authors have declared that no competing interests exist.
References
1. Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. 2016;101:396-406
2. San Miguel JF. Introduction to a series of reviews on multiple myeloma. Blood. 2015;125:3039-3040
3. Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9:52
4. Lonial S, Durie B, Palumbo A, San-Miguel J. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30:526-535
5. Driscoll JJ. Expression of E3 Ubiquitin Ligases in Multiple Myeloma Patients after Treatment with the Proteasome Inhibitor Bortezomib. Cancer Transl Med. 2015;1:153-157
6. Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O. et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30:1005-1017
7. Rajkumar SV. Myeloma today: Disease definitions and treatment advances. Am J Hematol. 2016;91:90-100
8. Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43:732-741
9. Dai C, Chen D, Jiang Y. Histone H2A and H2B Deubiquitinase in Developmental Disease and Cancer. Cancer Transl Med. 2015;1:170-175
10. Neri P, Bahlis NJ, Lonial S. New Strategies in Multiple Myeloma: Immunotherapy as a Novel Approach to Treat Patients with Multiple Myeloma. Clin Cancer Res. 2016;22(24):5959-5965
11. Terpos E, Kanellias N, Christoulas D, Kastritis E, Dimopoulos MA. Pomalidomide: a novel drug to treat relapsed and refractory multiple myeloma. Onco Targets Ther. 2013;6:531-538
12. Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A. et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947-959
13. Fouquet G, Bories C, Guidez S, Renaud L, Herbaux C, Javed S. et al. Pomalidomide for multiple myeloma. Expert Rev Hematol. 2014;7:719-731
14. Chen R, Chen B, Zhang X, Gao C. Efficacy of carfilzomib in the treatment of relapsed and (or) refractory multiple myeloma: a meta-analysis of data from clinical trials. Discov Med. 2016;22:189-199
15. Fall DJ, Stessman H, Patel SS, Sachs Z, Van Ness BG, Baughn LB. et al. Utilization of translational bioinformatics to identify novel biomarkers of bortezomib resistance in multiple myeloma. J Cancer. 2014;5:720-727
16. Chen R, Chen B, Ge Z. Efficacy of Carfilzomib in the Treatment of Relapsed and (or) Refractory Multiple myeloma: a Meta Analysis of Individual Patient Data from Clinical Trials. Blood. 2016;128:5675
17. Dimopoulos MA, Leleu X, Palumbo A, Moreau P, Delforge M, Cavo M. et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia. 2014;28:1573-1585
18. Laubach JP, Voorhees PM, Hassoun H, Jakubowiak A, Lonial S, Richardson PG. Current strategies for treatment of relapsed/refractory multiple myeloma. Expert Rev Hematol. 2014;7:97-111
19. Zou Y, Ma X, Yu H, Hu C, Fan L, Ran X. Carfilzomib/pomalidomide single-agent or in combination with other agents for the management of relapsed/refractory multiple myeloma: a meta-analysis of 37 trials. Oncotarget. 2016 Jul 21. doi: 10.18632/oncotarget.10768. [Epub ahead of print]
20. Sheng Z, Liu G. Pooled analysis of the reports of pomalidomide after failure of lenalidomide and (or) bortezomib for multiple myeloma. Hematol Oncol. 2016;34:102-107
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341
22. Tang PL, Wang HH, Chou FH. A Systematic Review and Meta-Analysis of Demoralization and Depression in Patients With Cancer. Psychosomatics. 2015;56:634-643
23. Higgins JP AD, Sterne JA. Chapter 8: Assessing risk of bias in included studies. Higgins JPT GS, ed. Cochrane Handbook for Systematic Reviews of Interventions Version 5 1 0. The Cochrane Collaboration 2011
24. Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S. et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27:5008-5014
25. Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S. et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia. 2010;24:1934-1939
26. Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F. et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970-2975
27. Leleu X, Karlin L, Macro M, Hulin C, Garderet L, Roussel M. et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125:1411-1417
28. Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G. et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968-1975
29. San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M. et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055-1066
30. Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S. et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832
31. Baz RC, Martin TG 3rd, Lin HY, Zhao X, Shain KH, Cho HJ. et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood. 2016;127:2561-2568
32. Fouquet G, Pegourie B, Macro M, Petillon MO, Karlin L, Caillot D. et al. Safe and prolonged survival with long-term exposure to pomalidomide in relapsed/refractory myeloma. Ann Oncol. 2016;27:902-907
33. Usmani SZ, Zhang Q, Stratton K, Qu P, Yaccoby S, Hansen E. et al. Phase II study of pomalidomide in high-risk relapsed and refractory multiple myeloma. Leukemia. 2014;28:2413-2415
34. Larocca A, Montefusco V, Bringhen S, Rossi D, Crippa C, Mina R. et al. Pomalidomide, cyclophosphamide, and prednisone for relapsed/refractory multiple myeloma: a multicenter phase 1/2 open-label study. Blood. 2013;122:2799-2806
35. San Miguel JF, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H. et al. Impact of prior treatment and depth of response on survival in MM-003, a randomized phase 3 study comparing pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed/refractory multiple myeloma. Haematologica. 2015;100:1334-1339
36. Cornell RF, Kassim AA. Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone marrow transplantation. 2016;51:479-491
37. Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O. et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2015;30:1005-1017
38. Moreau P, Touzeau C. Multiple myeloma: from front-line to relapsed therapies. Am Soc Clin Oncol Educ Book. 2015 e504-511
39. Sonneveld P, Asselbergs E, Zweegman S, van der Holt B, Kersten MJ, Vellenga E. et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015;125:449-456
40. Mikhael JR, Reeder CB, Libby EN, Costa LJ, Bergsagel PL, Buadi F. et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015;169:219-227
41. Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V. et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124:63-69
42. Treon SP, Tripsas CK, Meid K, Kanan S, Sheehy P, Chuma S. et al. Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom's macroglobulinemia. Blood. 2014;124:503-510
Author contact
![]() Corresponding authors: Baoan Chen, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. Tel: +86 25 83272006, Fax: +86 25 83272011. E-mail: cba8888com. Runzhe Chen, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. E-mail: runzhe.chenedu.cn. Xiaoping Zhang, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. E-mail: zhangxiaopingseucom
Corresponding authors: Baoan Chen, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. Tel: +86 25 83272006, Fax: +86 25 83272011. E-mail: cba8888com. Runzhe Chen, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. E-mail: runzhe.chenedu.cn. Xiaoping Zhang, Department of Hematology and Oncology, Zhongda Hospital, Medical School, Southeast University, Dingjiaqiao 87, Gulou District, Nanjing 210009, Jiangsu Province, P.R. China. E-mail: zhangxiaopingseucom

 Global reach, higher impact
Global reach, higher impact