3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(11):2104-2113. doi:10.7150/jca.19078 This issue Cite
Research Paper
CT-guided 125I brachytherapy for locally recurrent nasopharyngeal carcinoma
Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. ADD: 651 Dongfeng Road, East, Guangzhou, China.
#These authors contributed equally to this work.
Received 2017-1-6; Accepted 2017-4-14; Published 2017-7-5
Abstract
Purpose: The study evaluated the feasibility, clinical effectiveness, and quality of life of computed tomography (CT)-guided 125I brachytherapy for locally recurrent nasopharyngeal carcinoma (NPC).
Methods: We recruited 81 patients diagnosed with locally recurrent NPC after previous radiotherapy with or without chemotherapy. Thirty-nine patients received 125I brachytherapy (group A) and 42 received re-irradiation (IMRT, group B). The evaluated outcomes were local control, complications, and quality of life. Cox proportional hazards regression analysis was used to compare local tumor progression-free survival (LTPFS) and overall survival (OS) in the two treatment groups.
Results: The median follow-up was 30 months (range, 5-68 months), median LTPFS was 21 in group A and 17 months in group B. The 1-, 2-, and 3-year OS in group A were 84.6%, 51.3%, 30.7%, and 85.7%, 50.0%, and 32.6% in group B. In group A, 10/39 patients (25.6%) experienced at least one ≥grade III complication; no grade V complications occurred. In group B, 28/42 (66.7%) experienced at least one ≥grade III complication and 6/42 (14.3%) died of severe grade V complications. No significant between-group difference existed in the Quality of Life score on the EORTC QLQ-H&N35 questionnaire before treatment. In group A, quality of life was significantly improved after treatment; but did not improve, or even deteriorated in group B.
Conclusions: 125I brachytherapy was a feasible, safe, and effective treatment for locally recurrent NPC. 125I brachytherapy significantly reduced complications caused by re-irradiation and improved patients' quality of life.
Keywords: 125I brachytherapy, 125I seeds, recurrent nasopharyngeal carcinoma, quality of life, complications, local control
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignancy in China, especially in southern provinces. It was responsible for 60,600 new cases and 34,100 deaths in 2015(1). Undifferentiated nonkeratinizing nasopharyngeal carcinoma accounts for 95% of cases(2). Because of advances in radiotherapy, the 5-year overall survival (OS) of NPC has reached to currently 80%(3). However, local failure after primary radiotherapy has been reported to occur at rates of 15% to 58%(4). Decreased radiosensitivity and the complex anatomical characteristics of local recurrences lead to difficult treatment and a poor prognosis(5). As the 5-year OS of recurrent NPC is only 17% to 33%, the management of recurrent NPC is challenging(6).
The treatment options for local recurrence of NPC include re-irradiation with or without chemotherapy, surgery, or intracavitary brachytherapy(7,8). Some studies suggest that surgery can achieve better local control and overall survival than re-irradiation, but only patients with mucosal recurrence or limited recurrent lesions are considered suitable for surgery(9). Intracavitary brachytherapy offers benefits in early-stage, limited recurrent lesions because of fewer complications and better short-term local control than re-irradiation(10). Unfortunately, relatively few patients are suitable for these treatment modalities, because recurrent lesions often have a large volume and may have invaded the bone at the base of the skull, making it difficult to achieve good clinical results with these two modalities(11,12).
Re-irradiation with or without chemotherapy remains the most frequently used modality for locally recurrent NPC(13). However, a decrease of radiosensitivity after primary radiotherapy is unavoidable because of fibrosis, atrophy, edema, telangiectasia, or atrophy that develop in the radiated area(14). Some investigators have reported that re-irradiation achieved short-term local control of recurrent NPC in only 8% to 58% of patients(15). The responses to mono- and polychemotherapy are only 10% to 30% and 40% to 50%, respectively, and it is difficult to achieve long-term control(16). In addition, severe, late treatment-related complications of re-irradiation are a major challenge because of the unique anatomical location of recurrent NPC(17). Nearly all patients will undergo late or long-lasting complications after reirradiation; 65% patients will develop severe Radiation Therapy Oncology Group (RTOG) grade III or IV complications(18,33). Even Intensity-Modulated Radiotherapy (IMRT) is used, the detrimental side effects of radiotherapy cannot be avoided. Some patient deaths from fatal complications such as carotid blowout syndrome and temporal lobe necrosis have been reported(19).
Potentially fatal complications not only seriously degrade patient quality of life (QoL), but radiotherapists may reduce the re-irradiation dose after considering possible severe complications induced by the local cumulative radiation. Many studies have confirmed that high radiation doses usually achieve better local control but also leads to severe complications(20). Novel treatments of recurrent NPC are needed to overcome the drawbacks associated with increased local re-radiation doses.
125I brachytherapy is increasingly accepted as useful minimally invasive modality in recurrent cancers(21). It differs from intracavitary brachytherapy, as it involved direct implantation of radioactive 125I seeds into a tumor. The radiation dose decreases rapidly with distance from the seed, making it possible to deliver a high local radiation dose with few complications(22, 23). The purpose of this study was to evaluate the effectiveness and safety of computer tomography (CT)-guided 125I brachytherapy for locally recurrent NPC.
Materials and methods
Ethics
This retrospective study met the basic standards of the Declaration of Helsinki and was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center. All patients who participated in the study were fully informed of the potential risks and voluntarily gave their written informed consent.
Study Population
From June 2009 to July 2013, 81 patients who were diagnosed with recurrent NPC and met the inclusion criteria, participated in this study. All patients previously experienced radiotherapy with a prescription dose of 70 Gy, 35 times, and 7 weeks. The range was 64-88 Gy, 32-44 times, and 6.5-8.5 weeks. Eleven patients in group A (brachytherapy) and 13 in group B (re-irradiation) previously received chemotherapy including cisplatin (100-120 mg⋅m-2, intravenous drip) and 5-fluorouracil (800-1,000 mg⋅m-2, for 5 days, intravenous drip) at 3-week intervals. All patients had recurrences at relatively short interval from the end of the initial treatment (group A: 15 months, range of 8-29 months; group B, 17 months, range of 12-30 months). After being fully informed of the associated risks of brachytherapy, 31 patients consented to receive 125I brachytherapy (group A). Thirty-four patients received IMRT (group B). The patients' characteristics are shown in Table 1.
Inclusion and Exclusion Criteria
Patients were eligible for inclusion if they were 18-70 years of age, had pathological diagnosis of locally recurrent NPC, no invasion of the skull base and intracranial organs, a tumor size of ≤6 cm, fewer than three lesions, an East Coast Oncology Group (ECOG) performance status ≤2, and blood coagulation values within normal ranges. Exclusion Patients with primary NPC, serious bleeding tendency, coagulation function disorders, or severe liver, kidney, heart, or lung disease, or brain function deficit.
Patients' characteristics
| Characteristics | Group A (n = 39) | Group B (n = 42) | P |
|---|---|---|---|
| Gender | 0.875 | ||
| Male | 25(64.1%) | 28(66.7%) | |
| Female | 14(35.9%) | 14(33.3%) | |
| Age median age | 49(26-70) | 47(25-68) | 0.913 |
| ≤ 45 | 22(56.4%) | 23(54.8%) | |
| > 45 | 17(43.6%) | 19(45.2%) | |
| GTV1 mean±SD2 | 31±9.25 | 33±8.97 | 0.762 |
| ≤30cc | 21(53.8%) | 19(45.2%) | |
| >30cc | 18(46.2%) | 23(54.8%) | |
| Number of lesions | |||
| 1 | 30(76.9%) | 35(83.3%) | |
| 2 | 9(23.1%) | 7(16.7%) | |
| Histology3 | 0.968 | ||
| WHO I | 0(0.0%) | 0(0.0%) | |
| WHO II | 2(5.2%) | 3(7.2%) | |
| WHO III | 37(94.8%) | 39(92.8%) | |
| T stage at initial treatment | 0.613 | ||
| T1 | 9(23.1%) | 10(23.8%) | |
| T2 | 12(30.8%) | 13(40.0%) | |
| T3 | 11(28.2%) | 9(21.4%) | |
| T4 | 7(17.9%) | 10(23.8%) | |
| T stage at recurrence | 0.546 | ||
| rT1 | 9(23.1%) | 10(23.8%) | |
| rT2 | 11(28.2%) | 14(33.3%) | |
| rT3 | 7(17.9%) | 7(16.7%) | |
| rT4 | 12(30.8%) | 11(26.2%) | |
| Tumor stage at recurrence | 0.615 | ||
| I | 9(23.1%) | 10(23.8%) | |
| II | 8(20.5%) | 12(28.6%) | |
| III | 12(30.8%) | 11(26.2%) | |
| IV | 10(25.6%) | 9(21.4%) | |
| Disease-free interval (mo)4 | 15(8-29) | 17(12-30) | 0.314 |
| ≤ 24mo | 29(74.4%) | 30(71.4%) | |
| >24mo | 10(25.6%) | 12(28.6%) | |
| Previous radiotherapy | 39(100.0%) | 39(100.0%) | 0.868 |
| Median prior radiation dose, Gy | 70(64-82) | 70(64-80) | 0.763 |
| Previous chemotherapy | 11(28.2%) | 13(30.9%) | 0.824 |
Note: 1. Gross Tumor Volume; 2. Standard deviation; 3. WHO type I, keratinizing, type II, differentiated nonkeratinizing, type III, undifferentiated nonkeratinizing.
4. From the end of first course of radiotherapy to recurrence at diagnosis.
Treatment
125I brachytherapy
125I Seeds (Atom High Tech, Beijing, China) were included in 4.5-mm × 0.8-mm nickel-titanium tubes. The 125I was adsorbed on the surface of a 3-mm × 0.5-mm silver rod. The 125I seeds had an initial radioactivity of 0.8 mCi, a total administration dose of approximately 110-160 G, average energy of 27-32 KeV; half-life of 59.6 days, and an effective radiation radius of 1.7 cm. The 125I seeds continuously released low-dose γ-ray and soft X-rays (5% of 35 keV and 95% of 28 keV, respectively) within 3-4 half-lives of implantation.
Preoperative and postoperative treatment plans were developed for each patient using treatment planning system (TPS) software (YuanBo, Beijing, China). Approximately 1-2 weeks before seed implantation, images were imported by the TPS. We included outlines of the gross tumor volume (GTV), planning target volume (PTV), and the surrounding organs. The PTV was about 1-1.5 cm larger than the boundary of the GTV. Prescribed dose averaged 120 Gy (100-140 Gy), following American Brachytherapy Society recommendations for prostate cancer(24) and our previous 125I brachytherapy study(21,22,23,25). We also designed a puncture path, calculated the required number of seeds, and generated a dose-volume histogram with TPS, we continuously optimized the dose so that the mean peripheral dose was equal to or larger than the planned dose, and 90% of the GTV accepted 90% of the prescribed dose.
All 125I brachytherapy seeds were implanted by the same two radiologists, who had more than 10 years of experience in CT-guided 125I brachytherapy. Patients were placed in the supine position with the head fixed in place. A thin metal wire was attached to the skin as an orientation mark. An enhanced CT scan was performed to avoid damage to blood vessels, nerves, and other vital organs. We drew the puncture path on the CT images and located the skin puncture sites with reference to the wire. After local anesthesia with 5-10 ml 1% lidocaine, an 18-G puncture needle (Yunke Pharmaceutical Limited Liability Company, Chengdu, China) was inserted into the tumor. The direction of the needles was adjusted following the preoperative plan so that the seeds were separated by about 1 cm. A turntable or clip implant gun (Atom High Tech) was used to implant the seeds within the tumor, maintaining a space of 0.5-1 cm between seeds about 1 cm from blood vessels, nerves, and mucosa. After a CT scan confirmed the location of the seeds, the needles were removed. A repeat CT scan evaluated possible bleeding and again confirmed the seed location. Postoperative dose verification was performed in accordance with the actual location of seeds.
IMRT
Patients were placed in the supine position and wore thermoplastic masks. A radiotherapy physician sketched the GTV and CTV according to recent CT or MRI using the Monaco (Elekta, Stockholm, Sweden) system (version 5.0). The GTV of lesions was visualized on CT or MRI. The CTV was the GTV with an additional 1-1.5-cm margin. The PTV was developed by extending the margin another 3 mm relative beyond the CTV to allow for setup variability and internal motion.
The organs at risk (OAR) included the brainstem, spinal cord, optic nerves, optic chiasm, temporal lobes, and parotid glands. The OAR received a relatively low dose (50-60 Gy). The PTVs were treated with IMRT of 6 MV X-rays generated by a Clinac-600C linear accelerator (Elekta). The prescribed doses were 60-70 Gy to the GTV (2 Gy or 1.8 Gy per daily fraction, 5 days/week).
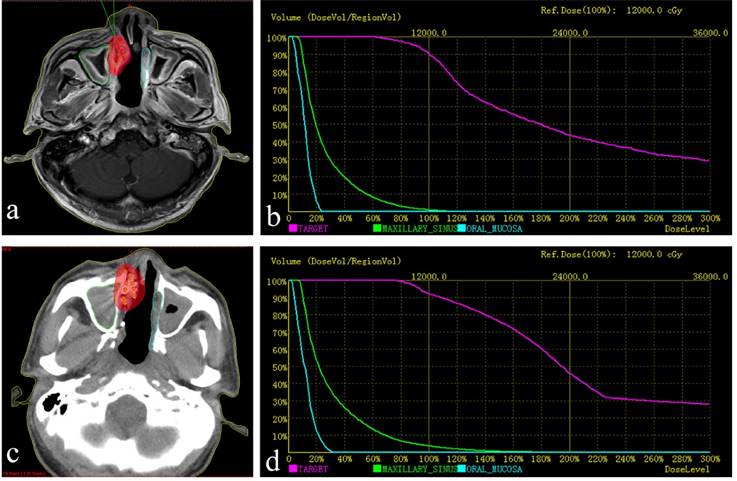
a. Red lines represent the tumor's contour; the purple area is covered by 90% of the prescribed dose. b. Preoperative dose volume histograms (DVH), target = tumor. The prescribed dose (PD) was 120 Gy. A total of 90% of the tumor target (D90=121.0 Gy) received 121.0 Gy, and 95.0% of the tumor target received 100% of the prescribed dose (V100 = 95.0%). c. postoperative practical distribution of seeds. d. Postoperative DVH, D90=130.9 Gy, V100 = 96.9%. The postoperative dose distribution coincided roughly with preoperative distribution.
Follow-Up and Evaluation Criteria
The major follow-up outcomes were OS, local control, QoL, and treatment-related complications. The European Organization for Research and Treatment of Cancer-Quality of Life-Head and Neck Questionnaire (EORTC-QLQ HN35) was used to evaluate QoL. Complications were evaluated using the RTOG radiation morbidity scoring criteria. During follow-up, enhanced MRI were obtained for the evaluation of curative effect at 1 month after treatment and then every 3 months. Blood testing was repeated once every 1-2 weeks for the first 3 months after treatment. All patients completed the EORTC-QLQ HN35 1-2 weeks before treatment and again at 0, 3, 6, and 12 months after treatment.
Statistical Analysis
Statistical analysis was performed by using SPSS version 20.0 (International Business Machines Corporation, New York, American). For between-group comparisons, P≤0.05 was considered significant. Kaplan-Meier analysis (Log- rank test) were used to compare OS and LTPFS in the two groups. A stratified Cox proportional hazards regression model with stepwise procedures were used to evaluate the relationship of study variables with LTPFS. Pearson's χ2 test, likelihood ratio, Fisher probabilities, or the Mann-Whitney U test were used to assess differences in local control, complications, and QoL.
Results
The median follow-up time was 30 months (range of 5-68 months). Thirty-nine patients with 48 lesions received 125I brachytherapy, and 36 of the 39 (92.3%) met the postoperative TPS dose verification. Three patients were re-implanted and eventually achieved the planned dose. The median of 24 125I seeds were implanted (range of 10-39), the median procedure duration was 55 min (range of 45-90 min). In group B, the median radiation dose was 64 Gy (range of 50-72 Gy). The median percentage of GTV receiving 100% of the prescribed dose (V100) and the dose encompassing 95% of the GTV (D95) were 91.6% (range of 80.1% to 100%) and 63.1 Gy (range of 56.5-71.0 Gy), respectively.
Local control
As shown in Table 2, local control at 3, 6, 12, 18, 24, and 36 months local control in group A was 92.3%, 82.1%, 71.7%, 51.3%, 41.0%, 23.1%, respectively, and was 85.7%, 78.6%, 66.7%, 47.6%, 35.7%, and 16.7%, respectively, in group B (P<0.05). Group A had overall better local control and the difference was statistically significant.
Clinical Efficacy of 125I Brachytherapy and reirradiation
| Local Control Efficacy (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | ||||||||||
| CR | PR | SD | PD | LC | CR | PR | SD | PD | LC | P | |
| 3m | 34 | 2 | 2 | 1 | 36/39(92.3%) | 29 | 7 | 2 | 4 | 36/42(85.7%) | 0.027 |
| 6m | 27 | 5 | 3 | 4 | 32/39(82.1%) | 26 | 7 | 3 | 6 | 33/42(78.6%) | 0.030 |
| 12m | 22 | 6 | 2 | 9 | 28/39(71.7%) | 20 | 8 | 4 | 10 | 28/42(66.7%) | 0.034 |
| 18m | 14 | 7 | 5 | 13 | 21/39(51.3%) | 11 | 9 | 5 | 17 | 20/42(47.6%) | 0.028 |
| 24m | 12 | 4 | 2 | 21 | 16/39(41.0%) | 9 | 6 | 4 | 23 | 15/42(35.7%) | 0.036 |
| 36m | 6 | 3 | 2 | 28 | 9/39(23.1%) | 3 | 4 | 5 | 30 | 7/42(16.7%) | 0.014 |
Note: CR= complete response, PR= partial response, SD= stable disease
PD=progressive disease; Based on the Response Evaluation Criteria in Solid Tumors (RECIST), LC defined as the proportion of patients with complete response and partial response.
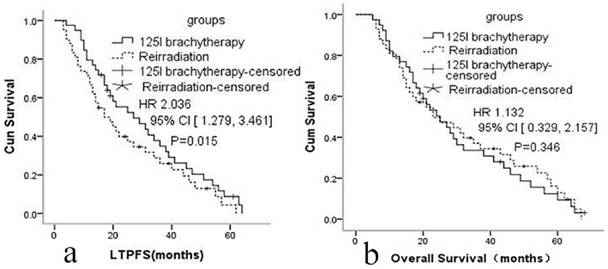
a: Local tumor progression-free survival in group A and group B. b: Overall survival in group A and group B
Local tumor progression-free survival
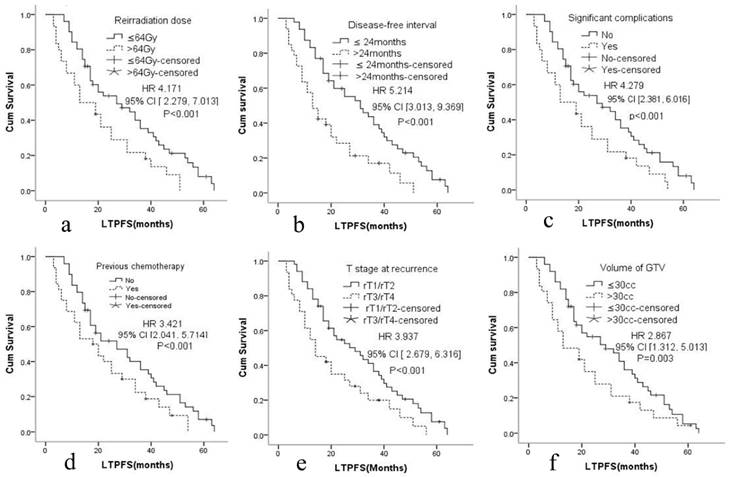
The median LTPFS was 21 months (95% CI: 9-51) in group A and 17 months (95% CI: 7-48) in group B. Cox proportional hazards regression analysis and the log-rank (Mantel-Cox) tests showed that LTPFS was significantly higher in group A than in group B. 125I brachytherapy was independently associated with LTPFS [P=0.015, HR=2.036 (95% CI: 1.279-3.461); Fig. 2a]. As shown in Fig. 3 and Table 3, patients without any clinically significant complications after re-irradiation had longer LTPFS [P<0.001, HR=4.279 (95% CI: 2.381-6.016)]. Patients with a GTV ≤30 cc (P=0.003, HR=2.867 [95% CI: 1.279-3.461]), disease-free interval >24 months [P<0.001, HR=5.214 (95% CI: 3.013, 9.369]), no previous chemotherapy [P<0.001, HR=3.421 (95% CI: 2.041-5.714]), re irradiation dose >64 Gy [p<0.001, HR=4.171 (95% CI: 2.279-7.013)], or rT1/rT2 [P<0.001, HR=3.937 (95% CI: 2.679-6.316)] had better LTPFS.
Overall Survival
The median OS was 25 months (95% CI: 7-64) in group A and 24 months (95% CI: 8-62) in group B. The 1-, 2-, and 3-year OS in group A was 84.6%, 51.3%, 30.7%; and 85.7%, 50.0%,32.6% in group B. Cox proportional hazards regression analysis found no significant difference in the OS of the two groups [p=0.346, HR=1.132 (95% CI: 0.329- 2.157)].
Stratified Cox proportional hazards regression analysis related LTPFS1
| Variable | P value | HR | 95%CI2 | |
|---|---|---|---|---|
| groups | 125I brachytherapy | 0.015 | 2.036 | 1.279, 3.461 |
| Reirradiation | ||||
| Volume of GTV3 | ≤30cc | 0.003 | 2.867 | 1.312, 5.013 |
| >30cc | ||||
| Disease-free interval | >24months | <0.001 | 5.214 | 3.013, 9.369 |
| ≤24months | ||||
| Previous chemotherapy | No | <0.001 | 3.421 | 2.041, 5.714 |
| Yes | ||||
| Significant complications4 | No | <0.001 | 4.279 | 2.381, 6.016 |
| Yes | ||||
| Reirradiation dose | >64Gy | <0.001 | 4.171 | 2.279, 7.013 |
| ≤64Gy | ||||
| T stage at recurrence | rT1/rT2 | <0.001 | 3.937 | 2.679, 6.316 |
| rT3/rT4 | ||||
1. Local tumor progression-free survival; 2. Confidence interval; 3. Gross Tumor Volume; 4. Complications ≥ grade III.
Complications
The major complications that occurred during or after treatment are summarized in Table 6. No severe complications occurred during the 125I brachytherapy treatment. Seed migration occurred in four of 39 patients (10.3%) but did not cause any severe complications during follow-up. In group A, ten of 39 patients (25.6%) experienced at least one grade III or higher complication; no grade V complications were seen. In Group B, 28 of 42 patients (66.7%) experienced at least one grade III or higher complication. Four patients (9.5%) with mucosal necrosis developed carotid blowout syndrome following involvement of the internal carotid artery and all died. Two patients (4.8%) died from severe radiation encephalopathy. Group A had fewer complications, especially complications ≥grade III(P<0.05). No participants in group A died because of severe complications, but six of the 42 patients (14.3%) in group B did die as a result of severe complications.
Cox proportional hazards regression model explored the factors related with local tumor progression-free survival. a. Reirradiation dose, b. Disease-free interval, c. Significant complications, d. Previous chemotherapy, e. T stage at recurrence, f. Volume of GTV.
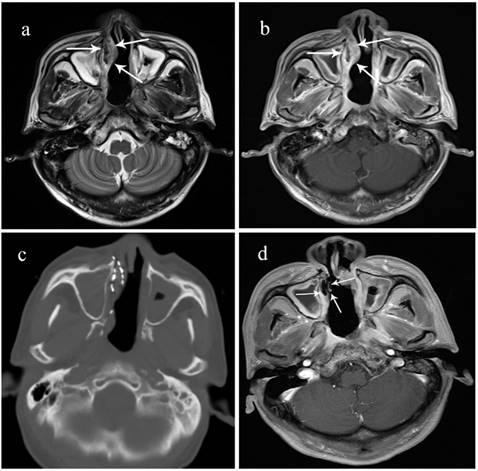
A 70-year-old male patient with locally recurrent NPC in the right nasal vestibular mucosa. He received concurrent chemoradiotherapy including cisplatin and 5-fluorouracil but recurrence was detected in the nasal septum after 15 months. A surgical resection was performed, but he had another recurrence after 10 months. a. Preoperative T2 MRI showing the tumor's boundary (arrow). b. Preoperative enhanced MRI sequence. c. Intraoperative CT scan. d. 3 months after 125I brachytherapy, the lesion has shrunk, enhanced MRI shows no activity.
Quality of life
The EORTC QLQ-N35 scores are summarized in Tables 4 and 5. The mean pretreatment scores were 76.34 in group A and 75.29 in group B(P-0.681). At the end of treatment, mean score in group B had increased by 5.92 to 79.21 but then got worse at 3, 6, and 12 months after treatment. The group A scores significantly improved by 10.56, 22.03, and 24.55 points between 3 and 12 months. The group B scores did not improve significantly, remaining at 70.12, 68.35, and 71.17. Thus QoL in group A was significantly better than in group B over 12 months of follow-up (P <0.001).
EORTC-QLQ H&N35 score in Each scales or items*
| Standard score of EORTC-QLQ H&N35(mean score±SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | |||||||||
| Before | 0 mo | 3 mo | 6 mo | 12 mo | before | 0 mo | 3 mo | 6 mo | 12 mo | |
| Pain | 65.46±26.28 | 70.26±25.67 | 46.23±23.23 | 40.25±21.01 | 34.12±13.76 | 62.15±28.24 | 74.55±24.25 | 59.23±22.14 | 55.09±23.46 | 53.34±18.21 |
| Swallowing | 83.24±13.34 | 80.21±10.56 | 74.67±16.89 | 65.21±20.22 | 60.24±11.90 | 79.23±17.12 | 80.23±7.90 | 82.23±12.89 | 84.21±16.34 | 79.46±16.18 |
| Senses | 83.24±19.21 | 82.29±15.35 | 60.21±19.34 | 48.23±15.98 | 46.13±18.34 | 82.19±19.93 | 89.34±15.98 | 80.23±18.76 | 75.21±15.95 | 69.59±20.34 |
| Speech | 72.25±11.45 | 73.39±9.98 | 57.25±8.21 | 49.34±7.45 | 44.65±23.90 | 71.23±9.04 | 77.36±8.91 | 76.21±9.23 | 78.34±47.34 | 80.12±14.17 |
| Social eating | 68.23±9.78 | 70.23±9.31 | 58.21±10.12 | 52.12±9.46 | 54.21±17.73 | 67.23±11.12 | 71.19±9.32 | 74.23±13.24 | 76.23±9.46 | 70.16±19.21 |
| Social contact | 71.14±15.23 | 70.14±12.15 | 60.34±8.90 | 52.13±14.23 | 48.21±23.19 | 72.23±18.13 | 76.24±18.9 | 78.21±19.12 | 80.12±9.32 | 85.19±8.97 |
| Sexuality | 61.23±15.23 | 63.12±9.78 | 60.12±7.98 | 62.98±9.12 | 60.14±11.23 | 59.12±18.21 | 63.21±11.98 | 60.23±9.19 | 60.01±8.12 | 59.09±25.13 |
| Teeth | 32.12±13.19 | 33.67±10.98 | 30.89±18.78 | 29.17±19.23 | 31.23±30.17 | 29.28±17.21 | 30.23±16.23 | 30.98±19.21 | 31.78±16.45 | 30.23±19.94 |
| Opening mouth | 63.35±26.19 | 60.12±21.82 | 53.12±23.12 | 46.56±19.89 | 45.21±17.24 | 62.19±24.78 | 69.23±20.19 | 75.21±19.98 | 81.12±15.21 | 86.34±9.34 |
| Dry mouth | 30.98±10.12 | 31.14±9.89 | 30.56±8.13 | 32.21±7.45 | 35.16±25.57 | 32.14±9.98 | 53.21±17.89 | 67.21±23.45 | 74.34±25.10 | 76.12±19.13 |
| Sticky saliva | 34.23±19.12 | 35.21±9.98 | 33.67±14.21 | 35.98±14.67 | 40.21±18.34 | 35.23±13.68 | 46.23±12.17 | 65.12±14.98 | 77.23±23.78 | 75.17±9.93 |
| Coughing | 36.78±19.21 | 35.21±17.19 | 34.90±10.78 | 37.12±13.56 | 34.23±19.56 | 35.21±10.34 | 34.67±12.56 | 40.23±19.54 | 50.56±17.79 | 48.21±23.16 |
| Feeling ill | 76.23±23.45 | 73.14±25.12 | 66.78±19.89 | 57.98±23.79 | 53.45±18.49 | 73.91±22.78 | 76.23±19.16 | 78.23±14.49 | 80.98±10.15 | 82.17±19.58 |
| pain killers | 67.23±28.91 | 68.12±26.18 | 64.19±18.39 | 61.21±21.79 | 57.13±30.14 | 60.34±16.34 | 63.89±27.19 | 62.98±17.90 | 65.23±19.80 | 60.16±18.10 |
| Nutritional supplement | 70.12±27.89 | 79.90±25.78 | 74.21±19.90 | 75.21±24.28 | 77.12±11.89 | 72.18±19.45 | 76.34±20.90 | 75.31±21.49 | 73.21±19.00 | 76.98±8.94 |
| feeding tube | 56.66±8.34 | 57.33±9.66 | 54.66±6.20 | 57.33±8.33 | 51.19±32.41 | 57.66±9.34 | 56.20±7.23 | 56.88±8.23 | 56.33±6.45 | 53.12±28.90 |
| Weight loss | 73.66±9.20 | 67.33±7.89 | 73.66±5.20 | 67.33±6.59 | 60.20±18.90 | 75.66±7.00 | 74.20±8.12 | 76.88±6.32 | 74.33±8.34 | 63.20±14.47 |
| Weight gain | 53.66±9.20 | 57.33±7.89 | 53.66±5.20 | 57.33±6.59 | 55.12±17.31 | 55.66±7.00 | 54.20±8.12 | 56.88±6.32 | 54.33±8.34 | 53.12±16.13 |
Note: * Compared all the scales assess symptoms with reirradiation during different period, P<0.05 (Mann-Whitney U Test); The EORTC Quality of Life Head and Neck Module (EORTCQLQ-H&N35) is a questionnaire specific to head and neck cancer patients consisting of 35 items designed to assess health-related QoL. According to the evaluation criteria, higher scores correspond to lower quality of life.
Mean EORTC-QLQ H&N35 scores of 35 items*
| Standard score of EORTC-QLQ H&N35(mean score±SD) | |||||
|---|---|---|---|---|---|
| Time | Group A | Group B | |||
| Score2 | Differenc3 | score | difference | P1 | |
| Before treatment | 76.34±17.21 | 75.29±16.79 | 0.681 | ||
| 0 month after treatment | 75.29±15.68 | -1.05±7.36 | 79.21±13.89 | +5.92±12.38 | 0.029 |
| 3 months after treatment | 65.78±20.67 | -10.56±9.56 | 70.12±15.67 | -3.17±9.59 | 0.001 |
| 6 months after treatment | 54.31±23.61 | -22.03±16.49 | 68.35±11.59 | -4.94±11.91 | 0.001 |
| 12 months after treatment | 51.79±20.94 | -24.55±18.79 | 72.17±16.21 | -8.04±21.93 | 0.001 |
Note: * This table represent the average score for each group. Calculated every patient's score in each item and converted into standardized score ranged 0 to 100, adding score of 35 items and then using the sum score of 35 items divided 35, higher scores correspond to lower quality of life; 1. Two independent samples t-test compared score in different periods; 2. Mean score of 35 items. 3. Score of 0, 3, 6, 12 months after treatment-score before treatment.
Complications in two groups
| Group A | Group B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1/2 | 3/4 | 5 | grade≥3 | 0 | 1/2 | 3/4 | 5 | grade≥3 | P | |
| Myelosuppression | 25 | 10 | 4 | 0 | 4/39(10.3%) | 20 | 15 | 7 | 0 | 7/42(16.7%) | 0.025 |
| Fever | 30 | 7 | 2 | 0 | 2/39(5.1%) | 30 | 10 | 2 | 0 | 2/42(4.8%) | 0.028 |
| Gastrointestinal response | 39 | 0 | 0 | 0 | 0/39(0.0%) | 29 | 10 | 3 | 0 | 3/42(7.1%) | <0.001 |
| Local skin reaction | 28 | 9 | 2 | 0 | 2/39(5.1%) | 18 | 20 | 4 | 0 | 4/42(9.5%) | <0.001 |
| Nasopharyngeal mucosa reaction | 22 | 13 | 4 | 0 | 4/39(10.3%) | 16 | 19 | 7 | 0 | 7/42(16.7%) | 0.006 |
| oral mucosa reaction | 30 | 8 | 1 | 0 | 1/39(2.6%) | 25 | 14 | 3 | 0 | 3/42(7.1%) | <0.001 |
| Alopecia | 36 | 3 | 0 | 0 | 0/39(0.0%) | 34 | 7 | 1 | 0 | 1/42(2.4%) | 0.008 |
| Dry eye | 35 | 4 | 0 | 0 | 0/39(0.0%) | 35 | 7 | 2 | 0 | 2/42(4.8%) | <0.001 |
| Epistaxis | 30 | 6 | 3 | 0 | 3/39(7.7%) | 28 | 10 | 4 | 0 | 4/42(9.5%) | 0.026 |
| Dysarthria | 35 | 3 | 1 | 0 | 1/39(2.6%) | 33 | 7 | 2 | 0 | 2/42(4.8%) | 0.023 |
| Radioactive otitis media | 36 | 3 | 0 | 0 | 0/39(0.0%) | 35 | 6 | 1 | 0 | 1/42(2.4%) | 0.016 |
| Headache | 29 | 7 | 3 | 0 | 3/39(7.7%) | 28 | 8 | 6 | 0 | 6/42(14.3%) | 0.021 |
| Soft tissue fibrosis | 35 | 3 | 1 | 0 | 1/39(2.6%) | 30 | 7 | 6 | 0 | 6/42(14.3%) | <0.001 |
| Hearing loss | 36 | 3 | 0 | 0 | 0/39(0.0%) | 30 | 9 | 3 | 0 | 3/42(7.1%) | <0.001 |
| Cranial nerve damage | 32 | 5 | 2 | 0 | 2/39(5.1%) | 28 | 9 | 5 | 0 | 5/42(11.9%) | <0.001 |
| Trismus | 33 | 5 | 1 | 0 | 1/39(2.6%) | 28 | 9 | 5 | 0 | 5/42(11.9%) | <0.001 |
| Osteoradionecrosis | 35 | 3 | 1 | 0 | 1/39(2.6%) | 32 | 7 | 3 | 0 | 3/42(7.1%) | <0.001 |
| Mucosal necrosis | 33 | 4 | 2 | 0 | 2/39(5.1%) | 26 | 7 | 5 | 4 | 8/42(19.0%) | <0.001 |
| Radiation encephalopathy | 33 | 4 | 2 | 0 | 2/39(5.1%) | 24 | 12 | 4 | 2 | 6/42(14.3%) | <0.001 |
Complications were evaluated using the RTOG radiation morbidity scoring criteria.
Discussion
125I brachytherapy has been successfully used in tongue cancer, hypopharyngeal cancer, salivary adenocarcinoma, intracranial tumors(26-28). Huang et al. reported 125I brachytherapy for cervical lymph node metastasis of head and neck cancer, the 1-, and 2-years local control rate were 64.51% and 45.16%, respectively(29). Zhu et al. and Yu et al. reported the 125I brachytherapy in recurrent head and neck cancer with local control of 73.7% and 52%, respectively(30,31). Their study only included partial recurrent NPC, but failed to show the role of 125I brachytherapy in the treatment of recurrent NPC. So far, there are few study to explore the role about 125I brachytherapy for recurrent NPC. So, our study is meaningful. In this study, we found that 125I brachytherapy was a feasible, safe and effective treatment for locally recurrent NPC. Local control was achieved more effectively with 125I brachytherapy, than with re-irradiation. Brachytherapy did not compromise of OS, had significantly reduced treatment-related complications and improved patient QoL.
Although many treatment modalities have been applied to recurrent NPC, most patients still receive re-irradiation(32). Advanced radiotherapy techniques of IMRT have improved OS and local control of recurrent NPC, but the risk of severe complications remains high and are the major cause of death. Therefore, the benefits of high-dose IMRT for disease control need to be weighed against the risk of severe complications(33). The incidence of severe and fatal complications such as cranial nerve damage, trismus, radiation encephalopathy, soft tissue fibrosis, hearing loss, and osteoradionecrosis is estimated at approximately 65%(34). Koutcher et al reported an incidence grade III or higher complications of up to 73%(35). Teo et al reported that hearing loss or trismus occurred in 50% to 70% of patients after re-radiation(36). These studies show that radiotherapy-induced complications seriously affect patient QoL. The significance of this study is the demonstration that 125I brachytherapy improved local control while significantly reducing the complications and improving patient QoL compared with re-irradiation.
The complication rate in our re-irradiation patients was similar to the above report. 66.7% patients treated with re-irradiation experienced at least one of complications ≥ grade III and 6/42(14.3%) died of severe complications. In the brachytherapy group, only 25.6% experienced complications of grade III or higher, and no deaths were caused by severe complications. The reasons for the difference can be explained as follows: 1. The energy of the 125I seeds decreased rapidly with distance. 2. Recurrent lesions located in deep positions were surrounded by complex structures and OARs that were intolerant to high radiation doses. 3. All patients had experienced previous radiotherapy, and some structures had reached the maximum tolerable cumulative dose(37).
The EORTC-QLQ HN35 scores confirmed the influence of complications on the patient QoL. Before treatment, mean scores in two group were similar (P-0.681). After treatment, mean scores in the re-irradiation group increased by 5.92 points at first but QoL became worse at 3 and 6 months after treatment. The mean scores of the re-irradiation improved inconspicuously, whereas the QoL scores in the 125I brachytherapy group improved. As Table 6 showed the scores in some individual items actually became worse in group B. At 12 months after treatment, the group A scores had decreased by 24.55 points and were not much changed from 6 months, but the 12-month scores in group B were not significantly improved, but were somewhat worse than at 6 months. The QoL scores were closely related to radiotherapy-induced acute complications. Over time, the gradual emergence of long-term complications caused by re-irradiation reduced the positive impact on clinical symptoms by decreasing QoL(38).
Another finding was that 125I brachytherapy for recurrent NPC achieved better local control, LTPFS was significantly better in group A than in group B. Dizman et al. reported progression free survival and OS of recurrent NPC treated with stereotactic body radiation therapy at 1-, 2- and 3-years were 60%, 30%, 17% and 83%, 43%, 31%, respectively(39). The group B results in this study were similar. li et al. reported local control and OS of 125I brachytherapy for recurrent NPC at 1-, 2- and 3-years were 73.7%, 26.3%, and 5.3% and 80%, 30%, and 6.7%, respectively(40). In our study, the group A results were better, which may have been related to tumor stage.
The Cox proportional hazards regression model revealed that patients with severe complications had shorter LTPFS, this may have been related to tolerance of high-dose re-irradiation. As patients with severe complications could not tolerate a higher dose of re-irradiation, local control was impacted, leading to relapse. Many studies have showed that higher doses of radiotherapy usually have better LTPFS(41), and our results are in line with those. Unfortunately, Proportional hazard regression found no significant difference in OS between two groups. The treatment of recurrent nasopharyngeal carcinoma is still a challenge, especially in improving the OS (42-45). So, the original intention of our study was to use the characteristic of 125I seed with a higher local radiation dose but rapid decay with increased distance to reduce complications caused by re-irradiation, to improve the local control and QoL. Therefore, although 125I brachytherapy did not improve the patient's OS, it has a positive effect in improving the patients QoL, reducing the complications associated with re-irradiation. So, it is still a meaningful treatment.
Our study was limited by its single center, retrospective design and small sample size. Second, despite strict adherence to the TPS treatment plan, inevitable change of posture and internal movements affected accuracy and dose. Three of the 39 patients (7.7%) experienced a second seed implantation before the planed dose could be verified. The overall good results do warrant a prospective multi-center randomized controlled trial, which we are planning.
Significant conclusion
125I brachytherapy was a feasible, safe, and effective treatment for locally recurrent NPC. 125I brachytherapy significantly reduced complications caused by re-irradiation and improved patients' quality of life.
Abbreviations
CT: computed tomography
NPC: recurrent nasopharyngeal carcinoma
IMRT: Intensity-Modulated Radiotherapy
LTPFS: local tumor progression-free survival
OS: overall survival
EORTC: QLQ-H&N35 European Organization for Research and Treatment of Cancer-Quality of Life-Head and Neck Questionnaire
QoL: Quality of Life
ECOG: East Coast Oncology Group
TPS: treatment planning system
GTV: gross tumor volume
PTV: planning target volume
RTOG: Radiation Therapy Oncology group
OAR: The organs at risk
Acknowledgements
We thank the patients enrolled in this study.
Funding
This work was supported by the National Natural Science Foundation of China (grant 81371654 to Fujun Zhang).
Authors' contributions
H.Y, Z.M and Z.X performed the study. D.R and Z.Z performed statistical analysis. Y.Z and G.C performed data collection. F.Z and F.G conceived the study.
Competing Interests
We declare that we have no conflict of interest.
References
1. Chen W, Zheng R, Baade P D. et al. Cancer statistics in China, 2015. Ca A Cancer Journal for Clinicians. 2016;66(2):115-132
2. Wei W I, Sham J S T. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041-54
3. Tian Y M, Xiao W W, Bai L. et al. Impact of primary tumor volume and location on the prognosis of patients with locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2015;34(6):1-7
4. Mould R F, Tai T H. Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. British Journal of Radiology. 2002;75(892):307-39
5. Chen C, Fee W, Chen J. et al. Salvage treatment for locally recurrent nasopharyngeal carcinoma (NPC). American Journal of Clinical Oncology. 2014;37(4):327-31
6. Smee RI, Meagher NS, Broadley K. et al. Recurrent nasopharyngeal carcinoma: current management approaches. American Journal of Clinical Oncology. 2010;33(5):469-473
7. Shin SS, Ahn YC, Lim DH, et a. High Dose 3-Dimensional Re-Irradiation for Locally Recurrent Nasopharyngeal Cancer. Yonsei Medical Journal. 2004;45(1):100-6
8. Yu K H, Leung S F, Tung S Y. et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Radiotherapy & Oncology. 2005;27(5):397-405
9. Hsu MM, Ko JY, Sheen TS. et al. Salvage surgery for recurrent nasopharyngeal carcinoma. Laryngoscope. 2007;110(9):1483-8
10. Cheah SK, Lau FN, Yusof MM. et al. Treatment outcome with brachytherapy for recurrent nasopharyngeal carcinoma. Asian Pacific Journal of Cancer Prevention Apjcp. 2014;14(11):6513-8
11. Chan YW, Chow VL, Wei WI. Quality of life of patients after salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Cancer. 2012;118(15):3710-8
12. Vlantis AC, Chan HS, Tong MC. et al. Surgical salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma: A multivariate analysis of prognostic factors. Head & Neck. 2011;33(8):1126-31
13. Qiu S, Lu J, Wei Z. et al. Advantages of intensity modulated radiotherapy in recurrent T1-2 nasopharyngeal carcinoma: a retrospective study. Bmc Cancer. 2014;14(1):797-797
14. Li J C, Hu C S, Jiang G L. et al. Dose escalation of three-dimensional conformal radiotherapy for locally recurrent nasopharyngeal carcinoma: a prospective randomised study. Clinical Oncology. 2006;18(4):293-299
15. Liu S, Lu T, Zhao C. et al. Temporal lobe injury after re-irradiation of locally recurrent nasopharyngeal carcinoma using intensity modulated radiotherapy: clinical characteristics and prognostic factors. Journal of neuro-oncology. 2014;119(2):421-8
16. Poon D, Yap S P, Wong Z W. et al. Concurrent chemoradiotherapy in locoregionally recurrent nasopharyngeal carcinoma. International Journal of Radiation Oncologybiologyphysics. 2004;59(5):1312-8
17. Mitsuhashi N, Sakurai H, Katano S. et al. Stereotactic radiotherapy for locally recurrent nasopharyngeal carcinoma. Laryngoscope. 1999;109(5):805-9
18. Ho A S, Zumsteg Z S, Meyer A. et al. Impact of Flap Reconstruction on Radiotoxicity After Salvage Surgery and Reirradiation for Recurrent Head and Neck Cancer. Annals of Surgical Oncology. 2016;23(Suppl 5):850
19. Eekers D B P, Roelofs E, Jelen U. et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiotherapy & Oncology. 2016;121(3):387
20. Ying G, Shuai L, Wang H Y. et al. Long-term outcomes of a phase Ⅱ randomized controlled trial comparing intensity-modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35(4):1-9
21. Wang G, Zhang F, Yang B. et al. Feasibility and Clinical Value of CT-guided (125)I Brachytherapy for Bilateral Lung Recurrences from Colorectal Carcinoma. Radiology. 2015:278 (3)
22. Zhang T, Lu M, Peng S. et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. Journal of Cancer Research & Clinical Oncology. 2014;140(8):1383-90
23. Xiang Z, Li G, Liu Z. et al. 125I Brachytherapy in Locally Advanced Nonsmall Cell Lung Cancer After Progression of Concurrent Radiochemotherapy. Medicine. 2015;94(49):S73-S73
24. Nag S. Brachytherapy for prostate cancer: summary of American Brachytherapy Society recommendations. Seminars in Urologic Oncology. 2000;18(2):133-6
25. Gao F, Li C, Gu Y. et al. CT-guided 125I brachytherapy for mediastinal metastatic lymph nodes recurrence from esophageal carcinoma: effectiveness and safety in 16 patients. European Journal of Radiology. 2013;82(2):70-5
26. Liu S M, Wang H B, Sun Y. et al. The efficacy of iodine-125 permanent brachytherapy versus intensity-modulated radiation for inoperable salivary gland malignancies: study protocol of a randomised controlled trial. BMC Cancer. 2016;16(1):1-8
27. Lei L, Jie Y, Li X. et al. 125I Seed Permanent Implantation as a Palliative Treatment for Stage III and IV Hypopharyngeal Carcinoma. Clinical & Experimental Otorhinolaryngology. 2016;9(3):185-191
28. Schwarz S B, Thon N, Nikolajek K. et al. Iodine-125 brachytherapy for brain tumours - a review. Radiation Oncology. 2012;7(1):30
29. Huang H, Xu S, Li F. et al. Clinical application of computed tomography-guided125I seed interstitial implantation for head and neck cancer patients with unmanageable cervical lymph node metastases. European Journal of Medical Research. 2016;21(1):18
30. Zhu L, Jiang Y, Wang J. et al. An investigation of 125 I seed permanent implantation for recurrent carcinoma in the head and neck after surgery and external beam radiotherapy[J]. World Journal of Surgical Oncology. 2013;11(1):60
31. Yu L J, Na M, Wang J J. et al. CT-guided iodine-125 seed permanent implantation for recurrent head and neck cancers. Radiation Oncology. 2010;5(1):68
32. Oksüz DC, Meral G, Uzel O. et al. Reirradiation for locally recurrent nasopharyngeal carcinoma: treatment results and prognostic factors. International Journal of Radiation Oncology Biology Physics. 2004;60(2):388-94
33. Han F, Zhao C, Huang S M. et al. Long-term Outcomes and Prognostic Factors of Re-irradiation for Locally Recurrent Nasopharyngeal Carcinoma using Intensity-modulated Radiotherapy. Clinical Oncology. 2012;24(8):569-76
34. Tian YM, Guan Y, Xiao WW. et al. Long-term survival and late complications in intensity-modulated radiotherapy of locally recurrent T1 to T2 nasopharyngeal carcinoma. Head & Neck. 2014;38(2):5573-5584
35. Koutcher L, Lee N, Zelefsky M. et al. Reirradiation of Locally Recurrent Nasopharynx Cancer With External Beam Radiotherapy With or Without Brachytherapy. International Journal of Radiation Oncology Biology Physics. 2010;76(1):130-7
36. Teo PM, Kwan WH, Chan AT. et al. How successful is high-dose (> or = 60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? International Journal of Radiation Oncology Biology Physics. 1998;40(4):897-913
37. Zhou G Q, Yu X L, Chen M. et al. Radiation-Induced Temporal Lobe Injury for Nasopharyngeal Carcinoma: A Comparison of Intensity-Modulated Radiotherapy and Conventional Two-Dimensional Radiotherapy. Plos One. 2013;8(7):e67488
38. Lee C C, Ho C Y. Post-treatment late complications of nasopharyngeal carcinoma. European Archives of Otorhinolaryngology. 2012;269(11):2401-9
39. Dizman A, Coskun M, Guney Y. et al. Reirradiation With Robotic Stereotactic Body Radiation Therapy for Locally Recurrent Nasopharyngeal Carcinoma. Asian Pacific Journal of Cancer Prevention Apjcp. 2013;87(2):3561-6
40. Shen X, Li Y, Zhang Y. et al. An analysis of brachytherapy with computed tomography-guided permanent implantation of Iodine-125 seeds for recurrent nonkeratin nasopharyngeal carcinoma. Nature Material. 2014;11(11):978-85
41. Kong L, Lu J J. Reirradiation of locally recurrent nasopharyngeal cancer: history, advances, and promises for the future. Chinese Clinical Oncology. 2016:5 (2)
42. Leung T W, Wong V Y W, Tung S Y. et al. Stereotactic Radiotherapy for Locally Recurrent Nasopharyngeal Carcinoma. The Laryngoscope. 1999;109(5):805
43. Lengyel E, Baricza K, Somogyi A. et al. Reirradiation of locally recurrent nasopharyngeal carcinoma. Strahlentherapie und Onkologie. 2003;179(5):298-305
44. Chua D T, Wei WI, Sham JS. et al. Treatment outcome for synchronous locoregional failures of nasopharyngeal carcinoma. Head Neck. 2003;25(7):585-94
45. Chua D T, Ma J, Sham J S. et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2005;23(6):1118-24
Author contact
![]() Corresponding authors: Fujun Zhang, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. ADD: 651 Dongfeng Road, East, Guangzhou, China. Postal address: Department of Minimally Invasive& Interventional Radiology, 12th Floor, Building 1, 651 Dongfeng Road, East, Guangzhou, China. Post code: 510060. Email: zhangfjorg.cn; Tel: +86-20-87343905; Fax: +86-20-87343905 and Fei Gao, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. ADD: 651 Dongfeng Road, East, Guangzhou, China. Postal address: Department of Minimally Invasive& Interventional Radiology, 12th Floor, Building 1, 651 Dongfeng Road, East, Guangzhou, China. Post code: 510060; Email: gaoforg.cn; Tel: +86-20-87343905; Fax: +86-20-87343905
Corresponding authors: Fujun Zhang, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. ADD: 651 Dongfeng Road, East, Guangzhou, China. Postal address: Department of Minimally Invasive& Interventional Radiology, 12th Floor, Building 1, 651 Dongfeng Road, East, Guangzhou, China. Post code: 510060. Email: zhangfjorg.cn; Tel: +86-20-87343905; Fax: +86-20-87343905 and Fei Gao, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. ADD: 651 Dongfeng Road, East, Guangzhou, China. Postal address: Department of Minimally Invasive& Interventional Radiology, 12th Floor, Building 1, 651 Dongfeng Road, East, Guangzhou, China. Post code: 510060; Email: gaoforg.cn; Tel: +86-20-87343905; Fax: +86-20-87343905

 Global reach, higher impact
Global reach, higher impact