Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(12):2212-2222. doi:10.7150/jca.18808 This issue Cite
Research Paper
Reelin promotes adhesion of multiple myeloma cells to bone marrow stromal cells via integrin β1 signaling
1. Key Laboratory of Medical Immunology, Ministry of Health. Department of Immunology, School of Basic Medical Sciences, Peking University Health Science Center, 38 Xue Yuan Road, Beijing, P. R. China, 100191
2. Peking University Institute of Hematology, People's Hospital, Beijing, China, 100044
# L.L. and X.Z. contributed equally to this work
Received 2016-12-18; Accepted 2017-4-25; Published 2017-7-15
Abstract
The close interaction between tumor cells and bone marrow stromal cells plays a crucial role in the tumorigenesis of multiple myeloma (MM). Reelin, an extracellular matrix protein, is found expressed in myeloma cells and is negatively associated with prognosis. We examined the role of Reelin in myeloma cell adhesion to bone marrow stromal cells and the signaling pathways involved. The results revealed that Reelin promoted the adhesion of myeloma cells to HS-5, a bone marrow stromal cell line, via the activation of β1 integrin. The resulting phosphorylation of focal adhesion kinase (FAK) led to the activation of Syk/STAT3 and Akt. Reelin's high affinity receptor ApoER2 indirectly modulated the adhesion of myeloma cells by promoting Reelin expression via Sp1. These findings indicate an important role for Reelin/integrin-β1-induced myeloma cell adhesion to bone marrow stromal cells and highlight the therapeutic potential of targeting Reelin/integrin/FAK axis.
Keywords: Reelin, multiple myeloma, bone marrow stromal cells, adhesion, integrin
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by clonal expansion of plasma cells within the bone marrow (BM). The behavior of myeloma cells such as tumor growth and drug resistance depends on the complex and reciprocal interactions of tumor cells with their BM microenvironment. Recent success in new classes of MM therapeutic agents is at least partially due to the fact that they can counteract certain aspects of MM-BM interactions (1).
It is well known that adhesion of MM cells to bone marrow stromal cells (BMSCs) renders the tumor cells resistant against cytotoxic and apoptotic stimuli (2-7). It also contributes to complications of the disease including osteolysis and angiogenesis (8-10). A variety of adhesive molecules, extracellular matrix (ECM), and soluble factors contribute to the adhesive interactions between MM cells and BMSCs. Identification of molecules involved in adhesion is critical for understanding MM biology and searching for novel therapeutic targets for this disease.
The extracellular matrix protein Reelin is an important regulator of proper migration and positioning of cortical neurons, differentiation of neuritis, and formation of spines and synapses during embryonic brain development (11-15). The interaction of Reelin with its high affinity receptor apolipoprotein E receptor 2 (ApoER2) also promotes the adhesion of migrating neurons to fibronectin (FN) via “inside-out” activation of integrin α5β1 (16).
Reelin is also found in multiple types of tumors including prostate cancer, esophageal carcinoma, and retinoblastoma (17-20). High Reelin level is reported to be associated with prostate cancer with high Gleason score (17). Whether Reelin plays a similar role in promoting tumor cell adhesion to their microenvironment, including extracellular matrix or stromal cells is not clear. However, increased cell migration and colony formation was found in a pancreatic cancer cell line or esophageal carcinoma cell line that received siRNAs specific for Reelin, its receptors VLDLR and ApoER2, or the key adaptor Dab1. This suggests that Reelin may play a role in suppressing cell migration or promoting firm cell adhesion to components in the microenvironment (20-21).
We recently found Reelin expression in myeloma cells and the association of high Reelin expression with poor prognosis in myeloma patients (22). We further found that Reelin promotes the adhesion of myeloma cells to FN-coated plates and protects the tumor cells from Doxorubicin-induced cell death. This MM cell-FN adhesion requires Dab1-independent activation of integrin β1 by Reelin. As the adhesion of MM cells to BMSCs is also mediated by the integrin family of adhesion molecules, we thus examined whether Reelin promotes the adhesion of myeloma cells to BMSCs and whether similar signaling pathway is involved.
Intrinsic or recombinant Reelin promotes the adhesion of H929 cells to HS-5 cells. (A-B) Intrinsic Reelin promotes MM cell adhesion to HS-5 cells. H929 cells labeled with Calcein-AM were pre-treated with functional blocking antibody CR-50 (20 μg/ml) or control antibody for 1 h. The cells were washed and added in triplicate to HS-5-seeded wells at 37 ºC for 1 hour. The unattached cells were removed by three washes and the attached H929 cells were analyzed by laser-scanning confocal microscopy (A) and by calculation of the average numbers of labeled cells per well (B). The values obtained in each group were calculated relative to H929 cells without treatment (Blank) and were shown as relative adhesion (cells) in (B). (C-D) Pre-incubation of recombinant Reelin (rReelin) alone or rReelin with blocking antibody CR-50 modulated the adhesion of H929 cells to HS-5. Calcein-AM-labeled H929 cells were incubated with rReelin, and/or CR-50 or isotype control (Ctrl) mouse IgG antibody 1 hour before being added into HS-5-seeded wells. The H929 cell adhesion was analyzed by laser-scanning confocal microscopy (C) and by calculation of the average numbers of labeled cells per well (D). The images are representative of three independent experiments. Error bars indicate the standard deviation. The data were evaluated by Student's unpaired t-test. *p<0.05, **p<0.01, ***p<0.005.
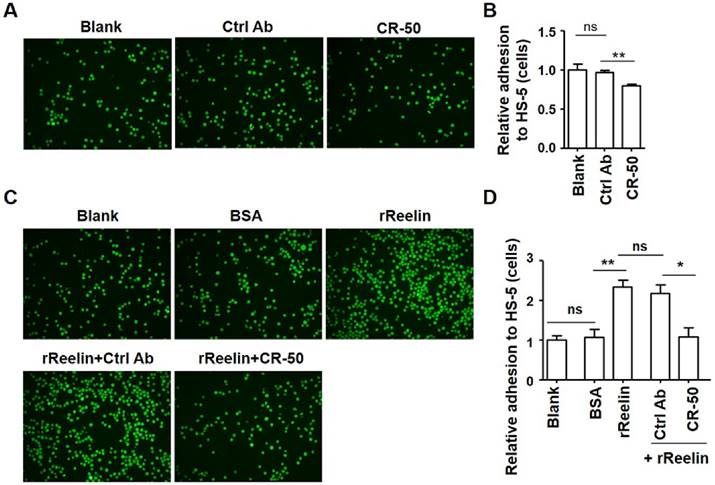
Results
Reelin promotes MM cell adhesion to BMSCs
To examine the effect of Reelin on the adhesion of MM cells to BMSCs, two human myeloma cell lines, H929 and U266 that secret Reelin were used. CR-50, a function-blocking anti-Reelin antibody that blocks Reelin-Reelin homopolymer formation was added to H929 cells to suppress the intrinsic Reelin activity (23). One hour later the CR50-pre-treated cells were co-cultured with a Reelin negative BMSC line (HS-5, data not shown). Compared to the control antibody, the addition of CR-50 inhibited H929 cell adhesion to HS-5 cells (Fig. 1A-B). To examine whether the adhesion of myeloma cells could be improved by Reelin, H929 or U266 cells were pre-incubated (incubated for an hour and then washed) with recombinant Reelin (rReelin) and the cell adhesion to HS-5 cells was measured. As shown in Fig. 1C-D and supplemental Fig. 1A-B, pre-incubation of rReelin significantly enhanced the adhesion of myeloma cells to BMSCs, whereas the addition of CR-50 in the presence of rReelin suppressed rReelin-mediated cell adhesion. No suppression of adhesion was found in the cells pre-incubated with rReelin and the control antibody (Fig. 1C-D and supplemental Fig. 1A-B). These results suggest that Reelin promotes MM cell adhesion to BMSCs.
Reelin facilitates H929 adhesion to HS-5 cells. (A-B) Overexpression or knockdown of Reelin changed the adhesion toward HS-5. H929 cells were transfected with Reelin-expressing plasmid pCrl or Reelin-specific siRNAs. Forty hours later, the cells were labeled with Calcein-AM and added in triplicate to HS-5 cells at 37 ºC for 1 hour. Cell adhesion was then analyzed by laser-scanning confocal microscopy (A) and by calculation of the average numbers of labeled cells per well (B). (C-D) The promotion of H929 cell adhesion to HS-5 cells is Reelin specific. H929 cells transfected with Reelin-specific siRNAs or m-siRNA1 were cultured in HS-5-seeded plates in the presence of BSA or rReelin. Cell adhesion was then analyzed by laser-scanning confocal microscopy (C) and by calculation of the average numbers of labeled cells per well (D). (E) H929 cells transfected with pCrl or control plasmid were measured for the transcription of FERMT2, DOCK8, and NRCAM by quantitative RT-PCR. The results are representative of three independent experiments. Error bars indicate the standard deviation. The data were evaluated by Student's unpaired t-test. *p<0.05, **p<0.01, ***p<0.005.
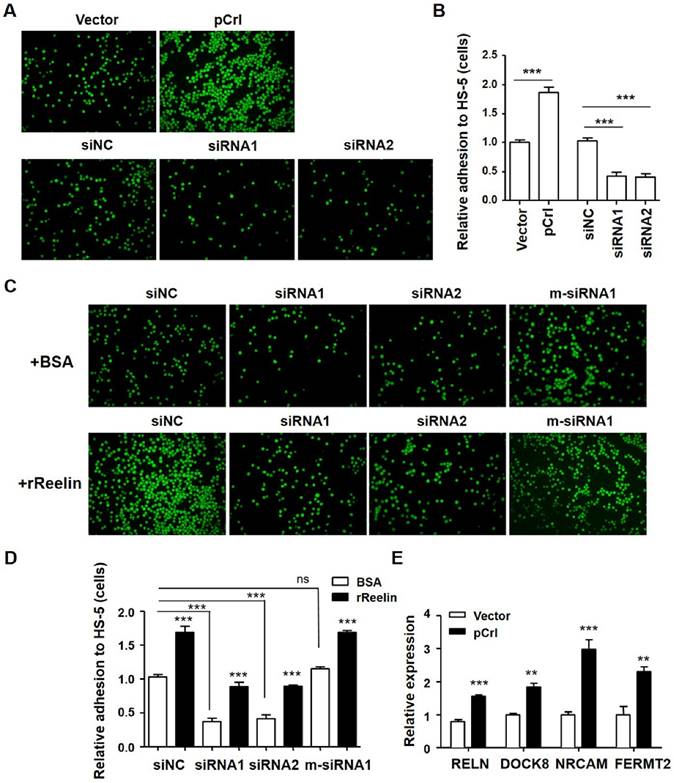
Reelin-mediated MM cell adhesion to BMSCs was further examined by RELN overexpression using the pCrl plasmid (Supplemental Fig. 2A-B) and by knockdown of intrinsic expression using Reelin-specific siRNAs (Supplemental Fig. 2C-D). As shown in Fig. 2A-B, a significant increase in adhesion to BMSCs was observed in pCrl-transfected H929 cells while a significant decrease in adhesion was found in siRNA-transfected H929 cells. The addition of rReelin to the cells transfected with Reelin-specific siRNAs partially alleviated the inhibition of MM cell adhesion to BMSCs (Fig. 2C-D). When mutations around the siRNA cleavage site were introduced into Reelin-specific siRNA (m-siRNA1), the knockdown of Reelin expression and the reduction of cell adhesion to BMSCs were both abolished (Fig. 2C-D, supplemental Fig. 2D). Similar BMSC-adhesion-promoting role of Reelin was also found in U266 cells (supplemental Fig. 2E-H). We also compared the expression of several adhesion-related molecules by real time PCR. As shown in Fig. 2E, Reelin overexpression led to the upregulation of essential genes involved in regulating cell adhesion, such as FERMT2 (Kindlin-2), DOCK8, and NRCAM. These results further confirm that Reelin is involved in enhancing MM adhesion to BMSCs.
Reelin promotes MM adhesion via the activation of integrin β1-FAK-Syk-STAT3/Akt pathways
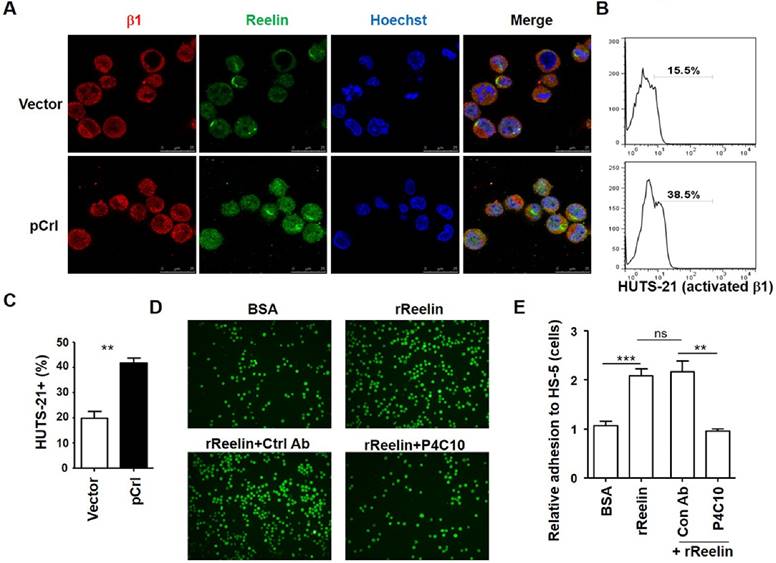
It has been reported that Reelin can activate integrin α3β1 and α5β1 directly or indirectly via inside-out activation (13, 16, 22, 24-26). We thus examined whether Reelin promotes MM adhesion to BMSCs via the activation of integrin β1 signaling. As shown in Fig. 3A, integrin β1 staining was positive in H929 cells and was co-localized with the staining of Reelin either in untransfected or pCrl-transfected cells (also seen in supplemental Fig. 3A). Compared to vector or BSA controls, pCrl transfection or rReelin treatment promoted the activation of integrin β1, resulting in more than 2-fold increase in HUTS-21+ MM cells when co-cultured with BMSCs (Fig. 3B-C and data not shown). When β1 inhibitory antibody P4C10 was applied to the cell culture, the MM cell adhesion to BMSCs caused by rReelin treatment or Reelin overexpression was abolished (Fig. 3D-E, supplemental Fig. 3B, and data not shown). These data suggest that Reelin likely enhances the binding capacity of the extracellular domain of integrin β1 to their ligands expressed in BMSCs (integrin activation), thereby promoting the adhesion and communication between MM cells and bone marrow stromal cells.
Reelin promotes integrin β1 activation and β1-mediated cell adhesion. (A) Reelin and integrin β1 expression in transfected H929 cells. H929 cells were transfected with pCrl or control plasmid pcDNA3 for 40 hours. The cells were then cultured in FN-coated plates overnight and stained with antibodies against integrin β1 (red) and Reelin (green). Nuclei were stained with Hoechst 33342. The cells were analyzed by laser-scanning confocal microscopy (160x). (B-C) Reelin induces the activation of integrin β1. pCrl- or pcDNA3-transfected H929 cells were cultured in HS-5-seeded plates for 1 hour. The cells were then stained with anti-activated integrin β1 antibody (HUTS-21) and analyzed by flow cytometry (B). The percentages of HUTS-21+ H929 cells were shown. The mean percentages of HUTS-21+ cells and standard deviation from each group were shown in (C). (D-E) Reelin-mediated H929 adhesion to HS-5 cells is blocked by P4C10. Labeled H929 cells were cultured in HS-5-seeded plate in the presence of BSA, rReelin, rReelin with control antibody or rReelin with β1 blocking antibody P4C10 for 1 hour. Cell adhesion was then analyzed by laser-scanning confocal microscopy (D) and by average adhesion cell numbers per well (E). The data were evaluated by Student's unpaired t-test.
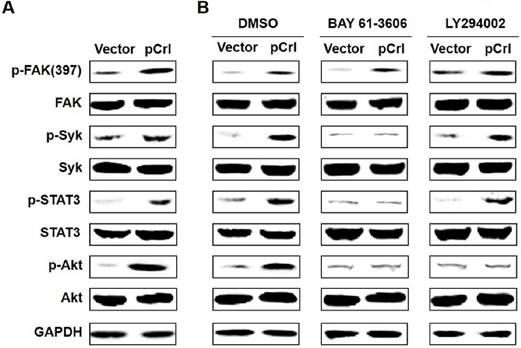
Previously, we found that Reelin promotes MM cell adhesion to FN by activating integrin β1 signaling pathway including focal adhesion kinase (FAK), Src, integrin-activated spleen tyrosine kinase (Syk), signal transducer and activator of transcription (STAT)3 and Akt(22). As Reelin-mediated integrin β1 activation was also found in MM cells when co-cultured with BMSCs, we thus examined whether Reelin activates a similar signaling pathway in MM cells in this co-culture system. As shown in Fig. 4A, overexpression of Reelin results in enhanced phosphorylation of FAK (Tyr397), Syk (Tyr525/526), STAT3 (Tyr705), and Akt (Ser473). The addition of Syk inhibitor BAY 61-3606 suppressed the phosphorylation of STAT3 and Akt but not that of FAK, suggesting that Syk phosphorylation is downstream of FAK and is required for STAT3 and Akt activation (Fig. 4B). The application of PI3K inhibitor LY294002 in the co-culture only inhibited Akt phosphorylation but not the phosphorylation of FAK, Syk, or STAT3, suggesting that PI3K-Akt activation is separate from STAT3 activation and is downstream of FAK and Syk activation (Fig. 4B). Together, these data indicate that Reelin promotes MM cell adhesion to BMSCs by activating the same integrin β1-FAK-Syk-STAT3/Akt signaling pathways.
Reelin promotes integrin β1 signaling via activation of FAK, Syk, STAT3, and Akt. (A) The activation of FAK, Syk, STAT3, and Akt by reelin. H929 cells transfected with Reelin expressing plasmid pCrl or control vector pcDNA3 were cultured in the presence of HS-5 cells for an hour. H929 cells were then harvested by pipetting and the cell lysates were subjected to western blotting with phospho-FAK (Tyr397), total FAK, phospho-Syk (Tyr525/526), total Syk, phospho-STAT3 (Tyr705), total STAT3, phospho-Akt (Ser473), and total Akt-specific antibodies. An antibody specific for GAPDH was used as the loading control. (B) The effects of Syk inhibitor and PI3K inhibitor on Reelin-mediated activation of β1 signaling pathway. Transfected H929 cells were cultured in HS-5-seeded plates and were treated with Syk inhibitor BAY 61-3606 or PI3K inhibitor LY 294002 for 1 hour. H929 cells were then lysed and subjected to western blotting.
ApoER2 is indirectly involved in Reelin-mediated cell adhesion
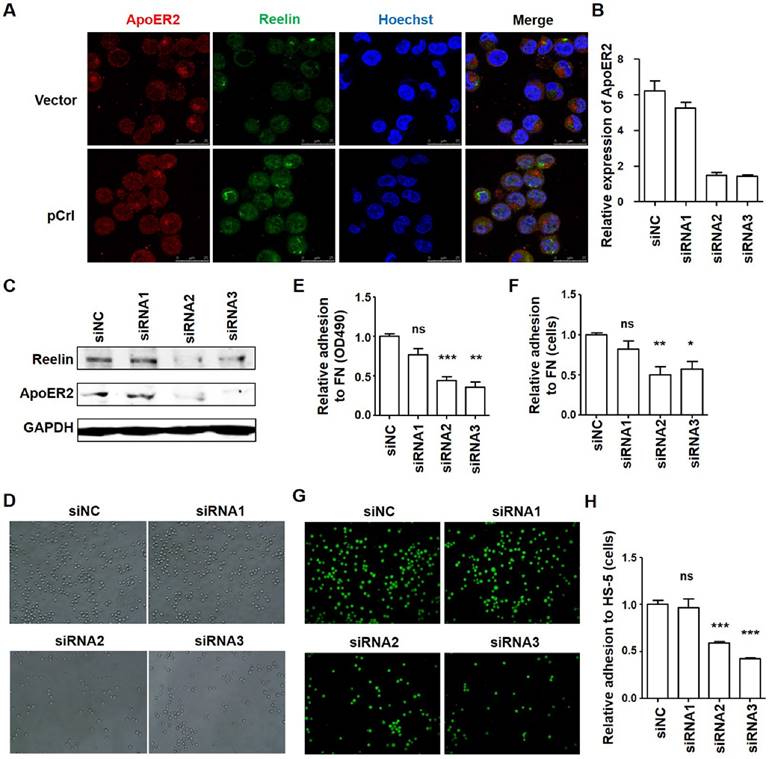
Although Reelin could bind to α3β1 and α5β1 directly, the binding affinity is much lower than ApoER2 (16, 27-28). An inside-out activation of α5β1 by Reelin was reported via ApoER2 and the intracellular Dab1-Crk/CrkL-C3G-Rap1 pathway (16). As ApoER2 is expressed in Reelin+ H929 cells and partially co-localizes with Reelin (Fig. 5A and supplemental Fig. 3C), we wonder whether ApoER2 is involved in Reelin-mediated integrin β1 activation and cell adhesion. Thus, ApoER2 was knocked down in H929 cells by specific siRNAs (Fig. 5B-C) and cell adhesion was examined. Compared to control siRNAs, MM cell adhesion to FN-coated plates (Fig. 5D-F) and HS-5 cells (Fig. 5G-H) were both significantly reduced in MM cells transfected with ApoER2 siRNAs (siRNA2 and siRNA3). This suggests that ApoER2 may be involved in Reelin-mediated cell adhesion.
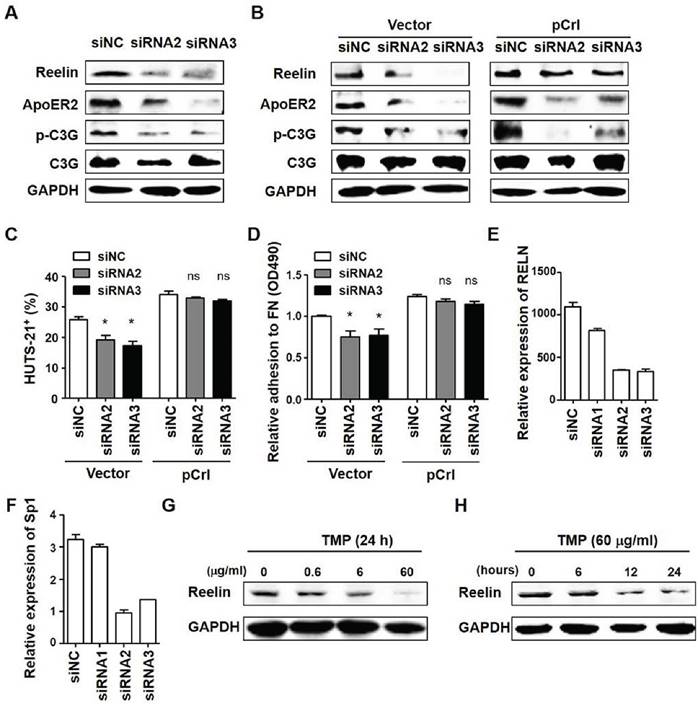
Unexpectedly, we also observed a down-regulation of Reelin expression in ApoER2 siRNA-transfected MM cells (Fig. 5C). To examine whether the suppression of MM adhesion by ApoER2 knockdown is due to the inhibition of inside-out activation of integrin β1 or inhibition of Reelin expression, we first measured the activation of key signaling molecule involved in inside-out signaling, C3G (16). Compared to the control siRNA, the transfection of ApoER2-specific siRNAs suppressed the phosphorylation of C3G, suggesting that ApoER2 may be involved in the activation of C3G signaling pathway (Fig. 6A). In these ApoER2 siRNA-transfected cells, the co-transfected Reelin-expressing plasmid could overcome ApoER2-siRNA-caused Reelin down-regulation but had minimal effect on the suppression of C3G phosphorylation (Fig. 6B). While ApoER2 knockdown itself led to a mild reduction in integrin β1 activation and cell adhesion, the co-transfection of ApoER2 siRNAs and Reelin-expressing plasmid resulted in comparable β1 activation and cell adhesion when compared to siRNA controls (Fig. 6C-D). This suggests that the suppression of MM cell adhesion by ApoER2 siRNA may result from the reduction of Reelin expression, which subsequently affects the activation of integrin. The inside-out activation of integrin induced by Reelin-ApoER2 interaction and C3G phosphorylation may not play a major role in Reelin-mediated MM cell adhesion.
It has been reported that the binding of ApoER2 with its ligand resulted in the activation of the zinc-finger transcription factor specificity protein 1 (Sp1) (29). Activated Sp1 could then bind to RELN promoter region and promote Reelin expression in retinoic acid-treated neuronal NT2 cells (30). As Sp1 is highly expressed in MM cells (31), we examined whether ApoER2 affects RELN expression in MM cells via Sp1. As shown in Fig. 6E-F, similar patterns of Sp1 and RELN down-regulation were found in ApoER2 siRNA-transfected H929 cells (Fig. 6E-F). Less Sp1 protein was also found in cells with ApoER2 knockdown (data not shown). When a small molecule, terameprocol (TMP) was used to inhibit Sp1 activity, the Reelin expression was suppressed (Fig. 6G-H), suggesting that Reelin expression is regulated by Sp1 activity and Sp1 expression is regulated by ApoER2-mediated signaling.
ApoER2 is involved in regulating H929 cell adhesion. (A) Reelin and ApoER2 expression in transfected H929 cells. H929 cells were transfected with Reelin expressing plasmid pCrl or control plasmid for 40 hours. The cells were cultured in FN-coated plates overnight and stained with antibodies against ApoER2 (red) and reelin (green). Nuclei were stained with Hoechst 33342. The cells were analyzed by laser-scanning confocal microscopy (160x). (B-C) Knockdown of ApoER2 reduces Reelin expression in H929 cells. H929 cells were transfected with 300 pmol ApoER2-specific or control siRNA for 24 or 40 hours. The cells were harvested and the mRNA (B) and protein expressions (C) of ApoER2 were determined. Reelin protein level in ApoER2 siRNA-transfected cells was also examined (C). (D-F) Knockdown of ApoER2 alters the adhesion of H929 cells toward FN. H929 cells were transfected with ApoER2-specific siRNAs for 40 hours. The cells were then incubated in FN-coated plates for 1 hour and cell adhesion was analyzed by light microscopy (D), colorimetric analysis (E), and calculation of the average cell numbers per well (F). (G-H) ApoER2 knockdown changes the adhesion of H929 cells toward HS-5 cells. H929 cells were transfected with ApoER2-specific siRNAs, labeled, and then incubated in HS-5-seeded plates for 1 hour. H929 adhesion to HS-5 cells was then analyzed by laser-scanning confocal microscopy (G) and by calculation of the average numbers of labeled cells per well (H). The experiments were repeated for three times. The data were evaluated by Student's unpaired t-test.
ApoER2 affects H929 cell adhesion via reducing reelin expression. (A-B) ApoER2 knockdown reduces the activation of C3G. H929 cells transfected with ApoER2-specific siRNAs alone (A) or co-transfected with ApoER2 siRNA and reelin-expressing plasmid/control vector (B) were cultured in the presence of FN for 1 hour. The cells were then harvested and cell lysates were subjected to western blotting with reelin, ApoER2, phospho-C3G (Tyr504), and total C3G-specific antibodies. An antibody specific for GAPDH was used as the loading control. (C) Overexpression of reelin alleviated the suppression of integrin β1 activation in ApoER2 siRNA-transfected cells. H929 cells were co-transfected with ApoER2-specific siRNA and pCrl/vector control for 40 hours. The cells were then cultured in FN-coated plates for 1 hour, stained with anti-activated integrin β1 antibody (HUTS-21), and analyzed by flow cytometry. Two independent experiments were performed and the mean percentages of HUTS-21+ cells and standard deviation were shown. (D) Reelin overexpression alleviated the suppression of cell adhesion in H929 cells transfected with ApoER2-specific siRNAs. H929 cells were co-transfected with ApoER2-specific siRNAs and pCrl/control vector. Cell adhesion to FN-coated plates was then analyzed by colorimetric analysis. The average and standard deviation of relative adhesion from three independent experiments were shown. (E-F) ApoER2 regulates the expression of transcription factor Sp1. H929 cells were transfected with ApoER2-specific or control siRNA for 24 hours. The cells were harvested and the mRNA expressions of RELN (E) and Sp1 (F) were determined by real time PCR. (G-H) Sp1 regulates the expression of RELN. H929 cells were treated with terameprocol (TMP) for various periods of time and dosages. The cells were harvested and the protein expressions of Reelin were determined. The data were evaluated by Student's unpaired t-test.
Discussion
The extracellular matrix protein Reelin plays an important regulatory role on the migration and adhesion of neurons and likely tumor cells. In a pancreatic cancer cell line, Reelin inhibits cell migration via its high affinity receptors ApoER2/VLDLR and intracellular adaptor protein Dab1 (21). Whether it promotes cell adhesion in tumors has not been thoroughly examined. Our previous study revealed that Reelin could be released from myeloma cells and induced cell adhesion to FN via integrin β1 signaling pathway (22). Blocking Reelin secretion by Brefeldin A diminished the activation of integrin β1. In this study, we further showed that myeloma cell adhesion to bone marrow stromal cells was also promoted by Reelin. Similar β1/FAK/Src/Syk/STAT3 and Akt signaling pathways were activated in Reelin-dependent but ApoER2-independent manner.
Many surface molecules contribute to the close interaction between myeloma cells and BMSCs, rendering the tumor cells resistant against cytotoxic and apoptotic stimuli (2-6). Among them, FN is a large heterodimeric glycoprotein that can be found on the surface of multiple types of stromal cells as well as plasma and extracellular matrix. It can bind to integrin β1 expressed on tumor cells, facilitating the stromal-tumor interaction (21). Our previous study indicates that Reelin promotes myeloma cell adhesion to FN-coated plates by activating integrin β1 (22). The current study shows that the adhesion of myeloma cells to HS-5 cells is also promoted by Reelin via similar β1 signaling pathway. The β1 integrin blockade suppresses Reelin-mediated myeloma cell adhesion not only to FN-coated plates but also to HS-5 cells. It is thus reasonable to suggest that Reelin facilitates the bindng of integrin β1 on myeloma cells to FN on the surface of HS-5 cells. Such myeloma-BMSC interaction subsequently promotes myeloma cell survival and drug resistance in the bone marrow (22, 32). The function-blocking antibody CR-50 may thus be used as a potential therapeutic agent to suppress myeloma-BMSC interaction and enhance drug resistance of myeloma cells.
Sekine, et al. showed that although Reelin could bind to integrin α5β1 with low affinity, the activation of α5β1 in neurons actually depends on the interaction of Reelin with its high affinity receptor ApoER2 which subsequently activates the intracellular Dab1-Crk/CrkL-C3G-Rap1 pathway (16). In myeloma cells, however, ApoER2 is not likely involved in Reelin-mediated activation of integrin β1 signaling. First, the transcription of key molecule Dab1 was undetectable (22). Second, although the knockdown of ApoER2 by siRNA results in reduced phosphorylation of C3G and reduced cell adhesion, it also reduced Reelin expression. The overexpression of Reelin in ApoER2 siRNA-transfected cells alleviated the suppression of β1 activation and cell adhesion but not that of C3G activation. It suggests that ApoER2 expression and C3G phosphorylation is probably not involved in Reelin-mediated cell adhesion. Instead, ApoER2 may indirectly improve cell adhesion via upregulating Reelin expression.
It has been reported that an intracellular fragment of ApoER2 negatively regulates RELN transcription. A γ-secretase activated by the binding of Reelin with ApoER2 was responsible for the cleavage of ApoER2 (33). Interestingly, our results with ApoER2 knockdown and Sp1 inhibition suggest that ApoER2 promotes the expression and activity of transcription factor Sp1 and thus facilitates RELN transcription in myeloma cells. In addition, a γ-secretase inhibitor N-[N-(3.5-difluorophenacetyl)-L-alanyl]-S-phenyl glycinet-butyl ester (DAPT) did not affect Reelin expression or Reelin-mediated myeloma cell adhesion (data not shown). Whether the disparity of RELN regulation between the two groups is due to the differences in cell types or culture conditions awaits further investigation.
Taken together, our results reveal that Reelin promotes myeloma cell adhesion to bone marrow stromal cells. This process is independent of its high affinity receptor ApoER2. Instead, Reelin activates integrin β1, facilitates its binding with fibronectin on the surface of bone marrow stromal cells, and activates FAK-Syk-STAT3/Akt signaling pathway. The expression of Reelin can be enhanced by ApoER2-mediated Sp1 upregulation. These findings further highlight the therapeutic potential of intervening with the complex interaction of myeloma cells with bone marrow microenvironment by targeting the Reelin/integrin/FAK axis.
Materials and Methods
Cell lines and cell culture
The human myeloma cell line NCI-H929 (shown as H929) and U266 were kindly provided by Prof. Jian Hou from Shanghai Chang Zheng Hospital and Prof. Yu Zhang from Peking University Health Science Center (Beijing, China), respectively. HS-5 was provided by Prof. Yuanzhong Chen from Affiliated Union Hospital of Fujian Medical University. H929 and U266 cells were cultured in RPMI 1640 (GibCo, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, ThermoFisher Scientific, Waltham, MA, USA). HS-5 was cultured in DMEM (GibCo) supplemented with 10% FBS. All the cell cultures were supplemented with 2 mmol/L glutamine and 1% penicillin/streptomycin (GibCo) and were cultured at 37 ºC in a humidified atmosphere of 5% CO2.
Quantitative RT-PCR
Total RNA was isolated from the indicated cells using TRIzol (Invitrogen, Grand Island, NY, USA), according to the manufacturer's instructions. The cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis kit (Tiangen, Beijing, China). Real-time PCR was performed using a PCR Master Mix (Roche, Basel Schweiz, Switzerland) according to the manufacturer's protocol. Quantitative PCR was performed on an iCycler (Bio-Rad Laboratories, Hercules, CA, USA). The primer sequences are shown in Table 1. The PCR conditions were 94 ºC for 12s, 60 ºC for 12s, and 72 ºC for 12s. The quantification was based on ∆∆CT calculations and was normalized to GAPDH as loading controls.
Primers used in qRT-PCR analyses (5'-3')
| RELN | forward | GATGGGCGGCGTCAGCTAAT |
|---|---|---|
| reverse | GGCTCTGCACGTGCTCAGAA | |
| GAPDH | forward | ACCCACTCCTCCACCTTTGA |
| reverse | CTGTTGCTGTAGCCAAATTCGT | |
| ApoER2 | forward | ACAATATTGAATGGCCCAACG |
| reverse | TCAATGCTGGACAGTTGGTGTA | |
| DOCK8 | forward | ACAAGACGCTTCCGAAACAGA |
| reverse | CCTGCCTATTGGTCCTCCG | |
| NRCAM | forward | TCCAACCATCACCCAACAGTC |
| reverse | TGAGTCCCATTACGGGTCCAG | |
| FERMT2 | forward | TCTGACCATGCTCTCTGGTG |
| reverse | AGTTCTTCGGGGTGTCTGATA |
Sequences of siRNAs.
| siRNAs | Sense strand (5'-3') |
|---|---|
| Reelin siRNA1 | CCAGCAUCAUCGUGUUAUAdTdT |
| Reelin siRNA2 | GGCGAUUGAUAAUGUUGUAdTdT |
| Reelin m-siRNA1 | CCAGCAGACGAUUGUUAUAdTdT |
| ApoER2 siRNA1 | CGAGGACGAUGACUGCUUAdTdT |
| ApoER2 siRNA2 | GUGACCUCUCCUACCGUAAdTdT |
| ApoER2 siRNA3 | CGAAGAUGAGCUCCAUAUAdTdT |
The difference between Reelin siRNA1 and m-siRNA1 is underlined.
Transient transfection
Full-length Reelin expression vector, pCrl, was a generous gift from Prof. Tom Curran (The Children's Hospital of Philadelphia, Philadelphia, PA). pcDNA3 was used as a control vector. siRNAs against RELN and APOER2 were purchased from RIBOBIO (Guangzhou, China). MM cells growing at logarithmic phase were transfected with either 10μg pCrl or control vector pcDNA3, or 300pmol Reelin- (or ApoER2-) specific siRNA, negative control siRNA (siNC), or a specific control siRNA with nucleic acid mutations around the cleavage site (m-siRNA1) using electroporation (Multiporator, Eppendorf, Hamburg, Germany). The sequences of siRNAs were shown in Table 2.
Cell adhesion assays
HS-5 cells at 2×105 cell/ml were allowed to attach to 96-well plates at 37 °C overnight. Plain myeloma cells (H929 or U266 cells) or myeloma cells transfected with plasmid and/or siRNAs 40 hours earlier were labeled with 10 μM calcein-acetoxymethyl ester (Calcein-AM, Life Technologies, Grand Island, NY) for 30 minutes at 37°C and washed for three times. The cells were pre-incubated at 5×105/ml in RPMI1640 with recombinant Reelin protein (1 μg/ml, R&D, Minneapolis, MN, USA) and/or Reelin-specific blocking antibody CR-50 (20 μg/ml, MBL International Corporation, Woburn, MA, USA), and/or the blocking antibody against integrin β1 (P4C10 clone, 20 μg/ml, Merck Millipore, Massachusetts, MA), and/or isotype control (Ctrl) mouse IgG antibodies (20 μg/ml, eBioscience). After 1 hour of pre-incubation at 37 ºC, the cells were washed and resuspended at 5×105/ml in RPMI1640 with 0.1% BSA (adhesion medium). The cells were then added in triplicate to HS-5 cells at 37 ºC for 1 hour. The unattached cells were removed by three washes with pre-warmed serum-free RPMI 1640 and the attached cells were counted for three microscopic fields (20×) and averaged. A laser-scanning confocal microscope (U-HGLGPS, Olympus, Shinjuku-ku, Tokyo, Japan) was used to distinguish the labeled H929 or U266 cells from unlabeled HS-5 cells.
For FN adhesion assays, a 96-well plate was coated with 40 μg/ml of FN (Sigma-Aldrich, Oakville, ON) in PBS at 4 ºC overnight. BSA in PBS (5%) was then used to block nonspecific binding sites in the wells at 37 ºC for 1 h before the experiment. Transfected myeloma cells were then added in triplicate to FN-coated 96-well plates at 37 ºC for 1 hour, and unbound cells were removed. The attached cell numbers were counted for three microscopic fields (20×) per well and averaged. The attached cells were also stained with crystal violet and measured by colorimetric cell adhesion assay at OD 490 using Multiskan MK3 (ThermoFisher Scientific).
Integrin activation assay
A 96-well plate was seeded with 2×105 cell/ml of HS-5 overnight. After washing, myeloma cells transfected with pCrl and/or ApoER2-specific siRNA were cultured in HS-5-seeded plates for 1 hour at 37°C. Myeloma cells were then separated from HS-5 cells by pipetting and stained with anti-CD29-PE (HUTS-21, activated integrin β1, BioLegend, San Diego, CA, USA). The cells were analyzed by FACS Arial II (BD PharMingen, San Diego, CA). To determine the level of nonspecific binding, the cells were stained in parallel with the PE isotype control mouse IgG antibodies (BD PharMingen, San Diego, CA, USA).
Immunoblotting and confocal microscopy
Following incubation under the indicated conditions, including culturing on FN or HS-5-coated plates and addition of DMSO, Syk inhibitor IV BAY 61-3606 (1 μmol/L, Merck Millipore), PI3K inhibitor LY 294002 (50 μmol/L, Cell Signaling Technology, Danvers, MA) or Sp1 inhibitor terameprocol (TMP, Sigma-Aldrich, Oakville, ON), H929 cells transfected with plasmid and/or siRNAs were collected by pipetting and washed twice with ice-cold PBS, and incubated for 10 minutes at 4 ºC in Triton X-100 lysis buffer (30 mM Tris-HCl pH7.5, 150 mM NaCl, 25 mM NaF, 1% Triton X-100, 10% glycerol, 2 mM Naorthovanadate). The whole-cell lysates were subjected to 6-8% gradient polyacrylamide gels and transferred to nitrocellulose membrane (Whatman, GE Healthcare Life Sciences, Pittsburgh, PA, USA). The primary antibodies used were anti-Reelin, anti-ApoER2 purchased from Abcam (Cambridge, MA, USA), anti-phospho-C3G (Tyr504), anti-C3G from Santa Cruz, anti-phospho-FAK (Tyr397), anti-FAK, anti-phospho-STAT3 (Tyr705), anti-STAT3, anti-phospho-Syk (Tyr525/526), anti-Syk, anti-phospho-Akt (Ser473), anti-Akt, and anti-GAPDH from Cell Signaling Technology. Goat-anti-mouse IRDye 800CW, Goat-anti-rabbit IRDye 800CW (LI-COR Biosciences, Lincoln, NE, USA), anti-mouse IgG HRP conjugate, anti-rabbit IgG HRP conjugate (Promega, Madison, WI, USA) were used as the secondary antibodies. The immunoreactive bands were detected by fluorescence with LiCor Odyssey Gel imaging Scanner or chemiluminescence with ECL detection reagents (ThermoFisher Scientific), and exposed to ImageQuantTM LAS 500 (GE Healthcare Life Sciences).
H929 cells transfected with plasmid and/or siRNAs were cultured on FN-coated plates overnight and stained with mouse anti-Reelin, rabbit anti-ApoER2, rabbit anti-Integrin beta 1 (Abcam), secondary anti-mouse AlexaFluor 488, anti-rabbit AlexaFluor 594 (ZSGB-BIO, Beijing, China) and H33324 (DOJINDO Molecular Technologies). A laser-scanning confocal microscope (TCS SP5, Leica, Germany) was used to assess the distribution and intensity. The objective lens used is HCX PL APO CS 40×. LAS AF was used to process the images.
Statistical analysis
The data were evaluated by Student's t-test. All data are presented as mean ± standard deviation. The following terminology is used to denote the statistical significance: *p<0.05, **p<0.01, ***p<0.005, ns, not statistically significant.
Supplementary Material
Supplementary figures.
Acknowledgements
The authors wish to thank Prof. Tom Curran (The Children's Hospital of Philadelphia, Philadelphia, PA) and Prof. Hongquan Zhang (Peking University Health Science Center) for kindly providing pCrl plasmid and integrin β1 antibodies. The human myeloma cell lines NCI-H929 (shown as H929) and U266 were kindly provided by Prof. Jian Hou from Shanghai Chang Zheng Hospital and Prof. Yu Zhang from Peking University Health Science Center. HS-5 was provided by Prof. Yuanzhong Chen from Affiliated Union Hospital of Fujian Medical University.
Grant Support
This work was supported by grants from the National Natural Science Foundation of China (31270935, 81471525, and 31671244, Q.G., 81670192, J.L.), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001, Q.G.), Natural Science Foundation of Beijing Municipality (5152010, Q.G.).
Author contributions
L.L., designed and performed the experiments, prepared figures; X.Z. performed the experiments and prepared figures. Q.A. performed the experiments; J.H., Y.C., and R.J. prepared the reagents and samples, helped with experimental design; X.H. and J.L. helped with experimental design and provided critical opinion on manuscript; Q.G., designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no conflict of interest.
References
1. Mitsiades CS, Mitsiades NS, Richardson PG, Munshi NC, Anderson KC. Multiple myeloma: a prototypic disease model for the characterization and therapeutic targeting of interactions between tumor cells and their local microenvironment. J Cell Biochem. 2007;101(4):950-68
2. Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17(6):1175-82
3. Wang X, Li C, Ju S, Wang Y, Wang H, Zhong R. Myeloma cell adhesion to bone marrow stromal cells confers drug resistance by microRNA-21 up-regulation. Leuk Lymphoma. 2011;52(10):1991-8
4. Markovina S, Callander NS, O'Connor SL, Xu G, Shi Y, Leith CP. et al. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176
5. Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103(9):3503-10
6. Perez LE, Parquet N, Shain K, Nimmanapalli R, Alsina M, Anasetti C. et al. Bone marrow stroma confers resistance to Apo2 ligand/TRAIL in multiple myeloma in part by regulating c-FLIP. J Immunol. 2008;180(3):1545-55
7. Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci. 2016
8. Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249-74
9. Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585-98
10. Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG. et al. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21(6):1007-34 vii-viii
11. D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719-23
12. D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17(1):23-31
13. Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D. et al. Reelin, Disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci U S A. 2002;99(20):13178-83
14. Forster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM. Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur J Neurosci. 2006;23(4):901-9
15. Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4(6):496-505
16. Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, Hattori M. et al. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin alpha5beta1. Neuron. 2012;76(2):353-69
17. Perrone G, Vincenzi B, Zagami M, Santini D, Panteri R, Flammia G. et al. Reelin expression in human prostate cancer: a marker of tumor aggressiveness based on correlation with grade. Mod Pathol. 2007;20(3):344-51
18. Wang Q, Lu J, Yang C, Wang X, Cheng L, Hu G. et al. CASK and its target gene Reelin were co-upregulated in human esophageal carcinoma. Cancer Lett. 2002;179(1):71-7
19. Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823-32
20. Yuan Y, Chen H, Ma G, Cao X, Liu Z. Reelin is involved in transforming growth factor-beta1-induced cell migration in esophageal carcinoma cells. PLoS One. 2012;7(2):e31802
21. Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006;130(2):548-65
22. Lin L, Yan F, Zhao D, Lv M, Liang X, Dai H. et al. Reelin promotes the adhesion and drug resistance of multiple myeloma cells via integrin beta1 signaling and STAT3. Oncotarget. 2016
23. Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, Nakajima K. et al. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc Natl Acad Sci U S A. 2000;97(17):9729-34
24. Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E. et al. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci U S A. 2003;100(9):5479-84
25. Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA. et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27(1):33-44
26. Magdaleno SM, Curran T. Brain development: integrins and the Reelin pathway. Curr Biol. 2001;11(24):R1032-5
27. Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J. et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689-701
28. D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24(2):471-9
29. Chen X, Guo Z, Okoro EU, Zhang H, Zhou L, Lin X. et al. Up-regulation of ATP binding cassette transporter A1 expression by very low density lipoprotein receptor and apolipoprotein E receptor 2. J Biol Chem. 2012;287(6):3751-9
30. Chen Y, Kundakovic M, Agis-Balboa RC, Pinna G, Grayson DR. Induction of the reelin promoter by retinoic acid is mediated by Sp1. J Neurochem. 2007;103(2):650-65
31. Fulciniti M, Amin S, Nanjappa P, Rodig S, Prabhala R, Li C. et al. Significant biological role of sp1 transactivation in multiple myeloma. Clin Cancer Res. 2011;17(20):6500-9
32. Kim I, Uchiyama H, Chauhan D, Anderson KC. Cell surface expression and functional significance of adhesion molecules on human myeloma-derived cell lines. Br J Haematol. 1994;87(3):483-93
33. Balmaceda V, Cuchillo-Ibanez I, Pujadas L, Garcia-Ayllon MS, Saura CA, Nimpf J. et al. ApoER2 processing by presenilin-1 modulates reelin expression. FASEB J. 2014;28(4):1543-54
Author contact
![]() Corresponding author: Qing Ge, Department of Immunology, School of Basic Medical Sciences, Peking University Health Science Center, 38 Xue Yuan Road, Beijing, 100191, P. R. China Tel: 86-10-82802593, Fax: 86-10-82801436, Email: qinggeedu.cn. Jin Lu, Peking University Institute of Hematology, People's Hospital, Beijing, P. R. China, 100044, jin1lucom.
Corresponding author: Qing Ge, Department of Immunology, School of Basic Medical Sciences, Peking University Health Science Center, 38 Xue Yuan Road, Beijing, 100191, P. R. China Tel: 86-10-82802593, Fax: 86-10-82801436, Email: qinggeedu.cn. Jin Lu, Peking University Institute of Hematology, People's Hospital, Beijing, P. R. China, 100044, jin1lucom.

 Global reach, higher impact
Global reach, higher impact