Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(14):2692-2698. doi:10.7150/jca.20409 This issue Cite
Research Paper
Updated Nomogram Incorporating Percentage of Positive Cores to Predict Probability of Lymph Node Invasion in Prostate Cancer Patients Undergoing Sentinel Lymph Node Dissection
1. University Hospital for Urology, Klinikum Oldenburg, School of Medicine and Health Sciences, Carl von Ossietzky University Oldenburg, Oldenburg, Germany;
2. Working Group Statistics and Econometrics, Georg-August University Göttingen, Göttingen, Germany;
3. Institute of Pathology Oldenburg, Oldenburg, Germany.
Received 2017-4-4; Accepted 2017-5-28; Published 2017-8-22
Abstract
Objectives: To update the first sentinel nomogram predicting the presence of lymph node invasion (LNI) in prostate cancer patients undergoing sentinel lymph node dissection (sPLND), taking into account the percentage of positive cores.
Patients and Methods: Analysis included 1,870 prostate cancer patients who underwent radioisotope-guided sPLND and retropubic radical prostatectomy. Prostate-specific antigen (PSA), clinical T category, primary and secondary biopsy Gleason grade, and percentage of positive cores were included in univariate and multivariate logistic regression models predicting LNI, and constituted the basis for the regression coefficient-based nomogram. Bootstrapping was applied to generate 95% confidence intervals for predicted probabilities. The area under the receiver operator characteristic curve (AUC) was obtained to quantify accuracy.
Results: Median PSA was 7.68 ng/ml (interquartile range (IQR) 5.5-12.3). The number of lymph nodes removed was 10 (IQR 7-13). Overall, 352 patients (18.8%) had LNI. All preoperative prostate cancer characteristics differed significantly between LNI-positive and LNI-negative patients (P<0.001). In univariate accuracy analyses, the proportion of positive cores was the foremost predictor of LNI (AUC, 77%) followed by PSA (71.1%), clinical T category (69.9%), and primary and secondary Gleason grade (66.6% and 61.3%, respectively). For multivariate logistic regression models, all parameters were independent predictors of LNI (P<0.001). The nomogram exhibited a high predictive accuracy (AUC, 83.5%).
Conclusion: The first update of the only available sentinel nomogram predicting LNI in prostate cancer patients demonstrates even better predictive accuracy and improved calibration. As an additional factor, the percentage of positive cores represents the leading predictor of LNI. This updated sentinel model should be externally validated and compared with results of extended PLND-based nomograms.
Keywords: prostate cancer, sentinel node, lymphadenectomy, lymph node invasion, nomogram, 99mtechnetium nanocolloid.
Introduction
Lymph node (LN) status is a crucial prognostic factor in prostate cancer and also has therapeutic relevance. The risk of progression can be calculated and appropriate adjuvant therapy can be planned. Despite recent advances in imaging, the histological detection of metastases, or pelvic LN dissection (PLND), is still the gold standard for LN staging in clinically localized prostate cancer. None of the available means of radiological imaging provides equal sensitivity in the detection of LN invasion (LNI) in prostate cancer patients. New imaging methods, like 68gallium prostate-specific membrane antigen (PSMA) positron emission tomography (PET)/computer tomography or PET/magnetic resonance imaging are suitable for the detection of small metastases, but the reliability of these procedures is limited by their spatial resolution, resulting in a low sensitivity (49%-66%) in detection of LN (micro)metastases [1].
LNI prevalence is directly related to the number of dissected LNs and extent of PLND [2]. For these reasons, the European Association of Urology (EAU) guidelines recommend an extended PLND (ePLND) approach for LN staging of prostate cancer patients with a >5% risk of LNI calculated by ePLND-based nomograms [3-5]. However, the rate of complications rises along with the number of LNs removed [6-8]. Furthermore, the extended template may not encompass all the lymphatic drainage sites. Studies using radioisotope-guided sentinel PLND (sPLND) demonstrated up to 35% of prostate lymphatic drainage sites outside of the standard extended anatomical template, which includes the external iliac, hypogastric, and obturator fossa regions [9, 10]. Because of the increased complication rates of ePLND and low detection rate of limited PLND (lPLND) procedures, the concept of radioisotope-guided sentinel lymph node (SLN) identification used in other tumor entities was transferred to prostate cancer [11]. In breast cancer, sentinel lymphadenectomy was shown to provide similar staging accuracy compared with nodal dissection while reducing morbidity [12]. SLN detection improved survival when compared with conservative management in melanoma patients [13].
In prostate cancer, the sentinel approach allows an individualized extension of LN dissection outside the boarders of ePLND with targeted removal of relatively few LNs, which also means lower morbidity [8]. Currently, a variety of new techniques (e.g., hybrid radioactive/fluorescent tracer methods, and marking with superparamagnetic iron-oxide nanoparticles) are showing promising results in the identification of SLNs in prostate cancer patients in open, laparoscopic, and robot-assisted approaches [14-16]. A current consensus report provides a basis for further studies in this field [17].
For radioisotope-guided sPLND using 99mtechnetium nanocolloid, studies involving several thousand prostate cancer patients demonstrated a high sensitivity for the identification of LN-positive patients [18-20]. However, until recently, nomograms predicting LNI for prostate cancer patients undergoing PLND were only developed based on data from conventional lPLND or ePLND and were not related to a sentinel-guided approach [4, 5, 21, 22, 23].
Therefore, a nomogram for predicting LNI in prostate cancer patients undergoing sPLND was developed [24]. This first sentinel nomogram could highly accurately predict LNI at a sPLND, and for the first time assist clinicians and patients in making important decisions upon indication of a sPLND. However, no consideration has yet been given to the percentage of positive cores as a predictor, as in other nomograms [4, 25]. In the updated Briganti Nomogram, the percentage of positive cores is the most accurate predictor of LNI [4].
The objective of the present study was an update of the first sentinel nomogram, taking into account the percentage of positive cores considering an even larger sentinel collective. With regard to the results of the ePLND series, we expected further improvement in predictability upon inclusion of the percentage of positive cores.
Patients and Methods
Patients
A total of 1,890 consecutive prostate cancer patients were identified who underwent sPLND in combination with radical retropubic prostatectomy carried out by four highly experienced surgeons in a single center between January 2006 and December 2013. We excluded patients with incomplete clinical information for prostate-specific antigen (PSA), clinical category, or biopsy Gleason score (n=4, 0.2%). Furthermore, we also excluded cases who had undergone a transurethral resection or laser therapy of the prostate, hormonal therapy prior to operative treatment, and those with cT4 tumors (n=16, 0.8%). The final sample comprised 1,870 patients. All patients were informed orally and in writing about a sPLND and radical retropubic prostatectomy, and all signed a consent form.
sPLND technique
99mtechnetium nanocolloid was transrectally injected 24 h before surgery into the prostate with ultrasound guidance [24]. Three injections were performed per prostate lobe. Activity attained approximately 100 MBq per lobe and total injection volume was about 1.2 ml. A few hours after injection, scintigraphy was carried out. The radioactivity of the LN was intraoperatively measured using two different gamma probe systems (C-Trak System, Care Wise, MorganHill, CA, USA; Crystal Probe SG04, Crystal Photonics GmbH, Berlin, Germany). LNs identified as SLNs by the gamma probe were dissected. For surgical reasons, LNs other than SLNs directly adjoining and adhering to SLNs were also removed if an in-situ separation was not possible. Furthermore, in the case of SLNs in the obturator fossa area, the surrounding non-radioactive lymphatic tissue of the fossa was also dissected. However, lymphatic tissue of the fossa was not resected if no SLN existed in the fossa area.
Histopathological examination
All LNs were initially cut into 3-mm-thick transverse sections, routinely processed and completely embedded in paraffin; 4-5-µm-thick sections were stained with hematoxylin-eosin (H&E). Selected cases of serial sections were analyzed. Immunohistochemistry with a pan-cytokeratin antibody (AE1/AE3) was carried out to confirm or exclude metastatic spread in rare cases with inconclusive conventional histology.
Statistical analyses
Univariable and multivariable logistic regression models were carried out to test the association between preoperative tumor characteristics and the probability of LNI. The predictor variables were preoperative PSA level, clinical T-category, primary and secondary biopsy Gleason grade, and the percentage of positive cores. The clinical category was classified per the 2002 Union for International Cancer Control TNM staging system.
Regression coefficients were used to develop the nomogram that predicts the probability of LNI at a sPLND. Bootstrapping (9,999 replications) was applied to generate reliable 95% confidence intervals for the predicted probabilities and for internal validation. Predictive accuracy was quantified using the area under the receiver operator characteristic curve (AUC). The performance characteristics were evaluated using a calibration plot of predicted probabilities against observed LNI rates.
Finally, we systematically assessed the sensitivity, specificity, positive predictive value, and negative predictive value of a range of nomogram thresholds from 1% to 10% to correctly predict histologically confirmed LNI.
Statistical analyses were performed using the GLM (generalized linear model) function of the open-source statistical software R (R Development Core Team 2016) [26].
Results
Table 1 summarizes the patient characteristics. The median number of LNs removed was 10 (IQR 7-13), encompassing a median of six (IQR 4-8) SLNs. Overall, 18.8% of patients (n=352) had LNI. The median number of positive LNs per patient was two (IQR 1-3).
Characteristics of patients according to lymph node invasion.
| Overall | pN0 | pN1 | p value | |
|---|---|---|---|---|
| Patients | 1,870 | 1,518 (81.2) | 352 (18.8) | |
| Age at surgery, yr | 67 (61-71) | 67 (61-71) | 67 (63-71) | 0.0252 |
| No. of LN removed | 10 (7-13) | 10 (7-13) | 12 (9-14) | <0.001 |
| No. of positive LN | - | - | 2 (1-3) | - |
| Total PSA, ng/ml | 7.68 (5.5-12.3) | 7.09 (5.3-10.7) | 12.3 (7.7-20.7) | <0.001 |
| T-category | <0.001 | |||
| T1c | 1,021 (54.6) | 933 (61.5) | 88 (25.0) | |
| T2 | 807 (43.2) | 577 (38) | 230 (65.3) | |
| T3 | 42 (2.2) | 8 (0.5) | 34 (9.7) | |
| Primary Gleason grade | <0.001 | |||
| ≤3 | 1,592 (85.1) | 1,387 (91.4) | 205 (58.2) | |
| ≥4 | 278 (14.9) | 131 (8.6) | 147 (41.8) | |
| Secondary Gleason grade | <0.001 | |||
| ≤3 | 1,098 (58.7) | 956 (63) | 142 (40,3) | |
| ≥4 | 772 (41.3) | 562 (37) | 210 (59.7) | |
| Biopsy cores taken | 12 (10-14) | 12 (10-14) | 12 (9-13) | <0.001 |
| Positive biopsy cores | 4 (2-6) | 3 (2-5) | 6 (4-10) | <0.001 |
| % of positive biopsy cores | 33.3 (16.7-50) | 25.8 (14.3-46.2) | 59.6 (39.4-100) | <0.001 |
| Postoperative Gleason sum | <0.001 | |||
| ≤6 | 324 (17.3) | 323 (21.3) | 1 (0.3) | |
| 7 | 1,377 (73.6) | 1,141 (75.2) | 236 (67.0) | |
| 8-10 | 169 (9) | 54 (3.6) | 115 (32.7) | |
| Pathologic stage | <0.001 | |||
| pT2 | 1,181 (63.2) | 1,141 (75.2) | 40 (11.4) | |
| pT3a | 357 (19.1) | 262 (17.3) | 95 (27) | |
| pT3b | 285 (15.2) | 101 (6.5) | 184 (52.3) | |
| pT4 | 47 (2.5) | 14 (0.9) | 33 (9.4) |
Data are given as median (IQR) or number (%).
IQR= Interquartile range; LN= lymph node; PSA= prostate-specific antigen
Nomogram predicting the probability of lymph node involvement (LNI) in patients undergoing sentinel-guided pelvic lymphadenectomy based on preoperative PSA, clinical T-category, primary and secondary biopsy Gleason grade, and percentage of positive cores. Instructions: Locate the pretreatment parameters (e.g. PSA, ng/ml) on the respective axis and draw a line straight up to the point axis. Sum the points for each of the predictors and locate the final sum on the total point axis. Draw a line straight down to find the patient's probability of having LNI (pN+).
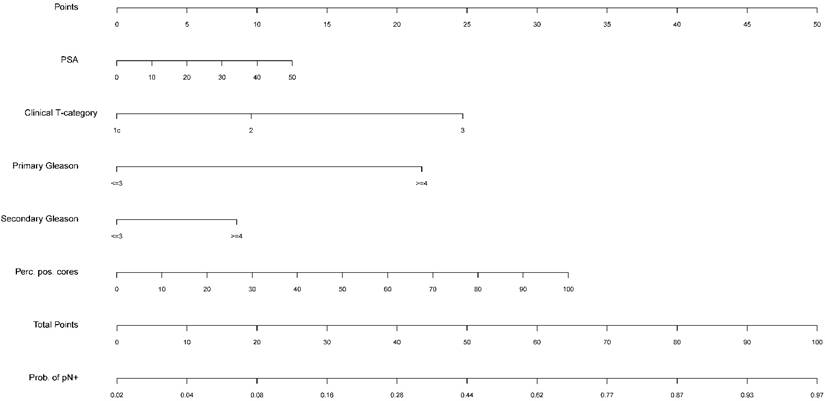
In the multivariate logistic regression analysis, all variables (preoperative PSA level, clinical T-category, primary and secondary biopsy Gleason grade, and the percentage of positive cores) were significantly associated (P<0.001) with LNI. The multivariate predictive accuracy (AUC) was 83.5% under consideration of all aforementioned predictors. Univariate analysis also showed a significant (P<0.001) association between each predictor and LNI. In the univariate predictive accuracy analysis, the percentage of positive cores was the most accurate predictor of LNI (77.0%), followed by PSA value (71.1%), clinical T-category (69.9%), and primary and secondary biopsy Gleason grade (66.6%; 61.3%). The results of the multivariate and univariate logistic regression analyses are detailed in Table 2.
Figure 1 illustrates the nomogram tool in a graphical form, generated by the multivariate analysis.
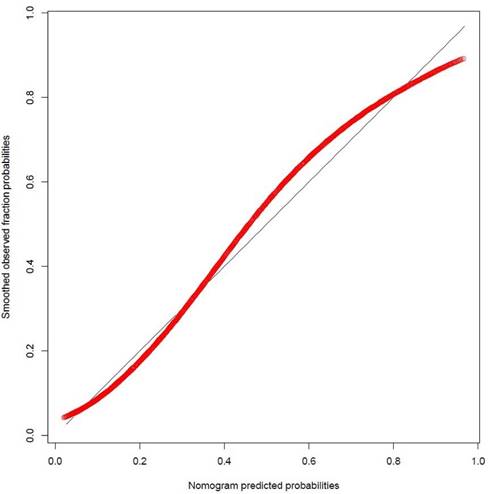
The calibration plot of predicted probabilities against observed LNI rates showed a high level of consistency between predicted and actual probabilities. The nomogram slightly underestimated the actual LNI risk when the predicted probability was between 40% and 80% (see Figure 2).
Table 3 presents the predictive accuracy and error associated with the updated sentinel nomogram when it calculates that a patient has a low risk of LNI (from 1% to 10%). Nomogram-derived predicted probabilities of LNI are categorized into strata.
Results of multivariate and univariate logistic regression analyses predicting lymph node invasion based on preoperative PSA, clinical T-category, biopsy Gleason grade, and percentage of positive cores.
| Predictors | Univariate model | Multivariate model | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Predictive accuracy, AUC | Odds ratio (95% CI) | p value | |
| Preoperative PSA, ng/ml | 1.04(1.03-1.06) | <0.001 | 71.1% | 1.02(1.01-1.03) | <0.001 |
| Clinical T-category | - | <0.001 | - | <0.001 | |
| T2 vs. T1 | 4.23(3.25-5.54) | <0.001 | 69.9% | 2.00(1.47-2.71) | <0.001 |
| T3 vs. T1 | 45.06(21.23-107.44) | <0.001 | 5.91(2.45-15.66) | <0.001 | |
| Primary Gleason grad | |||||
| ≥4 vs. ≤3 | 7.59(5.76-10.03) | <0.001 | 66.6% | 4.79(3.47-6.62) | <0.001 |
| Secondary Gleason grad | |||||
| ≥4 vs. ≤3 | 2.52(1.99-3.19) | <0.001 | 61.3% | 1.85(1.38-2.48) | <0.001 |
| % of positive biopsy cores | 1.04(1.03-1.04) | <0.001 | 77.0% | 1.02(1.02-1.03) | <0.001 |
| Predictive accuracy, AUC | 83.5% | ||||
PSA= prostate-specific antigen; AUC= area under the receiver operator characteristic curve.
The number of patients actually having negative LNs and those with positive nodes as well as sensitivity, specificity, and negative predictive values are depicted for each stratum. Accordingly, using a nomogram cutoff of 5%, 510 of 1.870 patients (27.3%) would be spared a sPLND. On the other hand, avoidance of sPLND in those 510 cases would have resulted in missing LNI in 17 patients or in 4.8% of all patients with histologically confirmed LNI.
Discussion
In 2015, we reported the first sPLND-based nomogram, including the preoperative PSA, clinical category, and the Gleason sum as predictors for LNI [24]. Until now, it was the only available nomogram predicting LNI in prostate cancer patients based on sPLND. With an AUC of 82%, the first sentinel nomogram presents a comparably accurate model for predicting LNI in prostate cancer patients. Nomograms based on lPLND or ePLND series that use the same preoperative parameters to predict LNI provided reliability between 76% and 86% [4, 5, 23]. Table 4 shows the comparison of the nomograms in detail.
Nomogram calibration plot. The red line indicates actual nomogram performance. The black line indicates the location of the ideal nomogram in which predicted and actual probabilities are identical.
Systematic analysis of thresholds used to discriminate between patients with or without histologically confirmed lymph node invasion, in 1,870 patients treated with radical retropubic prostatectomy and radioisotope guided sentinel lymphadenectomy between 2006 and 2013, at a single institution.
| Nomogram-calculatedprobabilityof LNI (threshold, %) | Patients in whomsPLND is notrecommandedaccording to the threshold(below threshold)* | PatientsbelowthresholdwithouthistologicalLNI* | PatientsbelowthresholdwithhistologicalLNI* | Patients in whomsPLND isrecommandedaccording to the threshold(above threshold)* | PatientsabovethresholdwithouthistologicalLNI* | PatientsabovethresholdwithhistologicalLNI* | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| ≥ 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1870 (100.0) | 1518 (100.0) | 352 (100.0) | 18.8 | 100.0 |
| ≥ 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1870 (100.0) | 1518 (100.0) | 352 (100.0) | 18.8 | 100.0 |
| ≥ 3 | 141 (7.5) | 138 (9.0) | 3 (0.9) | 1729 (92.5) | 1380 (90.9) | 349 (99.2) | 20.2 | 97.9 |
| ≥ 4 | 405 (21.7) | 393 (25.9) | 12 (3.4) | 1465 (78.3) | 1125 (74.1) | 340 (96.6) | 23.2 | 97.0 |
| ≥ 5 | 510 (27.3) | 493 (36.2) | 17 (4.8) | 1360 (72.7) | 1025 (67.5) | 335 (95.2) | 24.6 | 96.7 |
| ≥ 6 | 622 (33.3) | 599 (32.5) | 23 (6.5) | 1248 (66.7) | 919 (60.5) | 329 (93.5) | 26.4 | 96.3 |
| ≥ 7 | 745 (39.8) | 714 (47.0) | 31 (8.8) | 1125 (60.2) | 804 (53.0) | 321 (91.2) | 28.5 | 95.8 |
| ≥ 8 | 831 (44.4) | 789 (52.0) | 42 (11.9) | 1039 (55.6) | 729 (48.0) | 310 (88.1) | 29.8 | 95.0 |
| ≥ 9 | 893 (47.8) | 846 (55.7) | 47 (13.4) | 977 (52.3) | 672 (44.3) | 305 (86.7) | 31.2 | 94.7 |
| ≥ 10 | 954 (51.0) | 902 (59.4) | 52 (14.8) | 916 (49.0) | 616 (40.6) | 300 (85.2) | 32.8 | 94.6 |
* Data are given as number (%). PPV = Positive predictive value; NPV = negative predictive value; LNI = lymph node involvement, sPLND = sentinel-guided pelvic lymph node dissection.
Various models predicting lymph node invasion for patients undergoing pelvic lymphadenectomy at radical prostatectomy based on clinical tumour characteristics (e.g., PSA, clinical T-category, biopsy Gleason score).
| Reference | Number of patients | PLND technique | Prevalence of LNI | AUC |
|---|---|---|---|---|
| Eifler et al. [22] | 5,629 | lPLND | 1.0% | n.a. |
| Cagiannos et al. [23] | 5,510 | lPLND | 3.7% | 76.0% |
| Briganti et al. [21] | 781 | ePLND | 9.1% | 78.6% |
| Briganti et al. [4] | 588 | ePLND | 8.3% | 87.6% |
| Godoy et al. [5] | 4,176 | ePLND | 5.2% | 86.2% |
| Winter et al. [24] | 1,296 | sPLND | 17.8% | 82.0% |
| Winter et. al. [updated sentinel nomogram] | 1,870 | sPLND | 18.8% | 83.8% |
PLND= pelvic lymph node dissection; lPLND= limited pelvic lymph node dissection; ePLND= extended pelvic lymph node dissection; sPLND= sentinel-guided pelvic lymph node dissection; LNI= lymph node invasion; AUC= area under the receiver operator characteristic curve
Our updated sentinel nomogram added the percentage of positive cores as well as the primary and secondary Gleason grade as predictors, demonstrating an improved calibration and an even higher bootstrap-corrected predictive accuracy (AUC, 83.5%) than the first. These parameters are also included in the updated Briganti nomogram [4]. The Briganti nomogram and the updated Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram are some of the most commonly used LNI nomograms [4, 5]. Unlike most of the previous predictive tools, they were based on the results of ePLND. Both nomograms were included in the recommendation of the EAU guidelines. A calculated probability of LNI <5% had been determined as a threshold for not performing ePLND [3]. Based on the data of the Briganti nomogram, this cutoff would allow the avoidance of unnecessary PLND in about 65% of patients at the cost of missing 12% of patients with LNI [4]. In the updated sentinel nomogram, using a nomogram cutoff of 5%, approximately one-third of patients (27.3%) would be spared a sPLND. However, avoidance of sPLND in those cases would have resulted in missing LNI in only 4.8% of all patients with LNI. In this context, the low morbidity of sPLNDs should be weighted [8] and that patients with minimal LNI who particularly appear to benefit from removal of LN metastases should be noted [27].
As shown in Table 4, despite the removal of fewer LNs in the updated sentinel nomogram (median, 10) than in the Briganti (median, 16.5) and the MSKCC nomogram (median, 11), the proportion of LN-positive patients was significantly higher in the updated sentinel nomogram cohort than in that of these two ePLND series or other PLND series. Accordingly, it was possible to demonstrate that for the sPLND, the LNI rate was higher in a sentinel cohort than was expected from the Briganti nomogram [28]. One reason for this might be the advantage of targeted dissection of tumor-associated LNs or tailoring the extent of PLND to individual lymphatic drainage. Joniau et al. showed that 21% of SLNs could be found in the presacral and perirectal regions, and concluded that 8% of LN-positive patients would have been missed if a standard ePLND had been performed [29]. Results of a current systematic review indicate that for one in 20 patients who had undergone ePLND, metastatic LNs would have been left behind without performing sPLND [19]. Another explanation for the higher rate of positive LNs in patients undergoing sPLND might be a more detailed histopathologic evaluation of all removed LNs. In clinical routine or conventional PLND, the LNs are often provided to the pathologists in packages. The targeted sentinel procedure has the advantage that relatively few LNs are removed and can be more intensively examined. For example, more small or micrometastases can be detected by increasing the number of sections. This may contribute to the elevated detection rate of positive nodes in the sentinel guided procedure, too. Principally, this also offers the opportunity to use molecular procedures for LN evaluation, which can further increase the sensitivity in the detection of LN metastases in prostate cancer patients [30]. Taking into account all these aspects, the updated sentinel nomogram provides prostate cancer patients and surgeons a crucial basis to decide for or against a (s)PLND.
This study represents the first sPLND-based nomogram considering the percentage of positive cores as a main predictor for LNI. The strength of this study encompasses the high number of patients included, improved calibration, and the resulting high reliability for the prediction of LNI in prostate cancer patients undergoing sPLND.
The limitations of this study include those inherent to a retrospective analysis of prospectively collected data and the selection bias associated with a surgical series from a single institution. However, the staging accuracy and the rates of LNI patients detected by sPLNDs in the monitored sample compare well with data from other radio-guided sPLND-experienced centers [18]. Furthermore, prior to its application in everyday clinical practice, the updated sentinel nomogram should be externally validated in real practice [31, 32].
Conclusions
For radioisotope-guided sPLNDs, one can demonstrate a high staging accuracy accompanied by even low morbidity. We have updated the only available sentinel nomogram predicting the probability of LNI in patients undergoing a sPLND at radical prostatectomy. Comparable with ePLND models, the percentage of positive cores represents the leading predictor of LNI. Considering the percentage of positive cores and primary and secondary biopsy Gleason grade as additional predictors, the updated sentinel nomogram demonstrates a higher degree of accuracy with improved calibration. However, an external validation of the updated sentinel nomogram and an examination of its ability to predict LNI in patients undergoing ePLND is still pending.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Maurer T, Gschwend JE, Rauscher I. et al. Diagnostic efficacy of 68Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436-1443
2. Heidenreich A, Ohlmann CH, Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. 2007;52:29-37
3. Mottet N, Bellmunt J, Briers E. et al. Guidelines on Prostate Cancer 2016. Available from: http://uroweb.org/guideline/prostate-cancer/. Accessed January. 2017
4. Briganti A, Larcher A, Abdollah F. et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480-7
5. Godoy G, Chong KT, Cronin A. et al. Extent of pelvic lymph node dissection and the impact of standard template dissection on nomogram prediction of lymph node involvement. Eur Urol. 2011;60:195-201
6. Briganti A, Chun FK, Salonia A. et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. 2006;50:1006-13
7. Orvieto MA, Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel VR. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int. 2011;108:1185-90
8. Winter A, Vogt C, Weckermann D, Wawroschek F. Complications of pelvic lymphadenectomy in clinically localised prostate cancer: different techniques in comparison and dependency on the number of removed lymph nodes. Aktuelle Urol. 2011;42:179-83
9. Mattei A, Fuechsel FG, Bhatta Dhar N. et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008;53:118-25
10. Wawroschek F, Wagner T, Hamm M. et al. The influence of serial sections, immunohistochemistry, and extension of pelvic lymph node dissection on the lymph node status in clinically localized prostate cancer. Eur Urol. 2003;43:132-6
11. Wawroschek F, Vogt H, Weckermann D, Wagner T, Harzmann R. The sentinel lymph node concept in prostate cancer-first results of gamma probe-guided sentinel lymph node identification. Eur Urol. 1999;36:595-600
12. Krag DN, Anderson SJ, Julian TB. et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-33
13. Morton DL, Thompson JF, Cochran AJ. et al. MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609
14. KleinJan GH, van den Berg NS, de Jong J. et al. Multimodal hybrid imaging agents for sentinel node mapping as a means to (re)connect nuclear medicine to advances made in robot-assisted surgery. Eur J Nucl Med Mol Imaging. 2016;43:1278-87
15. Acar C, KleinJan GH, van den Berg NS. et al. Advances in sentinel node dissection in prostate cancer from a technical perspective. Int J Urol. 2015;22:898-909
16. Winter A, Woenkhaus J, Wawroschek F. A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide nanoparticles and a handheld magnetometer: the initial clinical experience. Ann Surg Oncol. 2014;21:4390-6
17. Van der Poel HG, Wit EM, Acar C. et al. Sentinel node biopsy for prostate cancer: report from a consensus panel meeting. BJU Int. 2017 doi: 10.1111/bju.13810. [Epub ahead of print]
18. Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J.Validation of sentinel lymph node dissection in prostate cancer. experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36:1377-82
19. Wit EM, Acar C, Grivas N. et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur Urol. 2017;71:596-605
20. Sadeghi R, Tabasi KT, Bazaz SM. et al. Sentinel node mapping in the prostate cancer. Meta-analysis. Nuklearmedizin. 2011;50:107-15
21. Briganti A, Chun FK, Salonia A. et al. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. 2006;98:788-93
22. Eifler JB, Feng Z, Lin BM. et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111:22-9
23. Cagiannos I, Karakiewicz P, Eastham JA. et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798-803
24. Winter A, Kneib T, Rohde M, Henke RP, Wawroschek F. First Nomogram Predicting the Probability of Lymph Node Involvement in Prostate Cancer Patients Undergoing Radioisotope Guided Sentinel Lymph Node Dissection. Urol Int. 2015;95:422-8
25. Heidenreich A, Pfister D, Thüer D, Brehmer D. Percentage of positive biopsies predicts lymph node involvement in men with low-risk prostate cancer undergoing radical prostatectomy and extended pelvic lymphadenectomy. BJU Int. 2011;107:220-5
26. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
27. Seiler R, Studer UE, Tschan K, Bader P, Burkhard FC. Removal of limited nodal disease in patients undergoing radical prostatectomy: long-term results confirm a chance for cure. J Urol. 2014;191:1280-5
28. Winter A, Kneib T, Henke RP, Wawroschek F. Sentinel lymph node dissection in more than 1200 prostate cancer cases: rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol. 2014;21:58-63
29. Joniau S, Van den Bergh L, Lerut E. et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013;63:450-8
30. Heck MM, Retz M, Bandur M. et al. Topography of lymph node metastases in prostate cancer patients undergoing radical prostatectomy and extended lymphadenectomy: results of a combined molecular and histopathologic mapping study. Eur Urol. 2014;66:222-9
31. Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14:4400-7
32. Dell'Oglio P, Abdollah F, Suardi N. et al. External validation of the European association of urology recommendations for pelvic lymph node dissection in patients treated with robot-assisted radical prostatectomy. J Endourol. 2014;28:416-23
Author contact
![]() Corresponding author: Alexander Winter, University Hospital for Urology, Klinikum Oldenburg, School of Medicine and Health Sciences, Carl von Ossietzky University Oldenburg, Rahel-Straus-Str. 10, D-26133 Oldenburg, Germany; Email: winter.alexanderde. Telephone 0049 (0)441 403 2302, fax 0049 (0)441 403 2303
Corresponding author: Alexander Winter, University Hospital for Urology, Klinikum Oldenburg, School of Medicine and Health Sciences, Carl von Ossietzky University Oldenburg, Rahel-Straus-Str. 10, D-26133 Oldenburg, Germany; Email: winter.alexanderde. Telephone 0049 (0)441 403 2302, fax 0049 (0)441 403 2303

 Global reach, higher impact
Global reach, higher impact