3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(14):2756-2764. doi:10.7150/jca.19545 This issue Cite
Research Paper
High Immunoreactivity of DUOX2 Is Associated With Poor Response to Preoperative Chemoradiation Therapy and Worse Prognosis in Rectal Cancers
1. Division of Clinical Pathology, Department of Pathology, E-DA Hospital, I-Shou University, Kaohsiung, Taiwan;
2. School of Medicine, I-Shou University, Kaohsiung, Taiwan;
3. Department of Medical Image, Chi Mei Medical Center, Tainan, Taiwan;
4. Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan;
5. Department of Leisure, Recreation, and Tourism Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan;
6. Department of Pharmacy, Chia Nan University of Pharmacy and Science, Tainan, Taiwan;
7. Division of General Surgery, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan;
8. Department of Radiation Oncology, Chi-Mei Medical Center, Tainan, Taiwan;
9. Division of Anatomical Pathology, Department of Pathology, E-DA Hospital, I-Shou University, Kaohsiung, Taiwan;
10. Department of Health & Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan.
* SC Lin and IW Chang contributed equally to this work
Received 2017-2-7; Accepted 2017-6-25; Published 2017-8-23
Abstract
Purpose: Colorectal cancer is the third most common cancer and also the fourth most common cause of cancer mortality worldwide. For rectal cancer, neoadjuvant concurrent chemoradiotherapy (CCRT) followed by radical proctectomy is gold standard treatment for patients with stage II/III rectal cancer. By data mining a documented database of rectal cancer transcriptome (GSE35452) from Gene Expression Omnibus, National Center of Biotechnology Information, we recognized that DUOX2 was the most significantly up-regulated transcript among those related to cytokine and chemokine mediated signaling pathway (GO:0019221). Hence, the aim of this study was to assess the DUOX2 expression level and its clinicopathological correlation and prognostic significance in patients of rectal cancer.
Materials and Methods: DUOX2 immunostain was performed in 172 rectal adenocarcinomas treated with preoperative CCRT followed by radical proctectomy, which were divided into high- and low-expression subgroups. Furthermore, statistical analyses were examined to correlate the relationship between DUOX2 immunoreactivity and important clinical and pathological characteristics, as well as three survival indices: disease-specific survival (DSS), local recurrence-free survival (LRFS) and metastasis-free survival (MeFS).
Results: DUOX2 overexpression was linked to post-CCRT tumor advancement, pre- and post-CCRT nodal metastasis and poor response to CCRT (all P ≤ 0.021). Furthermore, DUOX2 high expression was significantly associated with inferior DSS, LRFS and MeFS in univariate analysis (P ≤ 0.0097) and also served as an independent prognosticator indicating shorter DSS and LRFS interval in multivariate analysis (hazard ratio (HR) = 3.413, 95% confidence interval (CI): 1.349-8.633; HR = 4.533, 95% CI: 1.499-13.708, respectively).
Conclusion: DUOX2 may play a pivotal role in carcinogenesis, tumor progression and response to neoadjuvant CCRT in rectal cancers, and serve as a novel prognostic biomarker. Additional researches to clarify the molecular and biochemical pathways are essential for developing promising DUOX2-targeted therapies for patients with rectal cancers.
Keywords: CCRT, chemoradiotherapy, dual oxidase 2, DUOX2, rectal cancer.
Introduction
Colorectal cancer is the third leading cause of cancer-related deaths in Taiwan and in the United States [1], and the incidence is stepwise increasing every year worldwide [2]. Both preoperative concurrent chemoradiotherapy (CCRT) and adjuvant CCRT have been advocated by many institutions in locally advanced rectal cancer [3]. In the United States, preoperative CCRT of rectal cancer has been widely accepted. Preoperative CCRT has superior local control rate compared with preoperative radiotherapy alone for stage II and III resectable rectal cancer [4]. Moreover, in the randomized study of Sauer et al., neoadjuvant CCRT has a local control rate which is superior to adjuvant CCRT [5]. Therefore, now the neoadjuvant CCRT is the mainstream treatment of rectal cancer in Taiwan. Furthermore, the varied response of individual patients after neoadjuvant CCRT and surgery encourage us to identify the useful marker.
Cancer is one of diseases due to dysfunction of cytokine and chemokine mediated signaling pathway [6]. However, the genes related to cytokine and chemokine mediated signaling pathway have not been thoroughly evaluated in rectal cancer. Therefore, we analyzed a public transcriptomic dataset of rectal cancer (GSE35452) from Gene Expression Omnibus, National Center for Biotechnology Information (GEO, NCBI, Bethesda, MD, USA) and identified DUOX2 as the most significantly up-regulated gene associated with cytokine and chemokine mediated signaling pathway (GO:0019221).
DUOX2 gene encodes dual oxidase 2 (DUOX2) protein, which belongs to NOX/DUOX family or NOX family of NADPH oxidases. In the family of NOX/DUOX, there are seven members: NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase 1 (DUOX1), and dual oxidase 2 (DUOX2). All of them contain homologs to the catalytic element of phagocytic NADPH-oxidase, gp91phox [7]. DUOX1 and DUOX2 genes are located on the human chromosome 15 and their encoding proteins, DUOX1 and DUOX2, are two closely-related isoforms and originally found in the thyroid gland [8-10]. DUOX1 and DUOX2 are associated with the production of thyroid hormone. DUOX-derived H2O2 is important for the biosythesis of thyroid hormone and the host antimicrobial defense of the major respiratory tract, oral cavity and gastrointestinal tract [11, 12]. Thus, the name of dual oxidase is due to containing both NADPH-oxidase (gp91phox) domain and peroxidase homology domain. The impact of dual oxidase 2 in carcinogenesis is recently investigated, including colon cancer [13-18]. Nevertheless, no relationship between DUOX2 expression and clinical outcome is reported in patients with rectal cancer. Thus we retrospectively studied the association between the expression of DUOX2 protein by immunohistochemistry and significant clinical and pathological parameters, as well as different survival indices in patients with rectal cancer scheduled to receive preoperative CCRT.
Materials and Methods
Data mining of transcriptomic dataset of rectal cancers to identify the most up-regulated gene
A public transcriptomic database (GSE35452), comprising 46 patients of rectal cancer doctored with preoperative chemoradiation therapy from Gene Expression Omnibus, National Center for Biotechnology Information (GEO, NCBI, Bethesda, MD, USA), was selected for research. The tumors were subdivided into “responder” and “non-responder” according to the response to neoadjuvant CCRT. We downloaded the raw .cel file and performed comparative analysis without filtering or preselection by the software--Nexus Expression 3 (BioDiscovery, El Segundo, CA, USA). Under supervision, the statistical significance of each transcript by comparing responder and non-responder was examined, focusing on the genes related to cytokine and chemokine mediated signaling pathway (GO:0019221). The transcripts with expression fold change > ± 0.1 log2 ratio and P-value < 0.01 were selected for additional evaluation.
Study cohort of patients and specimens
The Institutional Review Board of Chi-Mei Medical Center approved this study. Totally 172 patients with primary rectal adenocarcinoma were enrolled from Chi-Mei Medical Center between 1998 and 2004. All of the selected patients received preoperative chemoradiation therapy followed by radical proctectomy. The primary clinical stage was determined by endoscopic ultrasound (EUS) and abdominal and pelvic computed tomography (CT). Patients with distant metastasis at initial diagnosis (cM1), screened by chest X-ray and abdominal and pelvic CT, were excluded. The clinical information was retrieved from the archives of medical records. The details of patient selection and the protocol of treatment were the same as preceding description [19].
Histopathological evaluation, immunohistochemical study and assessment of immunereactivity
Post-treatment stage was based on pathological examination of radical proctectomy specimen according to 7th edition of American Joint Committee on Cancer (AJCC) cancer staging system [20]. The grading system of tumor regression after preoperative chemoradiotherapy was evaluated in accordance with the description of Dworak et al. [21]. Dworak's tumor regression grade (TRG) is a five-tier system: grade 0 indicates no regression; grade 1 indicates dominant tumor mass with obvious fibrosis; grade 2 indicates dominantly fibrotic changes with few tumor cells or groups; grade 3 indicates very few tumor cells; grade 4 indicates no tumor cells. The method of immunohistochemistry is the same as that we reported previously [22-25]. Briefly speaking, paraffin-embedded tumor biopsy tissue specimens before neoadjuvant CCRT were administered the routine procedure of deparaffinization, rehydration, and epitope retrieval. Subsequently, the tissue sections were proceeded to incubation with primary antibody against DUOX2 (1:200, polyclonal, Abcam, Cambridge, United Kingdom) for one hour. Normal thyroid tissues with and without incubation of DUOX2 antibody were run parallel as positive and negative control, respectively. We assessed the expression of DUOX2 protein by combination of the intensity and percentage of immunoreactivity in the cytoplasm of tumor cells to produce an H-score. The equation is shown below: H-score = ΣPi(i+1), in which Pi symbolizes the percentage of stained tumor cells (0%-100%) and i symbolizes the intensity of immunostain (0-3+).
Statistical tests
IBM SPSS Statistics software, Version 22.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. After dividing the study cohort into high- and low-expression of DUOX2 by the median H-score of DUOX2 immunostaining, Pearson's Chi-square test was used for the relationship between DUOX2 immunoreactivity and categorical important clinical and pathological parameters. Three prognostic indices—disease-specific survival (DSS), local recurrent-free survival (LRFS) and metastasis-free survival (MeFS) were calculated from the days of surgery to those of events happened. Kaplan-Meier survival curves compared with log-rank test was used for univariate survival analysis. Parameters with statistical significance in univariate analyses were enrolled in multivariate ones, for which, Cox model was used. For all analyses, only P value < 0.05 was considered as statistically significant under two-tailed tests.
Results
DUOX2 identified as the most significantly up-regulated gene among those belonging to cytokine and chemokine mediated signaling pathway (GO:0019221)
In the public transcriptomic dataset of rectal cancer (GSE35452) from GEO, NCBI, 52.2% patients (n=24) revealed response to preoperative chemoradiotherapy (referred as responder), while 47.8% patients (n=22) showed resistance to preoperative chemoradiotherapy (referred as non-responder). Five probes covering four transcripts associated with cytokine and chemokine mediated signaling pathway (GO:0019221) were found. Of these, three probes covering DUOX2 and DUOX1 transcripts and two probes covering TGM2 and EREG transcripts exhibited significant up- and down-regulation in non-responders than in responders, respectively (Fig. 1). Among the up-regulated genes, DUOX2 was the most significantly up-regulated one, where the log2 ratio of DUOX2 mRNA by comparison between non-responders and responders was 1.1477 (P = 0.0001, Table 1).
Analysis of gene expression in rectal cancers with preoperative concurrent chemoradiotherapy by using a published transcriptome dataset (GSE35452). Conducting a clustering analysis of genes by focusing on cytokine and chemokine mediated signaling pathway (GO:0019221) revealed that DUOX2 is the most significantly up-regulated gene in non-responder comparing with responder. Tumors classified as responder (yellow) or non-responder (blue) were illustrated at the top of the heat map, and the up-regulation and down-regulation of gene expression were represented as a continuum of brightness of red or green, respectively. Tumors with unchanged transcriptional level were in black.
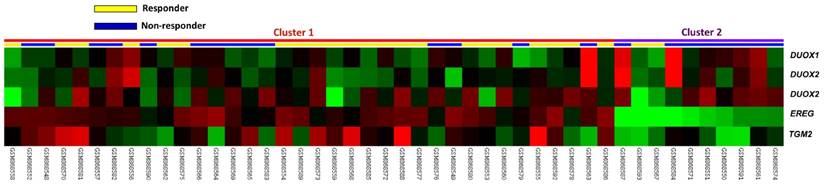
Significantly deregulated genes associated with cytokine and chemokine mediated signaling pathway (GO:0019221) based on CCRT response in rectal cancer
| Probe | Comparison log2 ratio | Comparison P-value | Gene Symbol | Gene Name | Biological Process | Molecular Function |
|---|---|---|---|---|---|---|
| 219727_at | 1.1477 | 0.0001 | DUOX2 | dual oxidase 2 | cuticle development, cytokine and chemokine mediated signaling pathway, electron transport, hormone biosynthetic process, hydrogen peroxide catabolic process, response to cAMP, response to virus | FAD binding, NAD(P)H oxidase activity, calcium ion binding, iron ion binding, metal ion binding, oxidoreductase activity, peroxidase activity |
| 215800_at | 0.2627 | <0.0001 | DUOX1 | dual oxidase 1 | cuticle development, cytokine and chemokine mediated signaling pathway, electron transport, hormone biosynthetic process, hydrogen peroxide biosynthetic process, hydrogen peroxide catabolic process, response to cAMP, superoxide release | FAD binding, NAD(P)H oxidase activity, NADP binding, calcium ion binding, iron ion binding, metal ion binding, oxidoreductase activity, peroxidase activity |
| 219597_s_at | 0.204 | 0.0033 | DUOX1 | dual oxidase 1 | cuticle development, cytokine and chemokine mediated signaling pathway, electron transport, hormone biosynthetic process, hydrogen peroxide biosynthetic process, hydrogen peroxide catabolic process, response to cAMP, superoxide release | FAD binding, NAD(P)H oxidase activity, NADP binding, calcium ion binding, iron ion binding, metal ion binding, oxidoreductase activity, peroxidase activity |
| 211003_x_at | -0.5527 | 0.0004 | TGM2 | transglutaminase 2 (C polypeptide; protein-glutamine-gamma-glutamyltransferase) | G-protein coupled receptor protein signaling pathway, anti-apoptosis, cAMP-mediated signaling, cytokine and chemokine mediated signaling pathway, isopeptide cross-linking via N6-(L-isoglutamyl)-L-lysine, peptide cross-linking, positive regulation of cell adhesion, programmed cell death | ATP binding, GTP binding, GTPase activity, acyltransferase activity, calcium ion binding, metal ion binding, protein-glutamine gamma-glutamyltransferase activity, transferase activity |
| 205767_at | -1.339 | 0.0001 | EREG | epiregulin | anatomical structure morphogenesis, angiogenesis, cell differentiation, cell proliferation, cell-cell signaling, cytokine and chemokine mediated signaling pathway, epidermal growth factor receptor signaling pathway, female meiosis, keratinocyte differentiation, keratinocyte proliferation, luteinizing hormone signaling pathway, mRNA transcription, multicellular organismal development, negative regulation of cell proliferation, negative regulation of epithelial cell proliferation, negative regulation of smooth muscle cell differentiation, negative regulation of transcription, oocyte maturation, organ morphogenesis, ovarian cumulus expansion, ovulation, positive regulation of DNA replication, positive regulation of cell proliferation, positive regulation of cytokine biosynthetic process, positive regulation of cytokine production, positive regulation of epidermal growth factor receptor activity, positive regulation of fibroblast proliferation, positive regulation of innate immune response, positive regulation of interleukin-6 biosynthetic process, positive regulation of mitosis, positive regulation of phosphorylation, positive regulation of protein kinase activity, positive regulation of smooth muscle cell proliferation, primary follicle stage; oogenesis, regulation of progression through cell cycle, wound healing | epidermal growth factor receptor binding, growth factor activity, protein binding, protein heterodimerization activity |
Clinicopathological characteristics of patients with rectal adenocarcinomas
As shown in Table 2, most of our cases of rectal adenocarcinoma were male (62.8%, n = 108) and less than 70 years old (61.6%, n = 106). The invasive depth of 47.1% cancers (n = 81) before neoadjuvant CCRT was limited to muscularis propria (cT1-2), and 52.9% (n = 91) was beyond the muscularis propria (cT3-4). 27.3% patients (n = 47) had nodal metastasis before chemoradiation treatment (cN1-2), and 72.7% patients (n = 125) didn't (cN0). The invasive depth of half tumors (n = 86) after neoadjuvant CCRT was limited to muscularis propria or showed complete remission (ypT0-2), and the other half (n = 86) was beyond the muscularis propria (ypT3-4). 28.5% patients (n = 49) had nodal metastasis after neoadjuvant CCRT, and 71.5% patients (n = 123) didn't. Lymphovascular and perineural invasion were observed in 8.7% (n = 15) and 2.9% (n = 5) tumors, respectively. The tumor response to neoadjuvant chemoradiotherapy varied from grade 0-1 (n = 37, 21.5%), grade 2-3 (n = 118, 68.6%) and grade 4 (n = 17, 9.9%).
Immunohistochemical expression of DUOX2 in rectal adenocarcinomas
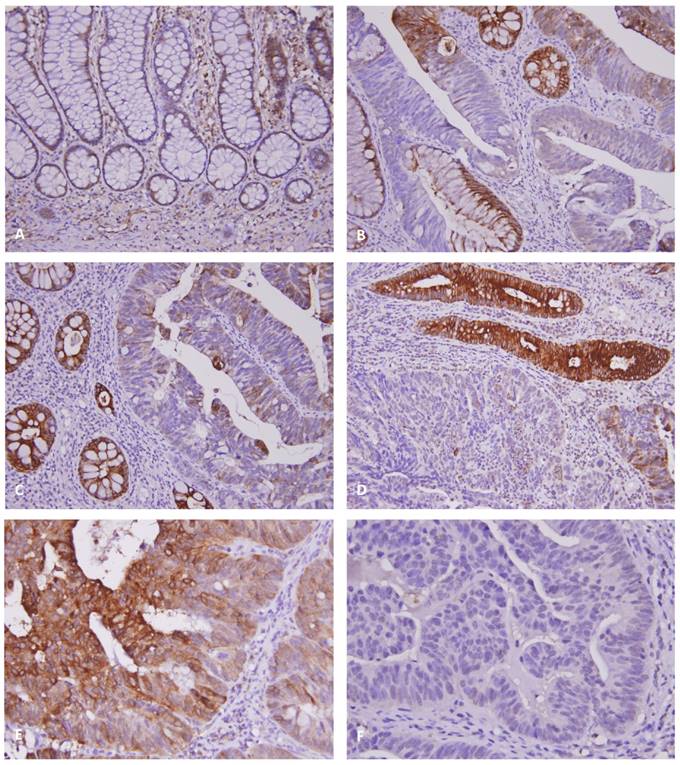
The representative result of DUOX2 immunohistochemical stain was illustrated in Fig. 2. In normal colonic mucosa, low level of DUOX2 immunoreactivity was noted (Fig. 2A). The adenocarcinoma in situ (AIS, Fig. 2B) or invasive adenocarcinoma (Fig. 2C-D) usually exhibited loss of expression or down-regulation of DUOX2, while increase of DUOX2 expression was found in adjacent adenomatous lesion (Fig. 2B-D). The H-score of DUOX2 immunoreactivity in adenoma was significantly higher than those in normal colonic mucosa, AIS and adenocarcinoma (all P < 0.01). However, tumors with high expression of DUOX2 (Fig. 2E) still showed resistance to neoadjuvant CCRT compared with low expression subgroup (Fig. 2F).
Association between DUOX2 immunoreactivity and clinicopathological variables
After dichotomizing the study cohort into DUOX2 high- and low-expression groups with cutoff point of median H-score, we applied Pearson's Chi-square test to examine the relationship between DUOX2 immunoreactivity and miscellaneous clinicopathological parameters. As demonstrated in Table 2, DUOX2 overexpression was significantly associated with more advanced post-CCRT ypT stage (P = 0.015), pre- and post-CCRT lymph node metastasis (P < 0.001 for both) and lower tumor regression grade after neoadjuvant chemoradiation therapy (P = 0.021).
DUOX2 immunostain on representative sections revealed (A) low level of immunoexpression in normal colonic mucosa, (B) loss of expression in adenocarcinoma in situ (note overexpression in adjacent adenomatous glands), and (C & D) relatively low immunoreactivity in the invasive cancers compared with high expression in adjacent adenomatous lesions. (E) Tumors with high DUOX2 immunoreactivity showed low tumor regression grade after preoperative chemoradiotherapy, while (F) tumors with low DUOX2 immunoreactivity showed high tumor regression grade.
Survival analyses for patients with rectal adenocarcinomas
In univariate analyses (Table 3), more advanced post-CCRT tumor invasiveness and lower tumor regression grade were significantly correlated with lower DSS, LRFS and MeFS rates (P ≤ 0.0090 for all). Higher pre-CCRT serum CEA level (>5 ng/ml) and presence of lymphovascular invasion were negatively linked to DSS and LRFS to statistical significance (P ≤ 0.0216 for all). Positive pre-CCRT nodal metastasis was significantly associated with adverse LRFS only (P = 0.0070).
In multivariate analyses (Table 4), tumor regression grade was an independent prognosticator for DSS (hazard ratio (HR) = 2.283, 95% confidence interval (CI): 1.101-4.739), LRFS (HR = 2.653, 95% CI: 1.193-5.882) and MeFS (HR = 2.331, 95% CI: 1.175-4.695). Lymphovascular invasion was an independent prognostic factor for DSS and LRFS only (HR = 2.892, 95% CI: 1.037-8.062; HR = 3.897, 95% CI: 1.345-11.292, respectively).
Prognostic significance of high DUOX2 immunoreactivity in patients with rectal adenocarcinomas
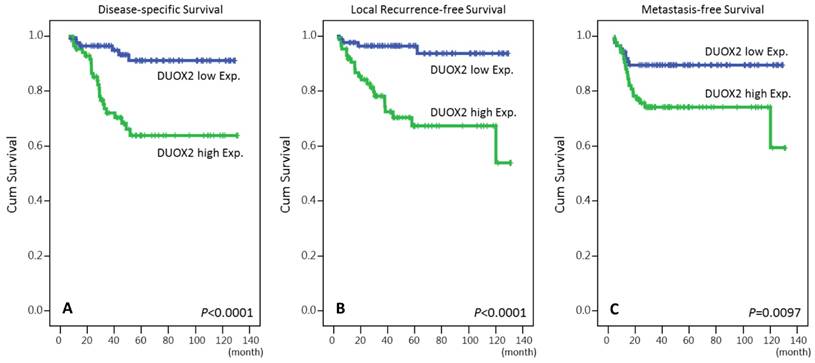
Overexpression of DUOX2 was negatively correlated with DSS, LRFS and MeFS in univariate analyses (P ≤ .0097 for all, Table 3 and Fig.3). High immunoexpression of DUOX2 still independently predicted shorter DSS and LRFS intervals in multivariate analyses (HR = 3.413, 95% CI: 1.349-8.633; HR = 4.533, 95% CI: 1.499-13.708, respectively) (Table 4).
Relationships between DUOX2 expression and clinicopathological factors in rectal cancer patients receiving preoperative CCRT
| Parameter | No. | DUOX2 Expression | P-value | ||
|---|---|---|---|---|---|
| Low Exp | High Exp. | ||||
| Gender | Male | 108 | 48 | 60 | 0.058 |
| Female | 64 | 38 | 26 | ||
| Age | <70 | 106 | 55 | 51 | 0.531 |
| ≥70 | 66 | 31 | 35 | ||
| Pre-CCRT cT stage | cT1-2 | 81 | 41 | 40 | 0.879 |
| cT3-4 | 91 | 45 | 46 | ||
| Pre-CCRT cN stage | cN0 | 125 | 73 | 52 | <0.001* |
| cN1-2 | 47 | 13 | 34 | ||
| Pre-CCRT CEA | ≤5 ng/ml | 114 | 61 | 53 | 0.197 |
| >5 ng/ml | 58 | 25 | 33 | ||
| Post-CCRT pT stage | ypT0-2 | 86 | 51 | 35 | 0.015* |
| ypT3-4 | 86 | 35 | 51 | ||
| Post-CCRT pN stage | ypN0 | 123 | 75 | 48 | <0.001* |
| ypN1-2 | 49 | 11 | 38 | ||
| Lymphovascular invasion | Absent | 157 | 82 | 75 | 0.059 |
| Present | 15 | 4 | 11 | ||
| Perineural invasion | Absent | 167 | 85 | 82 | 0.173 |
| Present | 5 | 1 | 4 | ||
| Tumor regression grade | Grade 0-1 | 37 | 12 | 25 | 0.021* |
| Grade 2-3 | 118 | 62 | 56 | ||
| Grade 4 | 17 | 12 | 5 | ||
*, statistically significant
Univariate log-rank analysis for important clinicopathological variables and DUOX2 expression
| Parameter | No. of case | Disease-specific survival | Local recurrence-free survival | Metastasis-free survival | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of event | P | No. of event | P | No. of event | P | ||||
| Gender | Male | 108 | 20 | 0.9026 | 7 | 0.2250 | 17 | 0.3520 | |
| Female | 64 | 11 | 20 | 14 | |||||
| Age | <70 | 106 | 19 | 0.8540 | 18 | 0.6615 | 20 | 0.7427 | |
| ≥70 | 66 | 12 | 9 | 11 | |||||
| Pre-CCRT cT stage | cT1-2 | 81 | 10 | 0.0776 | 10 | 0.2261 | 11 | 0.1745 | |
| cT3-4 | 91 | 21 | 17 | 20 | |||||
| Pre-CCRT cN stage | cN0 | 125 | 19 | 0.0711 | 15 | 0.0070* | 19 | 0.0973 | |
| cN1-2 | 47 | 21 | 12 | 12 | |||||
| Pre-CCRT CEA | ≤5 ng/ml | 114 | 15 | 0.0216* | 13 | 0.0179* | 17 | 0.1460 | |
| >5 ng/ml | 58 | 16 | 14 | 14 | |||||
| Post-CCRT pT stage | ypT0-2 | 86 | 7 | 0.0006* | 7 | 0.0040* | 8 | 0.0033* | |
| ypT3-4 | 86 | 24 | 20 | 23 | |||||
| Post-CCRT pN stage | ypN0 | 123 | 21 | 0.5998 | 16 | 0.1320 | 20 | 0.4634 | |
| ypN1-2 | 49 | 10 | 11 | 11 | |||||
| Lymphovascular invasion | Absent | 157 | 25 | 0.0184* | 21 | 0.0028* | 27 | 0.4470 | |
| Present | 15 | 6 | 6 | 4 | |||||
| Perineural invasion | Absent | 167 | 29 | 0.2559 | 25 | 0.0940 | 30 | 0.9083 | |
| Present | 5 | 2 | 2 | 1 | |||||
| Tumor regression grade | Grade 0-1 | 37 | 13 | 0.0038* | 10 | 0.0090* | 14 | 0.0006* | |
| Grade 2-3 | 118 | 17 | 17 | 16 | |||||
| Grade 4 | 17 | 1 | 0 | 1 | |||||
| DUOX2 expression | Low Exp. | 86 | 6 | <0.0001* | 4 | <0.0001* | 9 | 0.0097* | |
| High Exp. | 86 | 25 | 23 | 22 | |||||
*, statistically significant
Kaplan-Meier survival curves demonstrated significant prognostic impact of DUOX2 expression on disease-specific survival (P < 0.0001), local recurrence-free survival (P < 0.0001) and metastasis-free survival (P = 0.0097).
Multivariate survival analysis
| Parameter | Disease-specific survival | Local recurrence-free survival | Metastasis-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Tumor regression grade | 2.283 | 1.101-4.739 | 0.027* | 2.653 | 1.193-5.882 | 0.017* | 2.331 | 1.175-4.695 | 0.018* |
| DUOX2 expression | 3.413 | 1.349-8.633 | 0.010* | 4.533 | 1.499-13.708 | 0.007* | 1.900 | 0.851-4.238 | 0.117 |
| Lymphovascular invasion | 2.892 | 1.037-8.062 | 0.042* | 3.897 | 1.345-11.292 | 0.012* | - | - | - |
| Pre-CCRT CEA | 2.097 | 0.966-4.549 | 0.061 | 2.351 | 0.994-5.563 | 0.052 | |||
| Post-CCRT pT stage | 1.905 | 0.765-4.744 | 0.166 | 1.249 | 0.477-3.268 | 0.650 | 1.973 | 0.837-4.648 | 0.120 |
| Pre-CCRT cN stage | - | - | - | 1.210 | 0.510-2.874 | 0.665 | - | - | - |
CI, confidence interval; HR, hazard ratio; *, statistically significant
Discussion
DUOX2 was originally found in thyroid gland tissue by cloning from thyroid cDNA libraries [26, 27]. Two NADPH oxidase genes were identified and named thyroid oxidases (THOX1 and THOX2) due to considering thyroid specific. They localize at the apical membranes of the follicular cells and catalyze the oxidation of NADPH to generate hydrogen peroxide (H2O2), which is necessary for thyroid hormone biosynthesis by thyroperoxidase (TPO) [28]. Mutations of the THOX2 gene are also associated with congenital hypothyroidism [9]. However, two thyroid oxidases were later found in tissues other than thyroid glands, such as salivary glands, gastrointestinal tracts and respiratory tracts, where these two oxidases provide sources for mucosal host defense against microbiome [9, 30-32]. The amino acid sequences of both THOX1 and THOX2 conclude an extracellular peroxidase-like domain, in addition to NADPH-oxidase domain. Therefore, the terminology had been changed to dual oxidases (DUOX1 and DUOX2) [28]. Both human DUOX1 and DUOX2 genes are located on the chromosome 15q15.3 and share 83% similarity in the DNA sequences with each other [27]. Both DUOX1 and DUOX2 proteins contain seven transmembrane helices with two heme-binding sites, an extracellular peroxidase-like domain, two intracellular calcium-binding EF-hand motifs and a NADPH binding region [26, 27].
Although dual oxidases contribute to mucosal host defense against microorganism via generation of hydrogen peroxide (H2O2), the subsequent oxidative stress and reactive oxygen species (ROS) are also implicated in chronic inflammation [33]. DUOX2-induced ROS may cause chronic inflammatory pre-neoplastic disorders, e.g., inflammatory bowel disease and chronic pancreatitis [12, 34, 35]. Chronic inflammation and an imbalance between oxidants and antioxidants in organisms also play an important role in carcinogenesis of certain cancers [36-38]. In consequence, DUOX2 is highly expressed in several cancers, including colorectal cancer [13, 15-18]. By immunohistochemistry, overexpression of DUOX2 was observed in the majority of colon cancers (62%), breast cancers (66%), non-small cell lung cancers (86%) and prostate cancers (92%) [15]. In a later study, both mRNA and protein levels of DUOX2 revealed significant up-regulation in gastric and colorectal cancers compared with adjacent normal tissue (all P ≤ 0.01) [16]. High expression of DUOX2 was also an independent prognosticator predicting both lower recurrent-free and overall survival rates in patients with hepatocellular carcinoma after hepatectomy [17]. In pancreatic adenocarcinomas, the expression of both DUOX2 mRNA and protein was found significantly increased compared to the normal pancreatic tissue [18]. In contrast, the transcriptional level of DUOX2 was down-regulated in both human lung cancer cell lines and tissue specimens due to hypermethylation of CpG-rich promoter in the report of Luxen et al. [14]. The discrepancy maybe result from the dual enzymatic activity of DUOX2 per se, both NADPH-oxidase and peroxidase-like domain. Peroxidase serves as an antioxidative enzyme to protect the organism from oxidative stress. Down-regulation of glutathione peroxidase 2 (GPx2), an isoform of the common antioxidative enzyme of human bodies, was also significantly associated with poor prognosis in patients with urothelial carcinomas [38]. In the study of Luxen et al., therefore, the DUOX2 may act as a tumor suppressor gene in lung cancers [14]. In the present study, we also demonstrated the “double-edged sword” role in the carcinogenesis of rectal cancer. In normal colonic mucosa, low-level of immunoreactivity of DUOX2 was observed (Fig. 2A). Along with the transformation into adenomatous lesion, the expression of DUOX2 increased (Fig. 2B). After the carcinomatous change of the colonic adenoma into adenocarcinoma in situ (AIS) and invasive adenocarcinoma, down-regulation of DUOX2 was noted again (Fig. 2B-D). In normal colonic mucosa, DUOX2 play a role in host defense against microbiota. After that, oxidative stress and generation of ROS caused by DUOX2 overexpression may participate in the initial neoplastic transformation. Furthermore, the expression in AIS and invasive adenocarcinoma of rectum became complicated. The immunoreactivity was lower in AIS and invasive adenocarcinoma compared with adjacent adenoma, where down-regulation of the peroxidase-like domain in DUOX2 may contribute to the carcinogenesis. However, higher expression of DUOX2 in rectal cancer was still associated with pre- or post-CCRT disease advancement, and tumor response to CCRT, as well as poor prognosis.
In conclusion, the current work revealed that DUOX2 may play an imperial role in carcinogenesis of rectal cancers. DUOX2 overexpression was associated with adverse clinical and pathological parameters to statistical significance, including poor response to neoadjuvant CCRT. High immunoexpression of DUOX2 was also an independent prognostic factor of inferior DSS and LRFS for rectal cancer patients receiving neoadjuvant CCRT. Further evaluations to clarify the details of the biological and molecular role of DUOX2 in tumorigenesis of rectal cancer are essential for developing the potential DUOX2-targeted therapy for high-risk patients, as we illustrated the promising targets for new therapeutic strategies in patients with rectal cancers [39, 40].
Acknowledgements
The study was supported by grants from E-DA Hospital (EDAHS105003 and EDAHP106055) to I-Wei Chang.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29
2. Health promotion administration, Ministry of health, welfare, Executive Yuan. Cancer registry annual report: 2012. Taiwan. 2015
3. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291-304
4. Ceelen W, Fierens K, Van Nieuwenhove Y. et al. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer. 2009;124:2966-72
5. Sauer R, Becker H, Hohenberger W. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40
6. Kalvakolanu DV. Cytokine signaling in cancer: Novel players and pathways. Cytokine. 2017;89:1-3
7. Kawahara T, Lambeth JD. Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol Biol. 2007;7:178
8. Donkó A, Péterfi Z, Sum A, Leto T, Geiszt M. Dual oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2301-8
9. Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502-4
10. Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11-7
11. Katsuyama M. NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci. 2010;114:134-46
12. Lipinski S, Till A, Sina C. et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009;122:3522-30
13. Roy K, Wu Y, Meitzler JL. et al. NADPH oxidases and cancer. Clin Sci (Lond). 2015;128:863-75
14. Luxen S, Belinsky SA, Knaus UG. et al. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037-45
15. Wu Y, Antony S, Hewitt SM. et al. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol. 2013;42:1229-38
16. Qi R, Zhou Y, Li X. et al. DUOX2 Expression Is Increased in Barrett Esophagus and Cancerous Tissues of Stomach and Colon. Gastroenterol Res Pract. 2016 Epub 2015 Dec 29. doi: 10.1155/2016/1835684
17. Lu CL, Qiu JL, Huang PZ. et al. NADPH oxidase DUOX1 and DUOX2 but not NOX4 are independent predictors in hepatocellular carcinoma after hepatectomy. Tumour Biol. 2011;32:1173-82
18. Wu Y, Meitzler JL, Antony S. et al. Dual Oxidase2 and pancreatic adenocarcinoma: IFN-γ-mediated dual oxidase2 overexpression results in H2O2-induced, ERKassociated up-regulation of HIF-1α and VEGF-A. Oncotarget. 2016;7:68412-33
19. Lin CY, Lin CY, Chang IW. et al. Low thrombospondin 2 expression is predictive of low tumor regression after neoadjuvant chemoradiotherapy in rectal cancer. Am J Transl Res. 2015;7:2423-32
20. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edition. Berlin: Springer. 2011
21. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23
22. Chang IW, Wang YH, Wu WJ. et al. Necdin Overexpression Predicts Poor Prognosis in Patients with Urothelial Carcinomas of the Upper Urinary Tract and Urinary Bladder. J Cancer. 2016;7:304-13
23. Ma LJ, Wu WJ, Wang YH. et al. SPOCK1 Overexpression Confers a Poor Prognosis in Urothelial Carcinoma. J Cancer. 2016;7:467-76
24. Chang IW, Lin VC, Wu WJ. et al. Complement Component 1, s Subcomponent Overexpression is an Independent Poor Prognostic Indicator in Patients with Urothelial Carcinomas of the Upper Urinary Tract and Urinary Bladder. J Cancer. 2016;7:1396-405
25. Chang IW, Li CF, Lin VC. et al. Prognostic Impact of Thrombospodin-2 (THBS2) Overexpression on Patients with Urothelial Carcinomas of Upper Urinary Tracts and Bladders. J Cancer. 2016;7:1541-9
26. Dupuy C, Ohayon R, Valent A, Noël-Hudson MS, Dème D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265-9
27. De Deken X, Wang D, Many MC. et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227-33
28. Carvalho DP, Dupuy C. Role of the NADPH Oxidases DUOX and NOX4 in Thyroid Oxidative Stress. Eur Thyroid J. 2013;2:160-7
29. Moreno JC, Bikker H, Kempers MJ. et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95-102
30. Edens WA, Sharling L, Cheng G. et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol. 2001;154:879-91
31. El Hassani RA, Benfares N, Caillou B. et al. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933-42
32. Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454-61
33. Chu FF, Esworthy RS, Doroshow JH. et al. Deficiency in Duox2 activity alleviates ileitis in GPx1- and GPx2-knockout mice without affecting apoptosis incidence in the crypt epithelium. Redox Biol. 2016;11:144-56
34. Wu Y, Antony S, Juhasz A. et al. Up-regulation and sustained activation of Stat1 are essential for interferon-gamma (IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2 (DuoxA2) expression in human pancreatic cancer cell lines. J Biol Chem. 2011;286:12245-56
35. Fukushima N, Koopmann J, Sato N. et al. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod Pathol. 2005;18:779-87
36. Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655-63
37. Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37-56
38. Chang IW, Lin VC, Hung CH. et al. GPX2 underexpression indicates poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. World J Urol. 2015;33:777-89
39. Chai CY, Zhang Y, Song J, Lin SC, Sun S, Chang IW. VNN1 overexpression is associated with poor response to preoperative chemoradiotherapy and adverse prognosis in patients with rectal cancers. Am J Transl Res. 2016;8:4455-63
40. Lee YE, He HL, Shiue YL. et al. The prognostic impact of lipid biosynthesis-associated markers, HSD17B2 and HMGCS2, in rectal cancer treated with neoadjuvant concurrent chemoradiotherapy. Tumour Biol. 2015;36:7675-83
Author contact
![]() Corresponding author: Yu-Feng Tian, M.D., Division of General Surgery, Department of Surgery, Chi Mei Medical Center. 901 Chunghwa Road, Yung Kang Dist., Tainan City 710, TAIWAN. Tel: +886-6-2812811 ext. 53680. Fax: +886-6-2511235. E-mail: cmh7590chimei.org.tw
Corresponding author: Yu-Feng Tian, M.D., Division of General Surgery, Department of Surgery, Chi Mei Medical Center. 901 Chunghwa Road, Yung Kang Dist., Tainan City 710, TAIWAN. Tel: +886-6-2812811 ext. 53680. Fax: +886-6-2511235. E-mail: cmh7590chimei.org.tw

 Global reach, higher impact
Global reach, higher impact