3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(14):2828-2835. doi:10.7150/jca.19524 This issue Cite
Research Paper
Prognostic Impact of ABO Blood Group on Type I Endometrial Cancer Patients- Results from Our Own and Other Studies
1. Unit of Obstetrics and Gynaecology, Azienda Unità Sanitaria Locale - Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Reggio Emilia, Italy;
2. Laboratory of Translational Research, Azienda Unità Sanitaria Locale-Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Reggio Emilia, Italy;
3. Unit of Surgical Gynecol Oncology, Azienda Unità Sanitaria Locale - Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Reggio Emilia, Italy;
4. Laboratory of Molecular Biology, Azienda Unità Sanitaria Locale - Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Reggio Emilia, Italy;
5. Unit of Pathology, Azienda Unità Sanitaria Locale - Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Reggio Emilia, Italy;
6. Unit of Obstetrics and Gynaecology, University of Modena and Reggio Emilia, Reggio Emilia, Italy.
* These two authors contributed equally
# These two authors contributed equally
Received 2017-2-6; Accepted 2017-5-14; Published 2017-8-23
Abstract
Objectives: The ABO blood group antigens were found on most epithelial cells and in secretions. In the normal endometrium there is a variable expression of histo-blood group and related antigens suggesting a hormonal regulation. A relationship between ABO blood groups and endometrial cancer has been investigated with contradictory results. In this study we investigated the influence of blood types on clinical and pathological characteristics of endometrial cancer patients.
Method: Retrospective cohort study. Clinical and pathological data were extrapolated and their association with blood groups were assessed.
Results: A total of 203 type I endometrial cancer patients were included in the final analysis. Univariate analysis indicated that a lower frequency of G3 undifferentiated tumors was observed in patients with A blood group (P=0.027). Multivariate analysis, including also clinical features such as Age, BMI, parity, hypertension and diabetes confirmed that patients with A group present a lower risk of G3 tumors in comparison with not A patients. (OR=0.32, P=0.011).
Conclusions: Patients with A genotype have a lower risk to develop G3 type I endometrial cancer. ABO blood group might represent a useful, easy access and cheap biomarker for patients' selection and for management personalization of endometrial cancer patients.
Keywords: Endometrial cancer, Type I endometrial cancer, ABO blood group, Grading, A blood group.
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries, 319605 new cancer cases and 76160 cancer deaths worldwide were recorded in the 2012 [1-4].
Type I endometrial adenocarcinoma is the most common type, it can be classified into highly differentiated (G1), moderately differentiated (G2), or undifferentiated (G3) according to FIGO classification. Usually type I EC has a good prognosis because is a G1 tumour diagnosed at early stage [1]. Prolonged hyperestrogenism, unopposed by progesterone has a pivotal role in the pathogenesis of type I EC. Hyperestrogenism characterizes early menarche, late menopause, nulliparity, estrogen only hormone replacement therapy and obesity that are well known risk factors for type I EC [5,6]. Several data related to the genetic risk was also reported [7-9]. Single nucleotide polymorphisms (SNPs) of aromatase (CYP19A1) influenced susceptibility to EC, particularly among older and obese patients [7].
Some studies [10-13] have shown that the ABO blood group may influence the risk of EC.
The ABO blood group antigens were initially identified, by Landsteiner [10], as erythrocyte substances with a significance mainly ascribed to serology, but these antigens were found on most epithelial cells and in secretions [11-13].
The carbohydrate histo-blood group antigens are not primary gene products, but they are synthesized by the action of gene-encoded glycosyltransferases. The synthesis of histo-blood group antigens is stepwise, and each step is catalyzed by specific glycosyltransferases [14].
Several polymorphic genes are involved in the genetic regulation of carbohydrate synthesis which has to be taken into consideration when the expression of carbohydrates is evaluated. The pathway of the biosynthesis and the chemical structure of the antigens explains the interrelationships between the ABO and H systems [15].
This synthesis correlates with embryonic development and cellular differentiation [16]. The expression of histo-blood group antigens varies in this way from cell to cell and from organ to organ. The blood group antigens represent the terminal part of an oligosaccharide chain linked to proteins or lipids. The antigen determinants may be carried on many different core saccharide structures, and the general phenotype of the ABH epitopes is uninfluenced by the carbohydrates that carry them [16-17].
In the normal endometrium there is a variable expression of histo-blood groups and related antigens suggesting a hormonal regulation of glycosyltransferase activity. The expression of the A/B transferase proteins in human endometrial epithelial cells was shown to correlate with the level of oestradiol in serum [18] and is influenced by the secretor status [19]. Moreover, ABO carbohydrates seem to strictly regulate the adhesion and implantation of the blastocyst process [20].
A relationship between ABO blood groups with EC has been investigated with contradictory results (Table 1). In a study, A blood group had the highest frequency between the women with EC in the reproductive age. O phenotype group was the most frequent in case of menopause and post-menopause women with EC [21]. On contrary in another study no statistically significant correlations were obtained for EC and ABO blood groups [22].
In this study we investigated the influence of blood types on EC patients treated at our research institute, particularly we evaluated the impact of blood types on clinical and pathological characteristics.
Methods
Patient samples
The study was designed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [23]. The Local Ethical Committee approved the study design and all patients provided written informed consent to use personal non-sensitive data at hospital admission.
Clinical charts of EC patients treated and followed at the IRCCS - Santa Maria Nuova Hospital of Reggio Emilia (Italy) from 1997 to 2016 were checked for inclusion and exclusion criteria.
Clinical, pathological and genetic data were recorded in an electronic separate, anonymous, password-protected database. All relevant data were extrapolated and used for final analysis.
Patients with histological diagnosis of type I EC who received upfront adequate surgery treatment were electively included in the protocol study. Exclusion criteria were: histological diagnosis of non type I EC, inadequate EC management according to internal and international guidelines [24, 25], neoadjuvant chemotherapy performed before surgery, an age less than 18 years, non-Caucasian ancestry, a follow-up length less than of 6 months, inadequate follow-up according to internal guidelines, absence of written informed consent, diagnosis of a previous or concurrent cancer(s) and unavailable follow-up data.
An “adequate” management was considered as follows: total extrafascial hysterectomy (TEH) with bilateral salpingo-oophorectomy (BSO) was the standard staging procedure; whereas radical hysterectomy (RH) was performed only in stage II EC patients with gross cervical involvement; pelvic with/without paraaortic lymph node dissection were performed in case of myometrial invasion greater than 50 percent, large tumor (>2 cm in diameter) or filling the endometrial cavity. Vaginal brachytherapy alone was administered to the patients at stage IA G3 and IB G1 or G2 without negative prognostic factors. External beam radiotherapy plus vaginal brachytherapy was administered to the patients at stage IA G3 and IB G1 and G2 with negative prognostic factors, and to the patients at stage IB G3 and to all the patients at stage II, III and IV. Chemotherapy was administered to the patients at stage III C and IV. In all cases, chemoradiotherapy consisted of paclitaxel 175mg/m2 (P) and carboplatin AUC5 (C) on day 1 every three weeks, for a total of four to six cycles, and it was followed by external pelvic radiation therapy (1.8 Gy/d, d1-5) at a total dose of 45 Gy plus vaginal brachytherapy (3 × 5 Gy) [24, 26].
A follow-up was defined “adequate” in case of adherence to the following schedule: type I EC at stage IA and grading G1/G2 - physical and gynecological examination, and transvaginal ultrasound every 6 months for the first 2 years, and then every 12 months for at least 3 years; type I EC at stage IB and/or any grading G3 tumor - physical and gynecological examination, and transvaginal ultrasound every 6 months for the first 5 years. Further investigations such as abdominal ultrasound, chest X-ray, computed tomography scan, and serum CA 125 levels were performed if clinically indicated.
The same pathologist with long-time expertise in gynecological oncology reviewed all the histological samples in order to confirm formally the diagnosis.
Statistical analysis
For statistical analysis, R statistical software package version 2.15.1 (R foundation for Statistical Computing, Vienna, Austria) was used.
Fisher's exact test and generalized linear models were used to investigate univariate and multivariate association of blood groups with clinical and pathological parameters.
Overall survival (OS) was computed as the time period from the date of surgery to either the date of death or last follow up, whichever occurred first. Disease free survival (DFS) was computed as the time period from the date of surgery to either the date of diagnosis of recurrence or last follow up, whichever occurred first. The effects of genotypes on OS and DFS were evaluated using Cox regression hazard model and results were presented as hazard ratio (HR).
Significant statements referred P values lower than 0.05.
ABO blood groups and endometrial cancers: results reported in literature
| Year | Author (reference) | Ethnicity | Sample Size | Age (n) | BMI (n) | FIGO Stage (n) | Grading (n) | 0 % (n) | P value | A % (n) | P value | B % (n) | P value | AB % (n) | P value | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | Nakashidze I et al. (21) | Georgia | 60 | Reproductive Age (20) | - | - | - | 20% (4) | < 0.05 | 65% (13) | < 0.05 | 10% (2) | < 0.05 | 5% (1) | < 0.05 | A group is the most common in reproductive age |
| Menopause (20) | 55% (11) | 40% (8) | 5% (1) | 0% (0) | 0 group is the most common in menopause and post menopause age | |||||||||||
| Postmenopause (20) | 60% (12) | 30% (6) | 5% (1) | 5% (1) | ||||||||||||
| 2012 | Yuzhalin AE et al. (22) | Siberia | 440 | Premenopause (102) | - | - | - | 31.4% (32) | - | 36.3% (37) | 0.495 | 24.5% (25) | 0.435 | 7.8% (8) | 0.906 | No statistically significant correlation |
| Postmenopause (338) | 35.5% (120) | - | 37% (125) | 0.639 | 19.8% (67) | 0.409 | 7.7% (26) | 0.659 | ||||||||
| 1995 | Marinaccio M et al. (29) | Italy | 237 | - | - | I (119) | - | 23.5% (28) | 0.001 | 56.3% (67) | - | 16.0% (19) | - | 4.2% (5) | - | A group is the most common in the stage I |
| II (68) | 66.2% (45) | - | 25.0% (17) | - | 8.8% (6) | - | - | - | 0 group is the most common in the stage II | |||||||
| NA (50) | 24.0% (12) | - | 58% (29) | - | 18% (9) | - | - | - | ||||||||
| 2011 | Xu W et al. (30) | China | 1204 | 54,3 (mean) | 25,7 (mean) | - | - | 323 (26%) | 0.001 | 355 (29,4%) | 0.001 | 265 (22.0%) | 0.001 | 126 (126/1204) | 0.001 | A group is the most common in woman with endometrial cancer |
| 2017 | Mandato VD et al. | Italy | 203 | ≤ 64 (104) | 45.2% (33) | - | 56.7% (59) | 0.132 | 42.9% (9) | 0.849 | 60% (3) | 0.526 | No statistically significant correlation between blood group and age | |||
| > 64 (99) | 54.8% (40) | 43.3% (45) | 57.1% (12) | 40% (2) | ||||||||||||
| ≤ 28 (100) | 57.1% (40) | - | 46.1% (48) | 0.174 | 40% (8) | 0.180 | 805 (4) | 0.337 | No statistically significant correlation between blood group and BMI | |||||||
| > 28 (98) | 42.9% (30) | 53.4% (55) | 60% (12) | 20% (2) | ||||||||||||
| NA (5) | 3 | 1 | 1 | 0 | ||||||||||||
| I-II (187) | 90.4% (66) | - | 92.3% (96) | 0.656 | 95.2% (20) | 0.494 | 100% (5) | 0.989 | No statistically significant correlation between blood group and FIGO stage | |||||||
| III-IV (16) | 9.6% (7) | 7.7% (8) | 4.8% (1) | 0 | ||||||||||||
| G1 (88) | 37.0% (27) | - | 49.9% (51) | - | 28.6%(6) | - | 80% (4) | - | A group presents a lower risk of G3 endometrial cancer in comparison with not A group | |||||||
| G2 (81) | 41.1% (30) | 40.4% (42) | 0.375 | 38.1%(8) | 0.762 | 20% (1) | 0.194 | |||||||||
| G3 (34) | 21.9% (16) | 10.6% (11) | 0.027 | 33.3% (7) | 0.289 | 0 | 0.989 |
Results
After patients' selection for the inclusion and exclusion criteria, a total of 203 EC patients were studied and included in the final analysis. Total extrafascial hysterectomy was performed in 140 (69.0%) patients, whereas radical hysterectomy was performed in 45 (22.1%) patients. Salpingo-oophorectomy was performed in 195 (6.1%) patients. Omentectomy and appendicectomy were performed in 19 (9.4%) and 4 (2.0%) patients, respectively. One hundred thirty eight (68.0%) patients received pelvic lymphadenectomy and only 13 (6.4%) patients received lombo-aortic lymphadenectomy. In all patients a complete resection of the disease was obtained.
During a median follow-up time of 57 months (range, 7 to 151 months) 16 patients had a recurrence and 11 patients died because of the cancer.
In our population there were 73 patients with blood group 0 (36.0%), 104 with A (51.0%), 21 with B (10.3%) and 5 with AB (2.5%).
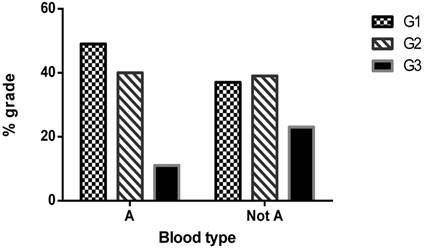
In table 2 blood groups AB0 were analyzed in association with clinical and pathological characteristics of EC patients. Interestingly a significant association was observed between tumor grading and patients' blood group, in particular a lower frequency of G3 undifferentiated tumors was observed in patients with A blood group (P=0.027) (Figure 1).
In table 3 the association was further investigated in multivariate analysis, including also clinical features such as Age, BMI, parity, hypertension and diabetes. Blood groups were clustered to investigate in particular the effect of O and A group on risk of high grade tumor. The analysis confirmed that patients with A group present a lower risk of G3 EC tumors in comparison with not A patients (OR=0.32, P=0.011) (Figure 2).
No association of blood groups with OS and DFS of EC patients was observed in our study population (Table 4).
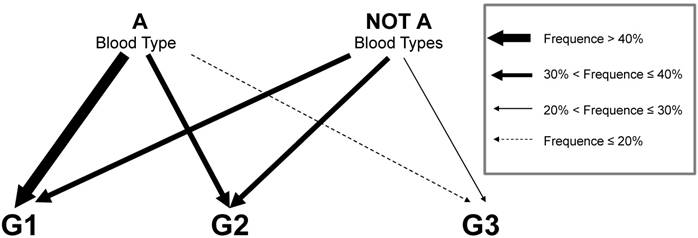
Representation in diagram of A and not A blood groups effect on type 1 EC. As indicated in legend arrows thickness express the range of frequency of each grade in the 2 group of patients.
Histogram of type 1 EC grades frequencies in A and not A population.
Association study of blood groups AB0 with clinical and pathological features of the type I endometrial cancer.
| Features | Patients (n%) (N=203, 100%) | 0 (n%) (N=73, 100%) | P value | A (n%) (N=104, 100%) | P value | B (n%) (N=21, 100%) | P value | AB (n%) (N=5, 100%) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| ≤ 64 | 104 (51.2) | 33 (45.2) | 59 (56.7) | 9 (42.9) | 3 (60.0) | ||||
| >64 | 99 (48.8) | 40 (54.8) | 45 (43.3) | 0.132 | 12 (57.1) | 0.849 | 2 (40.0) | 0.526 | |
| BMI | |||||||||
| ≤ 28 | 100 (50.5) | 40 (57.l) | 48 (46.6) | 8 (40.0) | 4 (80.0) | ||||
| >28 | 98 (49.5) | 30 (42.9) | 55 (53.4) | 0.174 | 12 (60.0) | 0.180 | 1 (20.0) | 0.337 | |
| NA* | 5 | 3 | 1 | 1 | 0 | ||||
| Parity | |||||||||
| No | 33 (16.4) | 11 (15.3) | 16 (15.5) | 5 (23.8) | 1 (20.0) | ||||
| Yes | 168 (83.6) | 61 (84.7) | 87 (84.5) | 0.963 | 16 (76.2) | 0.366 | 4 (80.0) | 0.779 | |
| NA* | 2 | 1 | 1 | 0 | 0 | ||||
| Hypertension | |||||||||
| No | 83 (41.3) | 36 (50.0) | 37 (35.9) | 6 (28.6) | 4 (80.0) | ||||
| Yes | 118 (58.7) | 36 (50.0) | 66 (64.1) | 0.064 | 15 (71.4) | 0.088 | 1 (20.0) | 0.225 | |
| NA* | 2 | 1 | 1 | 0 | 0 | ||||
| Diabetes | |||||||||
| No | 159 (79.1) | 62 (86.1) | 77 (74.7) | 15 (71.4) | 5 (100.0) | ||||
| Yes | 42 (20.9) | 10 (13.9) | 26 (25.3) | 0.071 | 6 (28.6) | 0.124 | 0 (0.0) | 0.989 | |
| NA* | 2 | 1 | 1 | 0 | 0 | ||||
| Figo Stage | |||||||||
| I-II | 187 (92.1) | 66 (90.4) | 96 (92.3) | 20 (95.2) | 5 (100.0) | ||||
| III-IV | 16 (7.9) | 7 (9.6) | 8 (7.7) | 0.656 | 1 (4.8) | 0.494 | 0 (0.0) | 0.989 | |
| Grading | |||||||||
| G1 | 88 (43.3) | 27 (37.0) | 51 (49.0) | 6 (28.6) | 4 (80.0) | ||||
| G2 | 81 (39.9) | 30 (41.1) | 42 (40.4) | 0.375 | 8 (38.1) | 0.762 | 1 (20.0) | 0.194 | |
| G3 | 34 (16.8) | 16 (21.9) | 11 (10.6) | 0.027 | 7 (33.3) | 0.289 | 0 (0.0) | 0.989 | |
| Adjuvant Treatment | |||||||||
| No | 141 (69.5) | 50 (68.5) | 76 (73.1) | 10 (47.6) | 5 (100.0) | ||||
| Yes | 62 (30.5) | 23 (31.5) | 28 (26.9) | 0.508 | 11 (52.4) | 0.084 | 0 (0.0) | 0.988 | |
| Death | |||||||||
| No | 178 (87.7) | 64 (87.7) | 92 (88.5) | 18 (85.7) | 4 (80.0) | ||||
| Yes | 25 (12.3) | 9 (12.3) | 12 (11.5) | 0.873 | 3 (14.3) | 0.813 | 1 (20.0) | 0.624 | |
| Death because of the tumor | |||||||||
| No | 192 | 70 (95.9) | 97 (93.3) | 20 (95.2) | 5 (100.0) | ||||
| Yes | 11 | 3 (4.1) | 7 (6.7) | 0.462 | 1 (4.8) | 0.896 | 0 (0.0) | 0.993 | |
| Recurrence | |||||||||
| No | 186 | 69 (94.5) | 94 (90.4) | 19 (90.5) | 4 (80.0) | ||||
| Yes | 17 | 4 (5.5) | 10 (9.6) | 0.322 | 2 (9.5) | 0.509 | 1 (20.0) | 0.235 | |
| Lymph node Metastasis (132 Lymphadenectomy) | 132 | 53 | 62 | 13 | 4 | ||||
| No | 121 | 48 (90.6) | 57 (93.4) | 12 (100.0) | 4(100.0) | ||||
| Yes | 9 | 5 (9.4) | 4 (6.6) | 0.572 | 0 (0.0) | 0.993 | 0 (0.0) | 0.996 | |
| NA* | 2 | 0 | 1 | 1 | 0 |
* Percentages were calculated from the total excluding NA patients. NA=Not Available.
Discussion
A relationship between ABO blood groups and EC cancer risk has been investigated in several studies. However, to our knowledge no study reported a significant favourable association between A genotype and tumor grading. Particularly, patients with A genotype have a lower risk (P = 0.011) to develop G3 EC in comparison with patients with other blood group combinations.
Previous studies reported that the A blood group was associated with high risk of EC [29] particularly in reproductive age [21].
A significant dose response relationship was observed for EC risk and level of antigen A. The positive association of blood type A with cancer risk was observed regardless of menopausal status, body mass index, oral contraceptive use, or family cancer history suggesting that ABO blood type may be involved in the development of EC [30].
On contrary, the O blood group was associated with high risk of EC in menopause and post-menopause women [21] and in another study no statistically significant correlations were obtained for EC and ABO [22].
Several mechanisms for the association of the ABO blood type with cancer risk have been proposed, including inflammation, immune surveillance for malignant cells, intercellular adhesion, and membrane signaling [31].
ABO antigens may interfere with cell adhesion, cell signaling and immune surveillance by altered levels of tumor necrosis factor-a, E-selectin and P-selectin [32, 33].
Multivariate analysis of association between patients' blood groups AB0 and clinical features and grade of type 1 endometrial cancer. Blood groups were also clustered to investigate in particular the effect of O and A group on tumor grades frequencies.
| Features | Patients (N=203, 100%) | Grade G1 (N=88) (n%) | Grade G2 (N=81) (n%) | Adjusted OR (95% CI) | Adjusted P value | Grade G3 (N=34) (n%) | Adjusted OR (95% CI) | Adjusted P value | |
|---|---|---|---|---|---|---|---|---|---|
| Blood group | 0 | 73 | 27 (37.0) | 30 (41.0) | - | - | 16 (21.9) | - | - |
| A | 104 | 51 (49.0) | 42 (40.4) | 0.73(0.36-1.47) | 0.378 | 11 (10.6) | 0.32 (0.11-0.86) | 0.028 | |
| B | 21 | 6 (28.6) | 8 (38.1) | 1.38 (0.38-5.41) | 0.628 | 7 (33.3) | 2.23 (0.58-9.18) | 0.249 | |
| AB | 5 | 4 (80.0) | 1 (20.0) | 0.25 ( 0.01-1.93) | 0.237 | 0 (0.0) | - | 0.990 | |
| Age (years) | ≤64 | 104 | 50 (48.1) | 36 (34.6) | - | - | 18 (17.3) | ||
| >64 | 99 | 38 (38.4) | 45 (45.4) | 1.42 (0.73-2.77) | 0.301 | 16 (16.2) | 0.84 (0.31-2.22) | 0.734 | |
| BMI | ≤28 | 100 | 47 (47.0) | 38 (38.0) | - | - | 15 (15.0) | - | - |
| >28 | 98 | 38 (38.8) | 42 (42.9) | 1.35 (0.67-2.71) | 0.398 | 18 (18.4) | 1.30 (0.51-3.35) | 0.588 | |
| NA | 5 | ||||||||
| Parity | No | 33 | 17 (51.5) | 7 (21.2) | - | - | 9 (27.3) | - | - |
| Yes | 168 | 69 (41.1) | 74 (44.0) | 2.76 (1.07-7.82) | 0.042 | 25 (14.9) | 0.78 (0.29-2.23) | 0.632 | |
| NA | 2 | ||||||||
| Hypertension | No | 83 | 38 (45.8) | 30 (36.1) | 15 (18.1) | ||||
| Yes | 118 | 48 (40.7) | 51 ( 43.2) | 0.98 (0.48-1.98) | 0.951 | 19 (16.1) | 0.99 (0.37-2.71) | 0.998 | |
| NA | 2 | ||||||||
| Diabetes | No | 159 | 70 (44.0) | 63 (39.6) | 26 (16.3) | ||||
| Yes | 42 | 16 (38.1) | 18 (42.9) | 1.17 (0.50-2.72) | 0.719 | 8 (19.0) | 1.67 (0.49-5.66) | 0.407 | |
| NA | 2 | ||||||||
| Clustered Blood groups | |||||||||
| 0 | 73 | 27 (37.0) | 30 (41.1) | 16 (21.9) | |||||
| Not 0 | 130 | 61 (46.9) | 51 (39.2) | 0.76 (0.39-1.50) | 0.434 | 18 (13.9) | 0.48 (0.20-1.12) | 0.089 | |
| Not A | 99 | 37 (37.4) | 39 (39.4) | 23 (23.2) | |||||
| A | 104 | 51 (49.0) | 42 (40.4) | 0.76 (0.40-1.44) | 0.393 | 11 (10.6) | 0.32 (0.13-0.76) | 0.011 | |
NA=Not Available; OR=Odd Ratio
Cox model evaluation of the effects of different blood groups on overall survival and disease free survival in type 1 endometrial cancer patients.
| Features | Patients (N=203, 100%) | Overall Survival | Disease free survival | ||||
|---|---|---|---|---|---|---|---|
| # Death (N=11) | HR (95% CI) | P value | # Recurrence (N=17) | HR (95% CI) | P value | ||
| Blood groups | |||||||
| 0 | 73 | 3 (4.1) | - | - | 4 (5.8) | - | |
| A | 104 | 7 (6.7) | 1.85 (0.48-7.16) | 0.373 | 10 (9.6) | 1.86 (0.58-5.95) | 0.293 |
| B | 21 | 1 (4.7) | 1.26 (0.13-12.07) | 0.844 | 2 (9.5) | 1.96 (0.36-10.73) | 0.436 |
| AB | 5 | 0 (0.0) | - | 0.999 | 1 (20.0) | 4.63 (0.51-41.82) | 0.172 |
| Clustered Blood groups | |||||||
| 0 | 73 | 3 (4.1) | - | - | 4 (5.5) | - | - |
| Not 0 | 130 | 8 (6.2) | 0.60 (0.44-6.30) | 0.450 | 13 (10.0) | 1.97 (0.64-6.04) | 0.236 |
| Not A | 99 | 4 (4.0) | - | - | 7 (7.1) | ||
| A | 104 | 7 (6.7) | 1.85 (0.54-6.34) | 0.325 | 10 (9.6) | 1.39 (0.53-3.66)\ | 0.501 |
HR=Hazard Ratio
The ABO gene is located on chromosome 9q34. ABO gene polymorphism has been implicated in susceptibility to several cancers across different populations, but a susceptibility to EC has not been reported [34].
This gene encodes for glycosyltransferases, which catalyze the step-by step transfer of single sugars to the H antigen to form the A and B antigen [35].
The peripheral part of the carbohydrates in the cell surface is strongly immunogenic, many carbohydrates, including the blood group ABO antigens, were initially identified as cell surface antigens [14].
Blood group O persons, who do not have the A and B gene coded glycosyltransferase, express a fucosylated variant (Ley) of the precursor structure [36]. The lack of expression of blood group antigens in tumours is correlated with lack of presence of the blood group coded glycosyltransferase [37]. Aberrant glycosylation patterns are a hallmark of cancer development and progression [38, 39] and aberrant glycosylation occurs early during oncogenic transformation and may represent a key event in invasion and metastasis.
Loss or reduction of A and B epitopes in human cancers is well documented, and loss of A and B antigens is correlated with the degree of malignancy and metastatic potential in EC, lung, bladder and oral carcinomas [33, 38, 40].
In vitro studies have demonstrated that loss or addition of a single glycosyl residue may affect tumor cell motility by altering glycosylation of integrin receptors and their interaction with 1 integrin. This may explain the observed correlation between glycosylation and prognosis [41].
Expression of A/B antigens in tumors is directly correlated with A and B glycosyltransferase activity [37]. An antigen negative tumors have reduced levels of ABO transcript as compared to A antigen positive tumors [38]. The regulatory mechanism of ABO gene transcription presents two promoter regions [42, 43].
Expression of the ABO gene in epithelial and erythroid cells lines was shown to be dependent on the methylation status of the proximal constitutive promoter encoding most of the ABO transcripts, as an inverse relationship was found between promoter hypermethylation and ABO gene expression [42].
Hence, poorly differentiated tumour contained high amounts of fully methylated alleles. The levels of DNA methylation have been shown to increase with the degree of malignancy. Hypermethylation in hyperplastic or dysplastic epithelium is found, it may therefore be an early sign of malignant transformation [38].
Both non-invasive (preneoplastic) endometrial lesions and EC show changes in histo-blood group phenotype, when this is compared with that of normal endometrium. In EC, the changes in histo-blood group phenotype are qualitatively influenced by the genetic status in terms of the ABO, Lewis and the ABH-secretor status predominantly. The malignant phenotype shows some resemblance to the luteal phase phenotype indicating that the responsible changes in glycosyltransferases and substrate levels may be alike. In both normal and malignant endometrial cells, the expression of A/B transferase protein is confined to endometrium from blood group A/B individuals and relate to serum estradiol levels. Loss of A/B transferase protein seems to be a late event in endometrial carcinogenesis, whereas the other changes in glycosyltransferase- activity responsible for the observed changes in histo-blood group phenotype seem to take place in premalignant endometrial cells [44]. At the precancerous stage, ABH antigens are highly expressed on epithelial cells. They participate in the phenomenon of apoptosis resistance. This would facilitate both cancerogenesis and immune escape. At more advanced stages of tumor progression, tumor cells that have lost A and B antigens would be potentially more metastatic since these antigens inhibit cell motility. Similarly, overexpression of sialyl-Lex and sialyl-Lea would increase metastatic potential by allowing adhesion to the vascular endothelium. In addition, at intermediate stages, the angiogenic and procoagulating activities of the H and Ley antigens would favor tumor development. Thus antigens of this family could have either a deleterious or a favorable impact on evolution of the disease. The presence of ABH antigen at early stages would be deleterious, while it would become favorable at latter stages by inhibiting cell motility on the one hand and synthesis of sialyl-Lewis antigens through the competition between al, 2fucosyltrasnferase and a 2.3sialyltransferase on the other [45].
In order to explore that influence, our population was studied according to well known risk factors such as age, body mass index (BMI), parity, diabetes, hypertension but at multivariate analysis, only AB0 blood group showed an influence on EC grade.
Tumor grade represents a well-established risk factor, according to tumor grade staging procedure such as systematic lymphadenectomy, and adjuvant treatment are performed or not [26]. Recently also rs5275 polymorphism CC of PTGS2 was reported to be associated with a lower risk to present G3 EC [8]. Moreover, with the widespread of fertility sparing surgery that can be proposed only in case of type 1 G1 EC at stage 1 [26], to know that the risk of G2-G3 EC is genetically reduced might be reassuring [8].
The strength of our study concern the selection of a very homogeneous population of type I EC patients who received an upfront surgery with adequate management and follow-up length. The centralization of ABO assessment, of treatment, of follow- up, and of pathology review are further study strengths that ensured a uniformity of treatment, of staging procedures, post-treatment monitoring, and of histological classification [8]. Instead, in other available studies the treatment and follow-up protocols widely varied [46,47]. Moreover, our study has also important limitations. Firstly, it had a retrospective design, and the potential and related biases/confounders are well known. Secondly, it might be underpowered due to the small cohort studied, and current sample size might not be sufficient to detect a synergistic effect in a replicate study, moreover this finding could be limited to the ethnicity.
In conclusion, current preliminary analysis demonstrates that the differentiation of the type I EC may be significantly and independently influenced by ABO blood group.
Patients with A genotype have a lower risk to develop G3 EC. If our results will be confirmed in large multicenter studies, ABO blood group might represent an useful, easy access and cheap biomarker for patients' selection and for management personalization of type I EC patients.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bakkum-Gamez JN, Gonzalez-Bosquet J, Laack NN, Mariani A, Dowdy SC. Current issues in the management of endometrial cancer. Mayo Clin Proc. 2008;83:97-112
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
3. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491-505
4. Bray F, Loos AH, Oostindier M, Weiderpass E. Geographic and temporal variations in cancer of the corpus uteri: incidence and mortality in pre- and postmenopausal women in Europe. Int J Cancer. 2005;117:123-31
5. Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69-102
6. Ohno S, Ohno Y, Suzuki N. et al. Multiple roles of cyclooxygenase-2 in endometrial cancer. Anticancer Res. 2005;25:3679-87
7. Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. Cyclooxygenase-2 expression correlates with apoptosis and angiogenesis in endometrial cancer tissue. Anticancer Res. 2007;27:3765-3770
8. Torricelli F, Mandato VD, Farnetti E. et al. Polymorphisms in cyclooxygenase-2 gene in endometrial cancer patients. Tumour Biol. 2015;36:7423-30
9. Mandato VD, Farnetti E, Torricelli F. et al. HNF1B polymorphism influences the prognosis of endometrial cancer patients: a cohort study. BMC Cancer. 2015;15:229
10. Watkins WM. Biochemistry and genetics of the ABO, Lewis, and P blood group systems. Harris H, Hirschhorn K, edtors. Advances in human genetics. New York: Plenum Press. 1980:1-136
11. Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108:1-28
12. Szulman AE. The histological distribution of the blood group substances in man as disclosed by immunofluorescence. IV. The ABH antigens in embryos at the fifth week post fertilization. Hum Pathol. 1971;2:575-85
13. Ito NHT. Histological and Cytochemical Localization of Blood Group Anti- gens. New York: Gustav Fischer Verlag/VCH Publishers. 1992:1-85 16, 17
14. Watkins WM. Blood-group substances. Science. 1966;152:172-81
15. Greenwell I. Blood group antigens: molecules seeking a function? Glycoconj J. 1997;14:159-73
16. Clausen H, Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1-20
17. Kouri M, Nordling S, Kuusela P, Pyrhonen S. Poor prognosis associated with elevated serum CA 19-9 level in advanced colorectal carcinoma, independent of DNA ploidy or SPE. Eur J Cancer. 1993;29A:1691-6
18. Ravn V, Mandel U, Svenstrup B, Dabelsteen E. Blood-group NB-defined glycosyltransferase and A/B blood-group antigens in human normal and malignant endometrium in relation to morphology, age and oestrogen levels. Glycosylation & Disease. 1994;1:153-63
19. Ravn V, Teglbjierg CS, Mandel U, Dabelsteen E. The distribution of type 1 chain ABO and related histoblood group antigens in normal cycling endometrium. Int J Gynecol Pathol. 1993;12:70-9
20. Zhu ZM, Kojima N, Stroud MR, Hakomori SI, Fenderson BA. Monoclonal antibody directed to Le(Y) oligosaccharide inhibits implantation in the mouse. Biol Reprod. 1995;52:903-12
21. Nakashidze I, Diasamidze A Baratashvili D. et al. Alteration of Sex and Non-Sex Hormones and Distribution Features of Blood ABO System Groups among the Women with Uterine Body Tumors. Journal of Cancer Therapy. 2014;5:411-419
22. Yuzhalin AE, Kutikhin AG. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13:5091-6
23. Von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLoS Med. 2007 e296 doi: 10.1371/journal.pmed.0040296
24. Mandato VD, Formisano D, Pirillo D. et al. Province wide clinical governance network for clinical audit for quality improvement in endometrial cancer management. Int J Gynecol Cancer. 2012;22:94-100
25. Colombo N, Preti E, Landoni F. et al. ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22:vi35-9
26. Palomba S, Mandato VD, La Sala GB. New Surgical Approaches to Early-Stage Endometrial Cancer. In: (ed.) Diaz-Padilla I, Garcia-Donas J. Endometrial Cancer: A Comprehensive Clinical and Translational Update. New York: Nova Science Publishers. 2014:117-146
27. Lee JC, Chang JG. ABO genotyping by polymerase chain reaction. J Forensic Sci. 1992;37:1269-75
28. Poulter M, Kemp TJ, Carritt B. DNA-based rhesus typing: simultaneous determination of RHC and RHD status using the polymerase chain reaction. Vox Sang. 1996;70:164-8
29. Marinaccio M, Traversa A, Carioggia E. et al. Blood groups of the ABO system and survival rate in gynecologic tumors. Minerva Ginecol. 1995;47:69-76
30. Xu WH, Zheng W, Xiang YB, Shu XO. ABO blood type is associated with endometrial cancer risk in Chinese women. Chin J Cancer. 2011;30:766-71
31. Wolpin BM, Chan AT, Hartge P. et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424-431
32. Melzer D, Perry JR, Hernandez D. et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4:e1000072
33. Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247
34. Duan YF, Zhu F, Li XD. et al. Association between ABO gene polymorphism (rs505922) and cancer risk: a meta-analysis. Tumour Biol. 2015;36:5081-7
35. Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev. 2005;19:200
36. Dabelsteen E. ABO blood group antigens in oral mucosa. What is new? J Oral Pathol Med. 2002;31:65-70
37. Mandel U, Langkilde NC, Orntoft TF. et al. Expression of histo-bloodgroup-A/B-gene-defined glycosyltransferases in normal and malignant epithelia: correlation with A/B-carbohydrate expression. Int J Cancer. 1992;52:7-12
38. Gao S, Worm J, Guldberg P. et al. Genetic and epigenetic alterations of the blood group ABO gene in oral squamous cell carcinoma. Int J Cancer. 2004;109:230
39. Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci. USA. 2002;99:10231
40. Kuemmel A, Single K, Bittinger F. et al. The prognostic impact of blood group-related antigen Lewis Y and the ABH blood groups in resected non-small cell lung cancer. Tumour Biol. 2007;28:340-9
41. Ichikawa D, Handa K, Withers DA, Hakomori S. Histo-blood group A/B versus H status of human carcinoma cells as correlated with haptotactic cell motility: approach with A and B gene transfection. Cancer Res. 1997;57:3092-6
42. Kominato Y, Hata Y, Takizawa H, Tsuchiya T, Tsukada J, Yamamoto F. Expression of human histo-blood group ABO genes is dependent upon DNA methylation of the promoter region. J Biol Chem. 1999;274:37240-50
43. Kominato Y, Hata Y, Takizawa H. et al. Alternative promoter identified between a hypermethylated upstream region of repetitive elements and a CpG island in human ABO histo-blood group genes. J Biol Chem. 2002;277:37936-48
44. Skovlund VR. ABH and related histo-blood group antigens in normal & malignant human endometrium in relation to genetic and hormonal factors. APMIS. Suppl. 1997;69:1-33
45. Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clément M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9-31
46. Matise TC, Ambite JL, Buyske S. et al. The Next PAGE in understanding complex traits: design for the analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. Am J Epidemiol. 2011;174:849-59
47. Diaz-Padilla I, Amir E, Marsh S, Liu G, Mackay H. Genetic polymorphisms as predictive and prognostic biomarkers in gynecological cancers: a systematic review. Gynecol Oncol. 2012;124:354-65
Author contact
![]() Corresponding author: Vincenzo Dario Mandato, Unit of Obstetrics and Gynecology, Azienda Unità Sanitaria Locale, IRCCS, Viale Risorgimento 80, 42123 Reggio Emilia (Italy). Tel: +39 3494640813, Fax: +39 0522296015, e-mail: dariomandatocom.
Corresponding author: Vincenzo Dario Mandato, Unit of Obstetrics and Gynecology, Azienda Unità Sanitaria Locale, IRCCS, Viale Risorgimento 80, 42123 Reggio Emilia (Italy). Tel: +39 3494640813, Fax: +39 0522296015, e-mail: dariomandatocom.

 Global reach, higher impact
Global reach, higher impact