Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(15):2876-2884. doi:10.7150/jca.21064 This issue Cite
Research Paper
Mechanisms of Breast Cancer in Shift Workers: DNA Methylation in Five Core Circadian Genes in Nurses Working Night Shifts
1. Department of Chemical and Biological Work Environment, National Institute of Occupational Health, Oslo, 0363, Norway
2. Department of Occupational Medicine and Epidemiology, National Institute of Occupational Health, Oslo, 0363, Norway
3. Department of Molecular Genetics and Epigenetics, Nofer Institute of Occupational Medicine, Lodz, Poland
4. Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland
Received 2017-5-17; Accepted 2017-7-28; Published 2017-8-24
Abstract
Shift work has been suggested to be associated with breast cancer risk, and circadian disruption in shift workers is hypothesized as one of the mechanisms of increased cancer risk. There is, however, insufficient molecular evidence supporting this hypothesis. Using the quantitative methodology of pyrosequencing, epigenetic changes in 5-methyl cytosine (5mC) in five circadian genes CLOCK, BMAL1, CRY1, PER1 and PER2 in female nurses working night shift work (278 breast cancer cases, 280 controls) were analyzed. In breast cancer cases, a medium exposure to night work was associated with increased methylation levels of the CLOCK (p=0.050), BMAL1 (p=0.001) and CRY1 (p=0.040) genes, compared with controls. Within the cases, analysis of the effects of shift work on the methylation patterns showed that methylation of CRY1 was lower in those who had worked night shift and had a high exposure (p=0.006) compared with cases that had worked only days. For cases with a medium exposure to night work, an increase in BMAL1 (p=0.003) and PER1 (p=0.035) methylation was observed compared with day working (unexposed) cases. The methylation levels of the five core circadian genes were also analyzed in relation to the estrogen and progesterone receptors status of the tumors in the cases, and no correlations were observed. Furthermore, nineteen polymorphisms in the five circadian genes were assessed for their effects on the methylation levels of the respective genes, but no associations were found. In summary, our data suggest that epigenetic regulation of CLOCK, BMAL1, CRY1 and PER1 may contribute to breast cancer in shift workers.
Keywords: shift work, breast cancer, circadian, polymorphism
Introduction
Breast cancer is the second most commonly diagnosed cancer worldwide and a leading cause of cancer-related mortality among women [1]. A number of biological, lifestyle and genetic risk factors is known to increase breast cancer risk [2-4]. Occupational exposure to work environment factors such as night work, may also influence the onset and progression of breast cancer [5]. Shift work involving work at night has been classified as probably carcinogenic (group 2A) by the International Agency for Research on Cancer (IARC) [6]. This classification is based on findings from several studies showing an increased risk for cancer, particularly breast cancer, with exposure to night work [7-10]. However, a recent publication did not support this association, and concluded that “prospective evidence shows little or no effect of night work, including long-term shift work, on incidence of breast cancer” [11]. Altogether, several animal and human studies indicate that exposure to night work resulting in disruption of the circadian rhythmicity may increase breast cancer risk, as reviewed in the IARC's monograph [12]. The influence of altered circadian rhythmicity on breast cancer risk was first described in the 1960s [13]. Since then, it has been hypothesized that alterations in the circadian genes and circadian time disruption may drive breast cancer development. Although, disruption of the circadian rhythm due to exposure to artificial light at night has been suggested as a potential mechanism of cancer development [14], there is inadequate molecular biological evidence for this hypothesis.
The circadian rhythm is characterized by oscillations in protein levels and is timely controlled by a series of auto-regulatory feedback loops [15]. The major components involved in this biological clock network include the transcriptional activators; Circadian Locomotor Output Cycle Kaput (CLOCK) and Brain and Muscle Arnt-like Protein 1 (BMAL1, also known as Aryl Hydrocarbon Receptor Nuclear Translocator Like, ARNTL), and Cryptochrome (CRY1 and CRY2) and Period (PER1, PER2 and PER3) proteins, which act in a negative feedback loop [15].
Epidemiological studies have shown associations between single nucleotide polymorphisms (SNPs) in the circadian genes and female breast cancer in shift workers [16]. Circadian gene variants may lead to individual differences in susceptibility to multiple cancer pathologies in shift workers in addition to breast cancer [17]. Further, a correlation between circadian gene variants and sex hormone levels has been shown [18]. Altered expression of the circadian genes has also been observed in breast cancer cells and breast tumor tissues [19, 20]. Changes in the expression of circadian genes may be involved in increased breast cancer risk by altering the cell cycle and apoptosis, and by changing the cellular metabolism [21]. In addition to genetic polymorphisms and changes in gene expression patterns, epigenetic variations in the levels of 5-methyl cytosine (5mC) in circadian genes may affect breast cancer susceptibility in shift workers, as shift work may lead to differential methylation of these genes [22-24]. Alteration of DNA methylation patterns of 5mC is emerging as a promising epigenetic biomarker of disease, particularly cancer, and is actively studied in breast cancer [25].
Transcriptional activity of the core circadian genes may also be influenced by female hormone levels. CLOCK gene expression can interact with estrogen receptor alpha (ERα/ERS1) and estrogen (ER) enhances both CLOCK and ERα driven transcription through CLOCK-dependent sumoylation [26]. Induction of the CLOCK gene by ER signaling promotes proliferation of breast cancer cells [27]. ER also influences epigenetic changes including 5mC level where methylation of CpG sites near ERα-binding regions is lower in ERα+ breast tumors than ERα- breast tumors [28, 29]. Interestingly, shift workers have increased levels of ER and progesterone (PR), but its association to shift work is inconclusive [30].
Epigenetics, in particular 5mC in DNA, is increasingly recognized as an important mechanism in the etiology of breast cancer [31]. However, only few studies have investigated the role of 5mC in a case-control setting of breast cancer in night shift workers. To address this, methylation patterns of 5mC in promoter regions of five core circadian genes, CLOCK, BMAL1, CRY1, PER1 and PER2, and their association to breast cancer risk were investigated in female nurses working night shifts.
Materials and methods
Study design and study population
A nested case-control study was carried out on a cohort of 49,402 female nurses graduated between 1914 and 1985. The study design is shown in the flow chart in Supplementary Figure 1. Further details on data collection and recruitment of subjects have been previously described [32]. Briefly, cases were included if diagnosed with breast cancer between 1990 and 2007, aged 35-74 years at diagnosis, and alive by February 2009. Of the 1,132 cases diagnosed in 1990-2007, 943 were alive in February 2009 and included in the study. Controls were frequency matched to cases in five-year age groups by diagnostic year of the case. Only controls, which were cancer-free at and prior to the year of diagnosis of the case were included. To be included, cases and controls must have had worked as a nurse for at least one year. Altogether, 74% of cases (699/943) and 65% of controls (895/1384) were interviewed. Testing for ER and PR status was restricted to cases diagnosed during the period from 1996 through 2007. Of these cases, ER status was determined for 203 (73%) subjects, of whom 178 (88%) were ER+, and PR status was determined for 200 (72%), of whom 138 (69%) were PR-positive (PR+). Of breast cancer cases with a known joint receptor status (n = 200), 134 (67%) were ER+/PR+ tumors, 42 (21%) were ER+/PR- tumors, 4 (2%) were ER-/PR+ tumors, and 20 (10%) were ER-/PR- tumors. Informed written consent was obtained from all study participants. The study was approved by the Regional Committee for Medical and Health Research Ethics, South-East region (S-08430a, 2008/10453).
Assessment of night work
For each job, information on job duration, workplace, proportion of fulltime work and work schedules (only day, only night, or both day and night shifts) were collected. Working periods including either rotating or permanent night shift were cumulated for each particular schedule. Night work included working periods from both rotating and permanent night schedules. Night shift was defined as a shift including work between 12 pm and 6 am. For each working period including night shift, information on number of consecutive night shifts (intensity) was obtained. For more detailed information of the exposure assessments reader is referred to our previous publication by Lie et al. [32]. In the current study, we focused on the combination of duration and intensity of night work. Exposure metrics for analysis of the combined effects of duration and intensity of night works included work for a minimum of three consecutive night shifts for less or more than five years. The workers were grouped into five groups based on their night work exposure patterns: no exposure (day workers, never), exposure (night shift workers, ever), low exposure (never ≥ three consecutive night shifts), medium exposure (≥ three consecutive night shifts < five years) and high exposure (≥ three consecutive night shifts ≥ five years).
Saliva samples, DNA extraction and pyrosequencing
Together with the information letter, a request for saliva samples using Oragene saliva sampling protocol (DNA Genotek Inc, Ottawa, Ontario, Canada) was sent out to all the cases and controls. A total of 563 cases (81%) and 619 controls (69%) returned a saliva sample. Briefly, 1 ml of saliva was transferred to an Eppendorf tube, and reagents provided by the supplier were added. DNA was successfully extracted from all saliva samples using Oragene DNA isolation kit as described by the manufacturer (DNA Genotek Inc.).
For the epigenetic analysis of 5mC, a random subset of cases (n=278) and controls (n=280) was included. The cases and controls were matched according to the work schedules across the different night work exposure groups (no night work, low, mid, and high exposure to night work). DNA samples were bisulfite treated using EpiTect Fast Bisulfite Conversion kit (Qiagen, Hilden, Germany) and PCR amplified using unbiased nested primers and the PyroMark PCR kit (Qiagen) according to the manufacturers' instructions. Five core circadian genes CLOCK, BMAL1, CRY1, PER1 and PER2 were selected in order to include both positive (CLOCK-BMAL protein complex) and negative regulators (CRY and PER proteins) of the circadian rhythm pathway. Pyrosequencing primers were designed using the PyroMark Assay Design software (Qiagen). The analysis focused on the proximal promoter region of each gene with emphasis on the functionality of the region based on information on transcription factor binding sites available from ENCODE project (www.encode.org) and the TRANFAC database (http://gene-regulation.com/pub/databases.html). For each gene, only one CpG target site containing 4 to 12 CpG dinucleotides was selected. The target CpG site for each of the genes was selected based on the putative transcription factor binding information available on the ENCODE project website. Additionally, the transcription factor binding analysis software PROMO (using TRANSFAC database) was used to investigate the strength of binding of each transcription factor, Supplementary Figure 2 [33]. Pyrosequencing was performed by Pyromark Q24 Advanced technology (Qiagen). Pyrosequencing primer sequences are shown in the Supplementary Table 1. The percentage methylation for each of the target CpG sites for each of the respective genes was calculated using the PyroMark CpG Software (Qiagen). A methylation index (MI) was calculated as the mean percentage of methylation across all CpG dinucleotides analyzed per CpG target site, varying between 4 to 12 CpG dinucleotides present on the respective gene.
Genotyping of single nucleotide polymorphisms
Nineteen SNPs (Supplementary Table 2) in the five circadian genes were genotyped using a custom made SNP microarray as described previously [34]. Briefly, the strategy for selection of the SNPs was based primarily on a candidate gene approach using information from a search of relevant studies. Polymorphisms were included using one or more of the following criteria: assumed functionality (located in the regulatory regions, for example, 3'-UTR, 5'-UTR or amino acid change), cancer genetic marker of susceptibility for breast cancer in epidemiological studies, candidate gene or genome-wide association studies (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?study_id=phs000147.v3.p1&phd=1395), minor allele frequency (MAF) ≥ 5%. The genotyping call rates were similar in cases and controls and were at least 80% for cases and 82% for controls. All SNPs were in Hardy-Weinberg equilibrium. Genotyping was performed by MassArray iPLEX technology (Sequenom Inc, San Diego, CA, USA) at the Centre for Integrative Genetics (CIGENE) genotyping core facility at the Norwegian University of Life Sciences, Ås, Norway.
Statistical analyses
Characteristics of the study subjects were assessed by Chi-square or Mann-Whitney U-test as appropriate in IBM SPSS software version 23.0. Differences in MI between cases and controls, and between different exposure categories were analyzed for each gene. Linear mixed models were analyzed in R, using the lme function. Associations between MI of the circadian genes and the ER or PR status of tumors from the patients were analyzed using the same linear mixed model. A linear mixed model was also applied to the statistical analysis of the association between SNPs and MI of the genes. A genotype model was used to analyze the effect of SNPs on MI. The genotypes were categorized as 0 = common homozygous genotype (reference), 1 = heterozygote genotype, and 2 = rare homozygous genotype. In all analyses, the list of potential confounders tested included: alcohol consumption, parity, duration of daily occupational exposure to x-rays, hormonal treatment last two years before diagnosis, age at saliva test, years since cancer diagnosis, and occurrence of familiar breast cancer. All possible combinations of adjustment variables were compared and the combination that minimized the AIC was chosen. The final correction variables are listed in the footnote of each table. In each mixed model crossed random intercepts were included for subject and CpG island to take into account the repeated observations for the CpG islands. P ≤ 0.05 was considered significant.
Results
The characteristics of the study subjects included in the study and the exposure metrics of night shift work are shown in Table 1. Cases and controls were not significantly different except for occurrence of breast cancer in first-degree family, which was significantly higher in cases (26%) compared to controls (11%, P=0.008). Alcohol consumption was also higher in cases (9%) than in controls (4.5%), and the number of children differed between controls (max 9 children) and cases (max 5 children). For night shift exposure, cases and controls were grouped into four exposure groups; never (only day workers = 0 night shift work), low (never ≥ 3 consecutive night shifts), medium (3 or more consecutive night shifts < 5 years) and high (3 or more consecutive night shifts ≥ 5 years) as shown in Table 1.
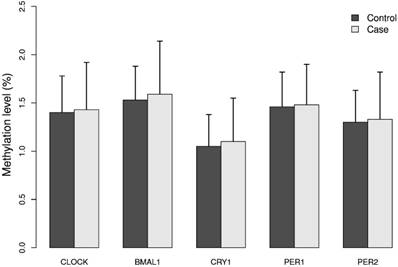
The mean methylation levels for each of the five genes analyzed in DNA from breast cancer cases and controls were low (approximately 2%), Figure 1. The promoter of the BMAL1 gene had the highest mean methylation level and the CRY1 gene the lowest level. The calculated MI did not differ significantly between cases and controls, Figure 1.
Characteristics of the study subjects and night work exposure parameters.
| Characteristics | Controls (N=280) | Cases (N=278) | P |
|---|---|---|---|
| Age (years), mean ± SD (min-max) | 54.34 ± 7.69 (36-74) | 54.46 ± 7.56 (36-74) | 0.924b |
| No. of children (min-max) | 0-9 | 0-5 | 0.055b |
| Age at first birth (years), mean ± SD (min-max) | 26.78 ± 4.17 (19-41) | 26.69 ± 4.18 (18-43) | 0.758b |
| Breast cancer in first-degree family (yes/no) | 28/253 | 49/227 | 0.008c |
| Alcohol consumption ≥twice/week (yes/no) | 12/269 | 23/256 | 0.052c |
| Daily exposure to x-rays (yes/no) | 51/230 | 57/222 | 0.494c |
| Hormone therapy in the past 2 years (yes/no) | 59/216 | 70/204 | 0.526c |
| Night shift exposure metrics | |||
| Never night work | 71 | 70 | |
| Ever night work | 210 | 209 | |
| Never ≥ 3 consecutive night shifts (low exposure group) | 21 | 28 | |
| 3 or more consecutive night shifts < 5 years (medium exposure group) | 49 | 41 | |
| 3 or more consecutive night shifts ≥ 5 years (high exposure group) | 140 | 140 | |
| Receptor status | |||
| ER+ | 178/203 | ||
| PR+ | 138/200 |
aAge at diagnosis (cases) and age at selection of reference (controls). bDerived from Mann-Whitney U-test (two-sided). cDerived from Pearsons Chi-square test (two-sided). dBreast cancer in mother or sister. eHormone replacement therapy in postmenopausal women.
Methylation index (MI) of the five circadian genes in cases and controls. Levels of 5mC of the five circadian genes CLOCK, BMAL1, CRY1, PER1 and PER2 was analyzed in breast cancer cases and matched controls by pyrosequencing. For each gene one CpG site containing 4 - 12 CpG dinucleotides was selected. Methylation index (MI) was calculated as the mean percentage of methylation across all CpG dinucleotides for each CpG site. Results represent mean methylation ± SD.
When assessing the effects of MI on cancer-status in individual shift work groups, an increased MI for CRY1 gene was observed in breast cancer cases that had worked only day shifts (E: 0.18, 95% CI: 0.04 - 0.31, p=0.009), and in cases that had had a medium exposure to night work (E: 0.17, 95% CI: 0.00 - 0.33, p=0.044) compared with controls with the same shift work schedules, Table 2. Similarly, cases with medium exposure to night work also had an increased MI of the CLOCK gene (E: 0.18, 95 % CI: 0.00 - 0.36, p=0.050) and BMAL1 gene (E: 0.33, 95 % CI: 0.14 - 0.52, p=0.001) compared with controls with the same work schedules.
Analysis of the effects of shift work on the methylation patterns in cases showed that MI of CRY1 was lower in those who had worked night shift ever (E: -0.11, 95% CI: -0.22 - -0.01, p=0.040), those that had been highly exposed (E: -0.16, 95% CI: -0.27 - -0.05, p=0.006) as well as those with a low exposure to night work (E: -0.19, 95% CI: -0.37 - -0.02, p=0.033), compared with cases that had worked only days, Table 3. For cases with a medium exposure to work at night an increase in MI for BMAL1 (E: 0.27, 95% CI: 0.09 - 0.45, p=0.003) and PER1 (E: 0.16, 95% CI: 0.01 - 0.31, p=0.035) was observed, compared with unexposed cases.
MI was also analyzed in regard to ER and PR receptor status of breast cancer cases. No association between MI of the circadian genes and the ER or PR status of the patients were observed, Table 4. We also investigated associations between nineteen SNPs in the five circadian genes and the MI of the genes, however, no associations between MI and SNPs in the genes were found, Table 5. The location of the SNPs, base change, minor allele frequency, Hardy-Weinberg equilibrium and the call rates of genotyping in cases and controls for each SNP are shown in Supplementary Table 2.
Differences in methylation index (MI) of the genes between cases and controls.
| Night work exposure | CLOCK | BMAL1 | CRY1 | PER1 | PER2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95 %CI) | Pa | Estimate (95 %CI) | Pa | Estimate (95 %CI) | Pa | Estimate (95 %CI) | Pa | Estimate (95 %CI) | Pa | |
| Neverb | 0.10 (-0.05 - 0.24) | 0.187 | 0.03 (-0.12 - 0.18) | 0.724 | 0.18 (0.04 - 0.31) | 0.009 | 0.13 (-0.02 - 0.28) | 0.090 | 0.04 (-0.10 - 0.18) | 0.553 |
| Everc | 0.01 (-0.07 - 0.10) | 0.733 | 0.07 (-0.01 - 0.16) | 0.101 | 0.00 (-0.07 - 0.10) | 0.962 | 0.07 (-0.04 - 0.18) | 0.118 | 0.02 (-0.06 - 0.10) | 0.645 |
| Lowb | 0.04 (-0.21 - 0.29) | 0.747 | 0.06 (-0.2 - 0.32) | 0.660 | -0.08 (-0.30 - 0.15) | 0.502 | 0.04 (-0.19 - 0.27) | 0.739 | 0.18 (-0.06 - 0.42) | 0.136 |
| Mediumb | 0.18 (0.00 - 0.36) | 0.050 | 0.33 (0.14 - 0.52) | 0.001 | 0.17 (0.00 - 0.33) | 0.044 | 0.23 (0.05 - 0.41) | 0.013 | 0.14 (-0.04 - 0.31) | 0.117 |
| Highb | -0.04 (-0.14 - 0.06) | 0.433 | 0.01 (-0.1 - 0.11) | 0.923 | -0.02 (-0.12 - 0.07) | 0.611 | 0.05 (-0.07 - 0.17) | 0.433 | -0.04 (-0.14 - 0.06) | 0.386 |
aDifferences in MI between cases and controls for each shift variable were analyzed using a linear mixed model with a random intercept to take into account the repeated observations for the CpG sites. Controls were used as reference. Adjustment variables were selected based on the AIC criterion. Adjustments were made for b alcohol (BMAL1), familiar breast cancer (CRY1), years since cancer and alcohol (PER1); and for c alcohol (BMAL1), familiar breast cancer and alcohol (CRY1), years since cancer and alcohol (PER1).
Effects of night work on DNA methylation patterns.
| Night work exposure | CLOCK | BMAL1 | CRY1 | PER1 | PER2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Pa | Estimate | Pa | Estimate | Pa | Estimate | Pa | Estimate | Pa | ||
| Cases | Everb | -0.04 (-0.16 - 0.08) | 0.520 | 0.06 (-0.06 - 0.19) | 0.320 | -0.11 (-0.22 - -0.01) | 0.040 | -0.01 (-0.12 - 0.10) | 0.849 | 0.00 (-0.11 - 0.12) | 0.985 |
| Lowc | -0.04 (-0.24 - 0.16) | 0.697 | 0.11 (-0.10 - 0.31) | 0.307 | -0.19 (-0.37 -- 0.02) | 0.033 | -0.10 (-0.27 - 0.07) | 0.234 | 0.07 (-0.12 - 0.25) | 0.470 | |
| Mediumc | 0.08 (-0.08 - 0.25) | 0.325 | 0.27 (0.09 - 0.45) | 0.003 | 0.07 (-0.08 - 0.22) | 0.352 | 0.16 (0.01 - 0.31) | 0.035 | 0.13 (-0.03 -0.29) | 0.113 | |
| Highc | -0.08 (-0.20 - 0.05) | 0.240 | -0.02 (-0.15 - 0.12) | 0.804 | -0.16 (-0.27 - -0.05) | 0.006 | -0.04 (-0.15 - 0.07) | 0.448 | -0.05 (-0.17 - 0.07) | 0.411 | |
| Controls | Everb | 0.04 (-0.08 - 0.16) | 0.466 | 0.01 (-0.11 - 0.14) | 0.857 | 0.06 (-0.05 - 0.16) | 0.295 | 0.04 (-0.07 - 0.15) | 0.459 | 0.02 (-0.09 - 0.14) | 0.678 |
| Lowc | 0.02 (-0.20 - 0.23) | 0.874 | 0.08 (-0.15 - 0.30) | 0.508 | 0.06 (-0.13 - 0.25) | 0.529 | -0.01 (-0.20 - 0.18) | 0.905 | -0.07 (-0.28 - 0.13) | 0.499 | |
| Mediumc | 0.00 (-0.16 - 0.16) | 0.993 | -0.03 (-0.20 - 0.14) | 0.732 | 0.08 (-0.07 - 0.22) | 0.288 | 0.06 (-0.08 - 0.20) | 0.406 | 0.03 (-0.12 - 0.19) | 0.667 | |
| Highc | 0.06 (-0.06 - 0.19) | 0.323 | 0.01 (-0.13 - 0.14) | 0.936 | 0.04 (-0.07 - 0.15) | 0.484 | 0.04 (-0.07 - 0.15) | 0.485 | 0.04 (-0.09 -0.16) | 0.568 | |
aEffects of night work on MI in cases and controls were analyzed using a linear mixed model with a random intercept to take into account the repeated observations for the CpG sites. Cases and controls working only days (Never) were used as reference. Adjustment variables were selected based on the AIC criterion. Adjustments were made for b alcohol (BMAL1), familiar breast cancer (CRY1), years since cancer and alcohol (PER1); and for c alcohol (BMAL1), familiar breast cancer and alcohol (CRY1), years since cancer and alcohol (PER1).
Analysis of the effect of estrogen and progesterone receptors status on the methylation of the circadian genes in breast cancer cases.
| Estrogen receptor status | Progesterone receptor status | |||
|---|---|---|---|---|
| Gene | Estimate | Pa | Estimate | Pa |
| CLOCK | 0.07 (-0.10 - 0.25) | 0.411 | 0.06 (-0.07 - 0.18) | 0.380 |
| BMAL1 | 0.04 (-0.31 - 0.22) | 0.631 | -0.03 (-0.15 - 0.10) | 0.670 |
| CRY1 | 0.04 (-0.11 - 0.18) | 0.606 | 0.08 (-0.02 - 0.18) | 0.134 |
| PER1 | 0.06 (-0.08 - 0.20) | 0.374 | 0.04 (-0.06 - 0.14) | 0.453 |
| PER2 | -0.14 (-0.29 - 0.01) | 0.066 | -0.07 (-0.17 - 0.04) | 0.242 |
aEstimated of effects of estrogen and progesterone receptor status on MI in cases were analyzed using a linear mixed model with crossed random intercepts for subjects and CpG islands. Cases and controls working only days (Never) were used as reference. Adjustment variables were selected based on the AIC criterion as described in materials and methods. For estrogen receptor status the following adjustments were made: years since cancer and hormonal treatment (BMAL1); number of children (CLOCK); number of children (CRY1); familiar breast cancer (PER1); For progesterone receptor status: years since cancer and hormonal treatment (BMAL1); number of children (CLOCK); number of children (CRY1); alcohol(PER1). The analyses for PER2 were unadjusted.
Effect of SNP genotype on the methylation of genes.
| Gene | SNP ID | Heterozygote (genotype 1) | Homozygote rare allele (genotype 2) | ||
|---|---|---|---|---|---|
| Estimate | Pa | Estimate | Pa | ||
| CLOCK | rs1048004 | 0.06 (-0.02 - 0.13) | 0.155 | 0.03 (-0.13 - 0.18) | 0.739 |
| rs11133373 | 0.05 (-0.03 - 0.13) | 0.222 | 0.05 (-0.08 - 0.17) | 0.475 | |
| rs11133376 | -0.05 (-0.13 - 0.03) | 0.208 | -0.08 (-0.19 - 0.03) | 0.171 | |
| rs13102385 | -0.03 (-0.11 - 0.05) | 0.500 | -0.09 (-0.21 - 0.03) | 0.133 | |
| rs17776421 | -0.06 (-0.14 - 0.02) | 0.170 | -0.06 (-0.17 - 0.05) | 0.304 | |
| rs1801260 | 0.05 (-0.03 - 0.13) | 0.184 | 0.04 (-0.11 - 0.19) | 0.597 | |
| rs3749474 | 0.05 (-0.04 - 0.13) | 0.277 | 0.00 (-0.12 - 0.12) | 0.986 | |
| rs7698022 | 0.05 (-0.03 - 0.13) | 0.182 | 0.03 (-0.13 - 0.19) | 0.715 | |
| BMAL1 | rs2278749 | -0.03 (-0.12 - 0.06) | 0.536 | -0.11 (-0.32 - 0.10) | 0.303 |
| rs2290035 | -0.03 (-0.12 - 0.06) | 0.536 | -0.06 (-0.17 - 0.06) | 0.346 | |
| rs7126303 | -0.06 (-0.14 - 0.03) | 0.227 | -0.07 (-0.20 - 0.05) | 0.243 | |
| rs969485 | -0.04 (-0.13 - 0.05) | 0.368 | 0.00 (-0.17 - 0.18) | 0.964 | |
| CRY1 | rs12315175 | -0.03 (-0.10 - 0.04) | 0.387 | 0.06 (-0.12 - 0.25) | 0.513 |
| rs3809235 | -0.01 (-0.09 - 0.07) | 0.756 | -0.03 (-0.14 - 0.07) | 0.537 | |
| PER1 | rs2253820 | 0.02 (-0.06 - 0.10) | 0.668 | -0.04 (-0.26 - 0.18) | 0.734 |
| rs2289591 | 0.04 (-0.03 - 0.11) | 0.223 | 0.01 (-0.14 - 0.14) | 0.945 | |
| rs885747 | 0.00 (-0.07 - 0.07) | 0.971 | 0.03 (-0.07 - 0.12) | 0.608 | |
| PER2 | rs11695472 | 0.04 (-0.03 - 0.12) | 0.272 | -0.07 (-0.20 - 0.07) | 0.334 |
| rs7602358 | -0.04 (-0.12 - 0.04) | 0.374 | -0.10 (-0.30 - 0.10) | 0.317 | |
aAnalyses were performed using a genotype model categorizing genotypes into three categories 0, 1, 2 where ref = genotype 0 (homozygote common genotype), 1 = heterozygote genotype and 2 = homozygote rare genotype. Adjustment variables were selected based on the AIC criterion as described in materials and methods. For rs13102385, rs11133376 and rs104800 (CLOCK), adjustments were made for age at saliva test. For rs2289591, rs885747 (PER1), adjustments were made for alcohol. For rs2290035, rs2278749, rs7126303 and rs969485 (BMAL1), adjustments were made for alcohol. For rs3809235 (CRY1) adjustments were made for years since cancer and familiar breast cancer. All other analyses were unadjusted.
Discussion
The present study is novel in the sense that it uses a relatively well-matched group of breast cancer cases and controls with night work exposure to investigate 5mC methylation levels at specific and biologically anticipated functional CpG sites in five core circadian genes. The selected five core circadian genes represent both positive (CLOCK and BMAL transcriptions factors) and negative regulators (CRY and PER proteins) of the circadian rhythm pathway. CLOCK and BMAL transcription factors represent the heterodimer that positively stimulates expression of the other CLOCK and CLOCK-controlled genes, whereas, CRY and PER represent the negative inhibitory complex that blocks the CLOCK-BMAL protein complex. Our analyses focused on the promoter region, especially the proximal promoter region, which is most important in regard to the regulation of gene expression through hypermethylation or hypomethylation of the local CpG sequences [35]. The CpG sequences in the promoter region may play an important role in altered gene transcription or gene silencing in many cancers including breast cancer [36].
We found overall low levels of 5mC in all genes and hypomethylation of CRY1 in night shift workers. Lower gene methylation has been previously reported in long term shift workers compared to the day workers, where a significantly lower methylation in shift workers versus day workers of DLX5, IGF2AS genes, and miRNAs 219 and 34b was observed [37-38]. Bhatti et al. performed genome wide methylation analyses, which included CpGs in both promoters and gene bodies, in 65 day workers and 59 current night shift workers [39]. They found a decreased average methylation of each of the loci, gene, CpG island or gene region, in the group of night shift workers compared to day shift workers. The 12 investigated circadian genes, including CRY1, were significantly hypomethylated in night shift workers compared to day workers and the 21 loci located in the circadian genes were found to be significantly hypomethylated in night shift workers [39]. A total of nine significant loci were found in the CSNK1E gene, most of which were located in a CpG island and near the transcription start site of the gene. Interestingly, the three loci located in gene body of the PER3 gene showed larger differences. Nevertheless, in agreement with the data presented for healthy controls in our study, they found no difference between night shift workers and day shift workers [39]. The results obtained in our study for women with breast cancer (significant for CRY and BMAL1) may suggest susceptibility of this group of subjects to night work exposure. However, given the novelty of the finding, relatively small number of subjects, limited power, and some inconsistency of the results i.e. (null finding for the medium exposure group for CRY1 and for high exposure in the BMAL1 methylation analysis) future studies are warranted to confirm these results. It should be noted that two independent studies have found an association of hypermethylation of the CLOCK gene in shift workers with reduced breast cancer. This is contradicting our results which indicate a slightly higher methylation of CLOCK in breast cancer cases compared to healthy controls [23, 24].
Studies have shown a correlation between circadian genes and the female hormones [40, 41], making the analysis of effects of ER and PR status on the MI of circadian genes in breast cancer cases interesting. ER is known to influence the epigenetic changes including 5mC methylation levels where methylation of CpG sites near ERα-binding regions tended to be lower in ERα+ breast tumors than ERα- breast tumors [29, 42]. Our data did not support such a role of ER on 5mC levels in the circadian genes investigated. Furthermore, the CLOCK and ER gene expressions may influence each other. However, this regulation may not be through CpG methylation but rather through other mechanisms such as CLOCK-dependent sumoylation [26]. So far, most of the attention has been on the ER status and no data are available on the relationship between DNA methylation and PR status. In a recent study, we found significant associations between breast cancer risk and long night work durations, with the highest risk observed for PR+ tumors [43]. Although, this observation may suggest that progesterone could play an important role in the detrimental effects of night work on breast cancer risk, no effects of PR status on the MI in circadian genes were observed in the present study.
We have previously reported that SNPs in the circadian genes may affect breast cancer risk among nurses who had worked at least three consecutive night shifts [34]. Specifically, noteworthy associations were found between night work, breast cancer and SNPs in BMAL1 and CLOCK genes. We therefore hypothesized that SNPs in these genes may also affect the methylation levels, particularly if they occur at or within the CpG dinucleotides. However, no significant associations between the analyzed SNPs and MI of the genes were observed in subjects working shift work. It has also been previously reported that healthy nurses and midwives who had worked either day or rotating night shifts had no differences in BMAL1, CLOCK, CRY1, CRY2 gene expression levels in peripheral blood leukocytes [44].
This study is strengthened as it includes breast cancer cases and healthy subjects with well-matched and characterized exposure to night shift work. The cases and controls were very similar with respect to night exposure parameters, and the only significant differences observed were for well-known risk factors such as breast cancer in mother or sister. Moreover, DNA sequencing (pyrosequencing), which is the gold standard for DNA methylation analysis was used. This approach enabled us to perform site-specific analysis of the CpG dinucleotides in the promoter region of each of the investigated genes. In contrast to other non-specific DNA methylation analyses that perform global but random genome methylation, our targeted and site specific approach enabled us to choose a biologically putative functional and relevant promoter region by using the information for binding motifs of transcription factors available in the ENCODE and TRANSFAC databases [45, 46]. Furthermore, pyrosequencing facilitated the quantitative analysis of 5mC levels at each individual CpG dinucleotide [47]. This approach allowed us to calculate an average of methylation level (methylation index) for the target CpG region based on the methylation level for each single methylated 5mC at each individual CpG dinucleotide.
There are, however, some limitations and weaknesses with this study that need to be taken into consideration upon interpretation of the findings. Transcriptional regulation is a complex process, limited not only to cis-acting elements but also to trans-acting elements such as enhancers, silencers, insulators and locus specific regions, and may involve several regions and CpG islands [48] not only in the promoter regions but also other sites in the gene bodies [39]. Furthermore, the results obtained in this study, such as hypermethylation of CLOCK gene in breast cancer cases, may not be comparable with previous studies due to various reasons including differences in study population, methodology and DNA source. Moreover, a generally low MI was found in both cases and controls. This could be due to tissue specificity of methylation in DNA from saliva. While sampling of DNA from saliva is a non-invasive method that may increase participation rate of subjects in epidemiological studies, it might have limitations for 5mC analysis. The study is also limited in that the number of cases and controls that had only worked day shifts (the reference group) was relatively low, thus the findings should be interpreted with caution and preferably be replicated in larger studies. This is particularly true for the analyses of ER and PR status which did not show any association with the MI of the breast cancer cases. This may indicate a passive role for ER or PR proteins in regulation of CpG methylation levels in the CpG sites investigated and could indicate that ER or PR may affect breast cancer risk in shift workers through mechanisms other than the regulation of 5mC levels. Furthermore, the applied night work exposure metrics are different from those used in other studies; therefore, the results may not be comparable. Finally, a limited number of genes and polymorphisms were selected in this study, and, to cover all genes and SNPs in the core circadian signaling pathway, a much larger study (e.g. GWAS/EWAS) is warranted.
Altogether, this study suggests that epigenetic regulation of BMAL1, CLOCK and CRY1 may contribute to increased breast cancer risk in shift workers. However, functional studies are warranted to investigate the correlation between 5mC levels and the expression of the circadian genes.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We thank Mrs. Kristine Haugen Anmarkrud for excellent technical assistance in isolation and organization of DNA samples. We are also grateful to the nurses participating in the project.
Funding
This project was supported by the Norway Grants, under the Polish-Norwegian Research Program (Grant no. Pol-Nor/196940/22/2013-clock shift). JSE acknowledges a postdoctoral fellowship from NIOH.
Ethics approval and consent to participate
Both cases and controls gave full informed written consent that their information could be used and published for research purposes, given that their personal details would remain anonymous. The Data Inspectorate and the Ethical Committee for Medical Research (REK-Sør) have evaluated and approved (project No. 6.2008/918) to conduct the study and establishment of a biobank (registration No. 2361, FHI).
Authors' contributions
JSE was involved in the planning and execution of the study, interpreted the results and drafted the initial version of the manuscript. HØN analyzed CpG data and performed pyrosequencing. ØS performed the statistical analysis. JAL established the case-control study and participated in the recruitment of subjects and establishment of the biobank, evaluated exposure metrics and contributed in writing the manuscript. MPØ was involved in pyrosequencing, analysis and interpretation of the results. ER and BP were involved in the study design and helped in drafting the manuscript. SZ planned the study and was a major contributor in drafting and finalizing the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11 (www.iarc.org). Lyon, France: International Agency for Research on Cancer. 2013
2. Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM. et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2016;103:454-64
3. Kar SP, Beesley J, Amin Al Olama A, Michailidou K, Tyrer J, Kote-Jarai Z. et al. Genome-Wide Meta-Analyses of Breast, Ovarian, and Prostate Cancer Association Studies Identify Multiple New Susceptibility Loci Shared by at Least Two Cancer Types. Cancer Discov. 2016;6:1052-67
4. Siddiq A, Couch FJ, Chen GK, Lindstrom S, Eccles D, Millikan RC. et al. A meta-analysis of genome-wide association studies of breast cancer identifies two novel susceptibility loci at 6q14 and 20q11. Hum Mol Genet. 2012;21:5373-84
5. Blakeman V, Williams JL, Meng QJ, Streuli CH. Circadian clocks and breast cancer. Breast Cancer Res. 2016;18:89
6. Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ. et al. Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154-62
7. Gyarmati G, Turner MC, Castano-Vinyals G, Espinosa A, Papantoniou K, Alguacil J. et al. Night shift work and stomach cancer risk in the MCC-Spain study. Occup Environ Med. 2016;73:520-7
8. Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Ardanaz E. et al. Breast cancer risk and night shift work in a case-control study in a Spanish population. Eur J Epidemio. 2016;31:867-78
9. Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Burgos J. et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137:1147-57
10. Papantoniou K, Castano-Vinyals G, Espinosa A, Turner MC, Alonso-Aguado MH, Martin V. et al. Shift work and colorectal cancer risk in the MCC-Spain case-control study. Scand J Work Environ Health. 2017;43:250-259
11. Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS. et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. J Natl Cancer Inst. 2016;108:djw169
12. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V. et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065-6
13. Hamilton T. Influence of environmental light and melatonin upon mammary tumour induction. Br J Surg. 1969;56:764-6
14. Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-8
15. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935-41
16. Reszka E, Przybek M, Muurlink O, Peplonska B. Circadian gene variants and breast cancer. Cancer Lett. 2017;390:137-45
17. Dai H, Zhang L, Cao M, Song F, Zheng H, Zhu X. et al. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res and treat. 2011;127:531-40
18. Chu LW, Zhu Y, Yu K, Zheng T, Chokkalingam AP, Stanczyk FZ. et al. Correlation between circadian gene variants and serum levels of sex steroids and insulin-like growth factor-I. Cancer Epidemiol Biomar Prev. 2008;17:3268-73
19. Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241-6
20. Reszka E, Przybek M. Circadian Genes in Breast Cancer. Adv Clin Chem. 2016;75:53-70
21. Kochan DZ, Ilnytskyy Y, Golubov A, Deibel SH, McDonald RJ, Kovalchuk O. Circadian-disruption-induced gene expression changes in rodent mammary tissues. Oncoscience. 2016;3:58-70
22. Fu A, Leaderer D, Zheng T, Hoffman AE, Stevens RG, Zhu Y. Genetic and epigenetic associations of circadian gene TIMELESS and breast cancer risk. Mol Carcinog. 2012;51:923-9
23. Hoffman AE, Yi CH, Zheng T, Stevens RG, Leaderer D, Zhang Y. et al. CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 2010;70:1459-68
24. Zhu Y, Stevens RG, Hoffman AE, Tjonneland A, Vogel UB, Zheng T. et al. Epigenetic impact of long-term shiftwork: pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol Int. 2011;28:852-61
25. How Kit A, Nielsen HM, Tost J. DNA methylation based biomarkers: practical considerations and applications. Biochimie. 2012;94:2314-37
26. Li S, Wang M, Ao X, Chang AK, Yang C, Zhao F. et al. CLOCK is a substrate of SUMO and sumoylation of CLOCK upregulates the transcriptional activity of estrogen receptor-alpha. Oncogene. 2013;32:4883-91
27. Xiao Z, Xue J, Sowin TJ, Zhang H. Differential roles of checkpoint kinase 1, checkpoint kinase 2, and mitogen-activated protein kinase-activated protein kinase 2 in mediating DNA damage-induced cell cycle arrest: implications for cancer therapy. Mol Cancer Ther. 2006;5:1935-43
28. Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL. et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer research. 2006;66:11954-66
29. Stone A, Valdes-Mora F, Gee JM, Farrow L, McClelland RA, Fiegl H. et al. Tamoxifen-induced epigenetic silencing of oestrogen-regulated genes in anti-hormone resistant breast cancer. PLoS One. 2012;7:e40466
30. Langley AR, Graham CH, Grundy AL, Tranmer JE, Richardson H, Aronson KJ. A cross-sectional study of breast cancer biomarkers among shift working nurses. BMJ open. 2012;2:e000532
31. Masri S, Orozco-Solis R, Aguilar-Arnal L, Cervantes M, Sassone-Corsi P. Coupling circadian rhythms of metabolism and chromatin remodelling. Diab Obes Metab. 2015;17(Suppl 1):17-22
32. Lie J-AS, Kjuus H, Zienolddiny S, Haugen A, Stevens RG, Kjærheim K. Night Work and Breast Cancer Risk Among Norwegian Nurses: Assessment by Different Exposure Metrics. Am J Epidemiol. 2011;173:1272-9
33. Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333-4
34. Zienolddiny S, Haugen A, Lie JA, Kjuus H, Anmarkrud KH, Kjaerheim K. Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res. 2013;15:R53
35. Esteller M. Epigenetics in cancer. The New England journal of medicine. 2008;358:1148-59
36. Klajic J, Fleischer T, Dejeux E, Edvardsen H, Warnberg F, Bukholm I. et al. Quantitative DNA methylation analyses reveal stage dependent DNA methylation and association to clinico-pathological factors in breast tumors. BMC Cancer. 2013;13:456
37. Jacobs DI, Hansen J, Fu A, Stevens RG, Tjonneland A, Vogel UB. et al. Methylation alterations at imprinted genes detected among long-term shiftworkers. Environ Mol Mutagen. 2013;54:141-6
38. Liu R, Jacobs DI, Hansen J, Fu A, Stevens RG, Zhu Y. Aberrant methylation of miR-34b is associated with long-term shiftwork: a potential mechanism for increased breast cancer susceptibility. Cancer Causes Control. 2015;26:171-8
39. Bhatti P, Zhang Y, Song X, Makar KW, Sather CL, Kelsey KT. et al. Nightshift work and genome-wide DNA methylation. Chronobiol Int. 2015;32:103-12
40. Dufour CR, Levasseur MP, Pham NH, Eichner LJ, Wilson BJ, Charest-Marcotte A. et al. Genomic convergence among ERRalpha, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS genetics. 2011;7:e1002143
41. Giguere V, Dufour CR, Eichner LJ, Deblois G, Cermakian N. Estrogen-related receptor alpha, the molecular clock, and transcriptional control of metabolic outputs. Cold Spring Harbor Symp Quant Biol. 2011;76:57-61
42. Fan M, Rickert EL, Chen L, Aftab SA, Nephew KP, Weatherman RV. Characterization of molecular and structural determinants of selective estrogen receptor downregulators. Breast cancer Res Treat. 2007;103:37-44
43. Lie JA, Kjuus H, Zienolddiny S, Haugen A, Kjaerheim K. Breast cancer among nurses: is the intensity of night work related to hormone receptor status? Am J Epidemiol. 2013;178:110-7
44. Reszka E, Peplonska B, Wieczorek E, Sobala W, Bukowska A, Gromadzinska J. et al. Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses. Scand J Work Environ Health. 2013;39:187-94
45. Oei SL, Babich VS, Kazakov VI, Usmanova NM, Kropotov AV, Tomilin NV. Clusters of regulatory signals for RNA polymerase II transcription associated with Alu family repeats and CpG islands in human promoters. Genomics. 2004;83:873-82
46. Weinstock GM. ENCODE: more genomic empowerment. Genome research. 2007;17:667-8
47. Harrington CT, Lin EI, Olson MT, Eshleman JR. Fundamentals of pyrosequencing. Arch Pathol Lab Med. 2013;137:1296-303
48. Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Ann Rev Genom Hum Genet. 2006;7:29-59
Author contact
![]() Corresponding author: S. Zienolddiny: Tel: +47 2319 5284; Email: shan.zienolddinyno
Corresponding author: S. Zienolddiny: Tel: +47 2319 5284; Email: shan.zienolddinyno

 Global reach, higher impact
Global reach, higher impact