3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(16):3190-3197. doi:10.7150/jca.20172 This issue Cite
Research Paper
Correlation of cancer stem cell markers and immune cell markers in resected non-small cell lung cancer
1. Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, China;
2. Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academic of Medical Science, Jinan, Shandong, China;
3. Department of Pathology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academic of Medical Science, Jinan, China;
4. Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, China.
Received 2017-3-20; Accepted 2017-8-30; Published 2017-9-15
Abstract
Background: Recent studies confirmed that immunotherapy showed prominent efficacy in non-small cell lung cancer (NSCLC). Cancer stem cells/cancer initiating cells are resistant to anticancer treatment. The purpose of the study was to analyze the correlation of cancer stem cells/cancer initiating cells and tumor-infiltrating immune cells in NSCLC.
Methods: CD133, octamer 4 (OCT-4), CD8, CD56, human leukocyte antigen (HLA) class I and programmed death ligand-1 (PD-L1) were assessed in 172 resected NSCLC samples. The staining was analyzed and scored by the pathologist who was blinded to the clinical pathological data of the patients.
Results: High CD8+ T cell infiltration was correlated significantly with squamous cell carcinoma histology (p=0.008). High PD-L1 expression (≥10%) was associated with high tumor status (p=0.043). Pearson's correlation test showed that CD56+ cells were negatively correlated with CD133 expression (r=-0.361, p<0.001) and weakly correlated with negative OCT-4 expression (r=-0.180, p=0.018). There was a strong positive correlation between CD8 and HLA class I (r=0.573, p<0.001). In the survival analysis, high CD8+ T cell infiltration is an independent predictor of improved disease-free survival and overall survival. Patients with low CD133 expression and high CD56 expression had a longer overall survival than those with high CD133 expression and/or low CD56 expression (p=0.013).
Conclusion: There is a negative correlation between CD56+ cells and cancer stem cell markers. This correlation may confirm the possibility that natural killer cells can target CD133+ cancer stem cells/cancer initiating cells in non-small cell lung cancer.
Keywords: Non-small cell lung cancer, CD133, OCT-4, CD8+ T cells, NK cells
Introduction
Lung cancer is the leading cause of death worldwide due to its high incidence and recurrence rates.[1] Cancer stem cells or cancer initiating cells (CSCs/CICs) are recognized as a small subpopulation of cancer cells that are able to self-renew and differentiate to heterogeneous lineages of cancer cells.[2] Lung CSCs/CICs possess properties against anticancer treatment, including chemotherapy [3], radiotherapy [4] and targeted therapy.[5] Recent studies confirmed that immunotherapy showed prominent efficacy in non-small cell lung cancer (NSCLC), successfully prolonged the survival of responding patients.[6, 7] However, it remains unclear if there is a correlation between cancer stem cell markers and tumor-infiltrating lymphocytes (TILs).
CD133, also known as prominin-1, has been widely used as a CSC/CIC marker in NSCLC.[8] Octamer 4 (OCT-4), a member of the POU domain transcription factor family, is normally expressed on pluripotent stem cells.[9] Some characteristics of OCT-4 also seem to serve as a functional switch related to the "stemness" of the CSCs/CICs. [10] A study revealed that OCT-4 played a crucial role in maintaining self-renewal and cancer stem-like properties in CD133+ lung cancer cells. [11]
The immune system includes innate compartments and adaptive compartments. [12] Among adaptive departments, most CD8+ T cells are cytotoxic T lymphocytes that recognize particular tumor-specific antigens and possess the ability to destroy cancer cells directly. [13] Human leukocyte antigen (HLA) class I displays tumor antigens to CD8+ T cells. The downregulation or absence of HLA class I expression is considered one of the mechanisms that allow tumor cells to escape from immunosurveillance.[14] Natural killer (NK) cells, with the most important surface marker CD56, are capable of recognizing and killing tumor cells in the absence of prior stimulation. [15] It was reported that human NK cells could selectively recognize and target cancer initiating cells by the presence of CD133 in colon cancer.[16]
The purpose of the present study was to evaluate the correlation of cancer stem cell/cancer initiating cells and tumor infiltration lymphocytes by analyzing the expression of CD133, OCT-4 and immune markers in resected NSCLC.
Materials and Methods
Patients
Patients diagnosed with stage I-IIIa NSCLC were included in this study. The tumor characteristics, such as tumor size, invasive depth, lymph node metastasis and differentiation, were confirmed by pathologists. Tumor staging was determined according to the International Association for the Study of Lung Cancer (IASLC). All patients had standard follow-ups. In total, 172 patients were included from Shandong Provincial Hospital affiliated to Shandong University and Shandong Cancer Hospital Affiliated to Shandong University. Tissue collection and analysis methods were conducted in accordance with the Institutional Ethics Committee of Shandong Cancer Hospital Affiliated to Shandong University and Shandong Provincial Hospital Affiliated to Shandong University.
Immunohistochemistry staining
All the markers were assessed in resected NSCLC samples. First, 5-μm-thick slides were deparaffinized and rehydrated. After antigen retrieval, serum was added to the slide to block nonspecific binding (Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China). The sections were incubated with primary rabbit anti-human CD133 antibody (Abcam, USA), rabbit anti-OCT-4 antibody (Abcam, USA), rabbit anti-CD8 antibody (Beijing Zhongshan Golden Bridge Biotechnology Company, China), mouse anti-CD56 antibody (Beijing Zhongshan Golden Bridge Biotechnology Company, China), mouse anti-HLA class I antibody (Abcam, USA) or rabbit anti-programmed death ligand-1 (PD-L1) antibody (28-8, Abcam, USA) at 37 ℃ for 60 minutes. After washing three times with phosphate buffer solution (PBS), secondary goat anti-mouse or anti-rabbit antibody (Beijing Zhongshan Golden Bridge Biotechnology Company, China) was added and incubated at 37 ℃ for 30 minutes. 3,3'-diaminobenzidine was then added to visualize the staining of the antibodies, and the nucleus was counterstained by hematoxylin.
Scoring of the staining
Immunostaining was analyzed under light microscopy at 400× magnification by a pathologist who was blinded to the clinical pathological data of the patients. For CD133, the staining was graded as positive at least with 5% stained cells.[17] While patients, sample with at least 10% stained cells was scored as positive for OCT-4. [18] For CD8 [19] and CD56, the staining was graded as either high expression or low expression at the median value. HLA class I staining was scored as positive when stained cells >80%, scored as moderate when stained cells 20%-80%, scored as negative when stained cells <20%. [15] For PD-L1 staining, the tumors were graded as positive at the level of 1% or 10% stained cells.
Statistical analysis
The chi-squared test was performed to assess the association between stained markers and clinical pathologic features. Pearson's correlation test was used to analyze the correlation between cancer stem cell markers and tumor infiltrating immune cells. Disease-free survival (DFS) was defined as from the date of surgery to the date of relapse or death or last follow-up. Overall survival (OS) was defined from the date of surgery to the date of death or last follow-up. The Kaplan-Meier method was used for the univariate analysis of DFS and OS. The Cox proportional hazards model was used for the multivariate survival analysis. The statistical analysis was performed using the Statistical Product and Service Solutions version 17.0 (SPSS Inc; Chicago, USA). Differences were considered as statistically significant when p<0.05.
Results
Patients' characteristics
Clinical and histopathologic variables of 172 NSCLC patients are presented in Table 1. Seventy-eight patients were ≥60 years at diagnosis. The majority of patients were male (65%). The tumors comprised 67 poorly differentiated, 72 moderately differentiated and 33 highly differentiated carcinomas. There was a higher frequency of nonsquamous carcinoma (58%) than squamous carcinoma (42%). There were 38 cases with tumor 1 (T1), 112 cases with T2 and 22 cases with T3 stage tumors. There were 106 patients without node metastasis (N0) and 66 patients with node metastasis.
Clinicopathological parameters
| Clinicopathological parameters | Cases (172) |
|---|---|
| <60 | 94 |
| ≥60 | 78 |
| Sex | |
| Male | 112 |
| Female | 60 |
| Smoking history | |
| Smoker | 95 |
| Non-smoker | 77 |
| Differentiation | |
| Low | 67 |
| Moderate | 72 |
| High | 33 |
| Pathology | |
| Squamous | 72 |
| Non-squamous | 100 |
| Tumor status | |
| T1 | 38 |
| T2 | 112 |
| T3 | 22 |
| Node status | |
| N0 | 106 |
| N1 | 31 |
| N2 | 35 |
CD133, OCT-4, CD8, CD56, HLA class I and PD-L1 expression and associations with clinicopathological parameters
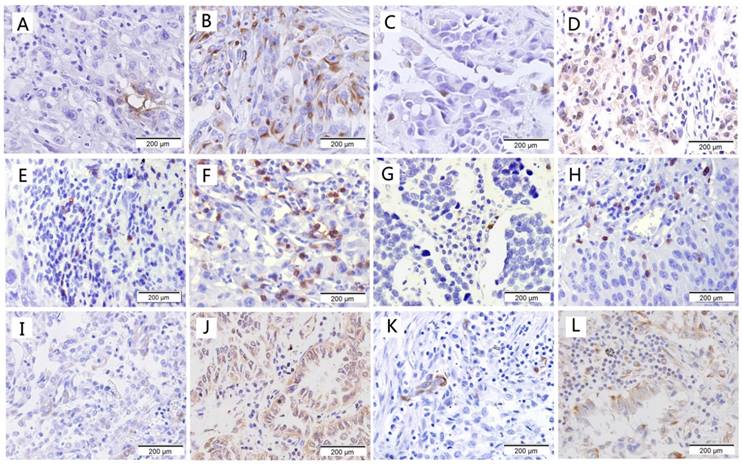
Representative examples of low and high staining for CD133, OCT-4, CD8, CD56, HLA class I and PD-L1 are shown in Fig. 1. The percentage of CD133 stained cells ranges from 0 to 25%, 86 cases were scored as positive;OCT-4 staining was observed in the nucleus and cytoplasm with 2%-65% tumor cells, 97 cases were scored as positive. Infiltration of CD8+ T cells and CD56+ cells was predominantly observed in tumor stroma compared to tumor nests. The median value of CD8 expression (9%) in the tumor was higher than that of CD56 expression (2.5%). For HLA class I, 17 cases were scored as negative; 127 cases were scored as moderate and 28 cases were scored as positive. There were 114 cases and 52 cases scored as positive for PD-L1 at the cutoff of 1% and 10%, respectively.
CD133, OCT-4, and CD56 showed no correlation with age, gender, differentiation, histology (squamous or not), tumor stage or nodal stage. No significant correlation was observed between CD8+ T cells with clinicopathological parameters, except high numbers of CD8+ T cells were significantly correlated with squamous carcinoma histology (p=0.008). HLA class I showed correlation with smoking history (p=0.002). PD-L1 expression was correlated with high tumor stage (p=0.043).
Correlation between cancer stem cell markers and immune cells
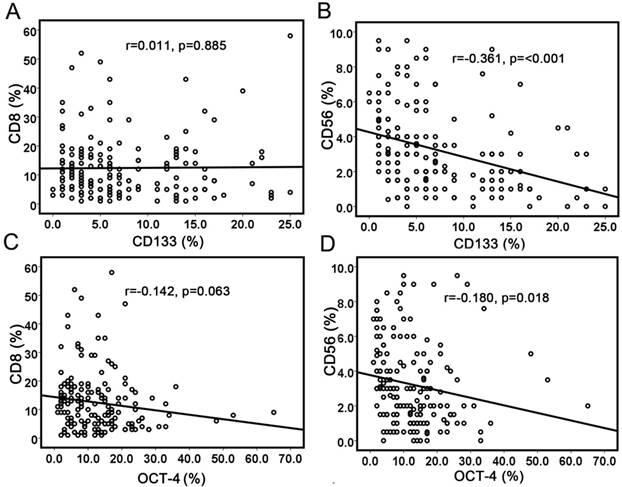
Pearson's correlation test was used to analyze the correlation between cancer stem cell markers and immune cell markers (Table 2). CD133 staining showed a positive correlation with OCT-4 expression (r=0.175, p=0.022). No correlation was observed between CD133 expression and CD8+ TILs (r=0.011, p=0.885) (Fig. 2a), while CD56 expression was negatively associated with CD133 expression (r=-0.361, p<0.001) (Fig. 2b). There was no correlation between OCT-4 expression and CD8+ TILs (r=-0.142, p=0.063) (Fig. 2c). OCT-4 expression showed a weakly negative correlation with CD56+ immune cells (r=-0.180, p=0.018) (Fig. 2d). The results demonstrated that increased NK cells were associated with less CSCs/CICs. HLA class I expression showed a strong positive correlation with CD8+ T-cell infiltration (r=0.573, p<0.001). No correlation was observed between HLA class I expression and CD133 or OCT-4.
The correlation between cancer stem cells markers and immune markers
| Markers | r value | p value |
|---|---|---|
| CD133 and OCT-4 | 0.175 | 0.022 |
| CD133 and CD8 | 0.011 | 0.885 |
| CD133 and CD56 | -0.361 | <0.001 |
| CD133 and HLA class I | 0.034 | 0.658 |
| CD133 and PD-L1 | 0.092 | 0.229 |
| OCT-4 and CD8 | -0.142 | 0.063 |
| OCT-4 and CD56 | -0.180 | 0.018 |
| OCT-4 and HLA class I | 0.034 | 0.654 |
| OCT-4 and PD-L1 | 0.154 | 0.044 |
| CD8 and HLA class I | 0.573 | <0.001 |
Immunohistological staining of cancer stem cell markers and immune markers. (a) Low expression of CD133; (b) High expression of CD133; (c) Low expression of OCT-4; (d) High expression of OCT-4; (e) Low expression of CD8; (f) High expression of CD8; (g) Low expression of CD56; (h) High expression of CD56; (i) Low expression of HLA class I; (j) High expression of HLA class I; (k) Low expression of PD-L1; (l) High expression of PD-L1.
Correlations between cancer stem cell markers with immune cell markers. (a) No significant correlation between CD133 expression and CD8+ TILs; (b) Significant correlation between CD133 expression and CD56+ cells; (c) No correlation was observed between OCT-4 expression and CD8+ TILs; (d) No correlation was observed between OCT-4 expression and CD56+ cells.
Univariate analysis between clinicopathological parameters and survival
| Parameters | Cases | 5-year DFS (%) | P value | 5-year OS(%) | P value |
|---|---|---|---|---|---|
| Age | |||||
| <60 | 94 | 54.8 | 0.581 | 60.2 | 0.339 |
| ≥60 | 78 | 57.2 | 67.5 | ||
| Sex | |||||
| Male | 112 | 57.5 | 0.373 | 66.9 | 0.172 |
| Female | 60 | 52.9 | 54.9 | ||
| Differentiation | |||||
| Low | 67 | 50.1 | 0.062 | 61.2 | 0.095 |
| Moderate | 72 | 52.3 | 60.6 | ||
| High | 33 | 75.6 | 78.2 | ||
| Pathology | |||||
| Squamous | 72 | 62.1 | 0.189 | 70.5 | 0.175 |
| Non-squamous | 100 | 51.4 | 57.6 | ||
| Pathological tumor status | |||||
| T1 | 38 | 78.4 | 0.004 | 81.6 | 0.004 |
| T2 | 112 | 50.7 | 59.4 | ||
| T3 | 22 | 43.6 | 48.5 | ||
| Pathological node status | |||||
| N0 | 106 | 72.1 | <0.001 | 77.2 | <0.001 |
| N1 | 31 | 41.9 | 50.6 | ||
| N2 | 35 | 20.0 | 30.8 | ||
| CD133 | |||||
| Negative | 86 | 59.9 | 0.359 | 67.1 | 0.133 |
| Positive | 86 | 51.9 | 58.7 | ||
| OCT-4 | |||||
| Negative | 75 | 64.9 | 0.063 | 70.5 | 0.047 |
| Positive | 97 | 48.9 | 56.9 | ||
| CD8 | |||||
| Low expression | 86 | 41.0 | <0.001 | 47.5 | <0.001 |
| High expression | 86 | 70.7 | 78.5 | ||
| CD56 | |||||
| Low expression | 86 | 51.7 | 0.315 | 57.5 | 0.084 |
| High expression | 86 | 60.1 | 68.4 | ||
| HLA I | |||||
| Negative | 17 | 35.3 | 0.009 | 41.2 | 0.010 |
| Moderate | 127 | 52.9 | 61.9 | ||
| Positive | 28 | 82.1 | 89.3 | ||
| PD-L1 (10%) | |||||
| Low expression | 120 | 59.6 | 0.172 | 67.1 | 0.033 |
| High expression | 52 | 47.3 | 53.2 | 0.036 | |
| PD-L1 (1%) | |||||
| Low expression | 58 | 48.7 | 0.278 | 56.1 | 0.336 |
| High expression | 114 | 59.4 | 62.0 | ||
| CD133 CD56 | |||||
| CD133 low and CD56 high | 59 | 63.9 | 0.141 | 74.4 | 0.013 |
| CD133 high and/or CD56 low | 113 | 51.8 | 57.1 | ||
| OCT-4 CD56 | |||||
| OCT-4 low and CD56 high | 50 | 65.5 | 0.114 | 71.9 | 0.098 |
| OCT-4 high and/or CD56 low | 122 | 51.3 | 59.2 |
Survival analysis
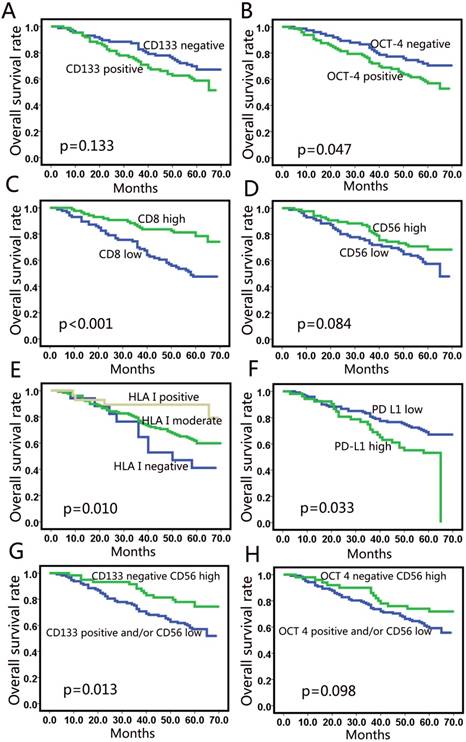
At the time of analysis, the median duration of follow-up was 60 months. The results of Kaplan-Meier analysis for 5-year DFS and 5-year OS are listed in Table 3. Patients with poorly differentiated tumors had shorter survival than those with moderately or highly differentiated tumors, but the difference was not significant. Among the molecular markers assessed, expression of CD133 (Fig. 3a) was not correlated with survival. High expression of OCT 4 was correlated with poor 5-year OS (p=0.047). High CD8+ TILs were significantly associated with favorable 5-year DFS (p<0.001) and 5-year OS (p<0.001) (Fig. 3c). High HLA class I expression correlated with longer 5-year DFS (p=0.009) and 5-year OS (p=0.010) (Fig. 3e). Patients with high PD-L1 expression based on the 10% cut-off value showed worse 5-year OS (p=0.033) (Fig. 3f). However, no correlation was observed between PD-L1 expression and survival when 1% was taken as the cutoff value.
Patients with high CD56 expression showed a tendency of better OS than those with low expression (p=0.084) (Fig. 3d), but the tendency was not significant. We used a sub-analysis to examine the factors associated with CD56 expression. We found that patients with low CD133 expression and high CD56 expression had a longer OS than those with high CD133 expression and/or low CD56 expression (p=0.013) (Fig. 3g). However, the combination of CD56 and OCT-4 expression did not predict prognosis (Fig. 3h).
In multivariate analyses, T stage and N stage were independent predictors for 5-year DFS and 5-year OS (p<0.01). (Table 4) CD8 expression was an independent predictive factor for longer DFS (HR 0.496, 95% CI 0.288-0.854, p=0.011) and OS (HR 0.486, 95% CI 0.267-0.884, p=0.018).
Multivariate analysis between clinicopathological parameters and survival.
| Parameters | 5-year DFS | 5-year OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 0.956 | 0.592-1.553 | 0.852 | 0.789 | 0.464-1.343 | 0.383 |
| Sex | 1.305 | 0.766-2.223 | 0.327 | 1.430 | 0.798-2.563 | 0.230 |
| Differentiation | 0.808 | 0.582-1.122 | 0.202 | 0.804 | 0.561-1.152 | 0.235 |
| Squamous or not | 1.347 | 0.770-2.356 | 0.297 | 1.357 | 0.728-2.532 | 0.337 |
| Tumor | 1.893 | 1.275-2.810 | 0.002 | 2.044 | 1.308-3.195 | 0.002 |
| Node | 2.183 | 1.656-2.877 | <0.001 | 2.154 | 1.595-2.908 | <0.001 |
| CD133 | 0.982 | 0.566-1.704 | 0.947 | 1.054 | 0.577-1.926 | 0.865 |
| OCT-4 | 1.298 | 0.762-2.211 | 0.337 | 1.268 | 0.705-2.281 | 0.428 |
| CD8 | 0.496 | 0.288-0.854 | 0.011 | 0.486 | 0.267-0.884 | 0.018 |
| CD56 | 0.727 | 0.417-1.266 | 0.259 | 0.582 | 0.315-1.077 | 0.085 |
| HLA I | 0.905 | 0.552-1.484 | 0.693 | 0.879 | 0.510-1.514 | 0.641 |
| PD-L1 | 0.961 | 0.566-1.630 | 0.883 | 1.057 | 0.598-1.867 | 0.850 |
Discussion
In the current study, we observed heterogeneous expression of CD133, OCT-4, CD8, CD56, HLA class I and PD-L1 in NSCLC regimens. We found that CD56 correlated negatively with CD133 expression (r=-0.361, p<0.001) and OCT-4 expression (r=-0.180, p=0.018). Positive correlations were observed between CD8+ T-cell infiltration and HLA class I expression (p<0.001). However, no correlations were found between OCT-4 and CD8+ TILs or between CD133 and CD8+ TILs. In survival analysis, patients with low CD133 expression and high CD56 expression had a longer OS than other patients (p=0.013). CD8 expression was an independent predictive factor for longer DFS and OS.
Univariate analysis for overall survival. CD133 (a) and OCT-4 (b) did not correlate with OS in NSCLC; (c) CD8+ T cell-infiltration was a positive predictor; (d) High CD56 expression showed a tendency for a better OS; (e) Patients with high expression of HLA class I showed better OS; (f) High PD-L1 expression showed correlation with poor OS (10%); (g) The survival curve for the combination of CD56 and CD133 levels; (h) The survival curve for the combination of CD56 and OCT-4 level.
In the current study, the expression of CD133 correlated positively with OCT-4 staining. The result was consistent with the previous study, which also showed positive correlation of CD133 and OCT-4. [20] The results suggest an interplay of these two biomarkers in tumor initiation. OCT-4 plays a crucial role in maintaining cancer stem-like properties of CD133+ CSCs/CICs.[9, 12, 21] Preclinical studies demonstrated that forced reduction of OCT-4 expression induced apoptosis of CSCs.[12] Transfection of the OCT-4 gene promoted CSC properties, including self-renewal ability, tumorigenicity in vivo and resistance to targeted therapy.[21]
CD8+ T cells are crucial for cell-mediated antitumor immune responses.[22] In this study, we demonstrated that high numbers of CD8+ T cells correlated significantly with squamous carcinoma histology (p=0.001). In a study by Hiraoka et al., the number of CD8+ TILs within the cancer nest was higher in squamous carcinoma than in other histologic subtypes, while the number of CD8+ TILs within the stroma showed no difference.[14] In CheckMate 017 and CheckMate 057 trials, nivolumab demonstrated no statistically significant improvement in PFS for non-squamous-NSCLC [23] while demonstrated significantly better PFS over docetaxel among squamous-NSCLC patients. [24] Our results showed the higher CD8+ T cells infiltration in squamous carcinoma, which may explain partly why nivolumab demonstrated better PFS improvement in squamous NSCLC.
Under the appropriate antigen stimulation, CD8+ T cells undergo proliferation and differentiation into cytotoxic T lymphocytes (CTLs).[25] It was reported that CSCs⁄CICs expressed several tumor-associated antigens which could be recognized by CTLs. [25] However, our findings demonstrated no correlation between CD8+ TILs and CD133 or OCT-4 expression. Possible explanations may be that CSCs/CICs cannot initiate a cytotoxic immune response without the enhancement of other cells or cytokines, or the immune suppression of CSCs/CICs is predominant over the activation of immune responses. Several reports suggest that CSCs/CICs seem to be able to evolve strategies to escape from T-cell attacks.[26, 27]
Our data showed a strong positive correlation between HLA class I and CD8+ T cells. This result was consistent with that in previous studies.[15, 28] A significantly lower number of cancer nest CD8+ T cells was observed in areas with negative expression of HLA class I than in areas with strongly positive expression of HLA class I in early stage NSCLC,[15] and much more CD8+ T cell-infiltration was observed in HLA class I-positive tumors compared to HLA class I-negative tumors. [28]
Previous reports have indicated that NK cells are capable of exhibiting their cytotoxic functions toward CSCs/CICs. [29, 30] In osteosarcoma, the cytotoxicity of activated and expanded NK cells could target and eliminate tumor-initiating cells. [29] In oral squamous cancer, increased NK cell function was observed when cells were co-cultured with primary CSCs/CICs compared to more differentiated tumor cells.[30] Data from a colon cancer study demonstrated that freshly purified allogeneic NK cells can recognize and kill CICs, whereas the non-CIC counterpart of the tumor is less susceptible to NK cells.[31] Our data firstly demonstrated that the number of CD56+ cells is negatively related with CD133+ CSCs/CICs, suggesting that more NK cells results in less CD133+ cells in NSCLC. This is suggestive of the potency of NK cells as a possible key player in immunotherapy by targeting CSCs/CICs in NSCLC.
Our data demonstrated that patients with high HLA class I expression achieved better 5-year DFS and 5-year OS. The reason for improved outcome with high HLA class I expression is thought to be due to less tumor cells escaping from the immune surveillance.[32] In a previous study, patients with low expression of HLA class I also displayed worse prognosis in stage I NSCLC. [15] In this study, high PD-L1 expression was correlated with high tumor stage and worse prognosis at the cut-off of 10%. The prognostic value of PD-L1 expression remains controversial in NSCLC. Two meta-analyses showed opposite results regarding the correlation between PD-L1 expression and prognosis in NSCLC.[19, 33] Multivariate analysis showed that CD8+ T-cell infiltration is an independent prognostic factor for long-term DFS and OS. The results suggest that the cytotoxic CD8+ T cells in NSCLC tumor microenvironment play an important role in antitumor progression.
Patients with high CD56 expression showed a tendency of better OS than those with low expression (p=0.084). This result agrees with previous observations demonstrating that the survival time of lung cancer patients was positively related to NK-cell infiltration in lung cancer (p=0.030).[34] However, the data in the current study did not reach statistical significance. When combining with CD133 and CD56 expression levels, we found that patients with low CD133 expression and high CD56 expression had longer survival than those with high CD133 expression and/or low CD56 expression (p=0.013). These results suggest that high CD56 expression inhibit CD133 expression. Hence, the combination of NK cell infiltration and CD133 expression may be more important than CD56 or CD133 expression alone for predicting survival.
In conclusion, this is the first study to evaluate the association between CSC/CICs and tumor-infiltrating immune cells. There is a significant negative correlation between the numbers of NK cells and CD133+ CSCs/CICs. The patients with low CD133 expression and high CD56 expression had longer survival than other patients. The results confirmed the possibility that CD56+ cells may target CD133+ CSCs/CICs in NSCLC. Increasing NK cells infiltration may help overcome the resistance to antitumor therapy caused by CSCs/CICs. However, the current study is a retrospective study. Additional basic and clinical studies are needed before NK cells can be used to treat NSCLC patients by targeting CSCs/CICs.
Acknowledgements
The study was funded by National Natural Science Foundation of China (No. 81301868 and 81572970) and was funded by the Special Fund for Scientific Research in the Public Interest (201402011).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mizugaki H, Sakakibara-Konishi J, Kikuchi J. et al. CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol. 2014;19:254-9
2. Jordan CT. Cancer stem cell biology: from leukemia to solid tumors. Curr Opin Cell Biol. 2004;16:708-12
3. Sourisseau T, Hassan KA, Wistuba I. et al. Lung cancer stem cell: fancy conceptual model of tumor biology or cornerstone of a forthcoming therapeutic breakthrough? J Thorac Oncol. 2014;9:7-17
4. Lundholm L, Haag P, Zong D. et al. Resistance to DNA-damaging treatment in non-small cell lung cancer tumor-initiating cells involves reduced DNA-PK/ATM activation and diminished cell cycle arrest. Cell Death Dis. 2013;4:e478
5. Ghosh G, Lian X, Kron SJ. et al. Properties of resistant cells generated from lung cancer cell lines treated with EGFR inhibitors. BMC Cancer. 2012;12:95
6. Fehrenbacher L, Spira A, Ballinger M. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-46
7. Reck M, Rodriguez-Abreu D, Robinson AG. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-33
8. Eramo A, Lotti F, Sette G. et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504-14
9. Chen YC, Hsu HS, Chen YW. et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637
10. Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372-6
11. Zhong B, Lin Y, Lai Y. et al. Relationship of Oct-4 to malignant stage: a meta-analysis based on 502 positive/high Oct-4 cases and 522 negative/low case-free controls. Oncotarget. 2016;7:2143-52
12. Hu T, Liu S, Breiter DR. et al. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008;68:6533-40
13. Hald SM, Bremnes RM, Al-Shibli K. et al. CD4/CD8 co-expression shows independent prognostic impact in resected non-small cell lung cancer patients treated with adjuvant radiotherapy. Lung Cancer. 2013;80:209-15
14. Hiraoka K, Miyamoto M, Cho Y. et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275-80
15. Kikuchi E, Yamazaki K, Torigoe T. et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424-30
16. Carrega P, Morandi B, Costa R. et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863-75
17. Alamgeer M, Ganju V, Szczepny A. et al. The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax. 2013;68:1095-104
18. Li X, Wang J, Xu Z. et al. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012;13:7663-75
19. Tokito T, Azuma K, Kawahara A. et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7-14
20. Cortes-Dericks L, Galetta D, Spaggiari L. et al. High expression of octamer-binding transcription factor 4A, prominin-1 and aldehyde dehydrogenase strongly indicates involvement in the initiation of lung adenocarcinoma resulting in shorter disease-free intervals. Eur J Cardiothorac Surg. 2012;41:e173-81
21. Kobayashi I, Takahashi F, Nurwidya F. et al. Oct4 plays a crucial role in the maintenance of gefitinib-resistant lung cancer stem cells. Biochem Biophys Res Commun. 2016;473:125-32
22. Fridman WH, Pages F, Sautes-Fridman C. et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306
23. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-39
24. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-35
25. Pan Q, Li Q, Liu S. et al. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085-92
26. Volonte A, Di Tomaso T, Spinelli M. et al. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol. 2014;192:523-32
27. Schatton T, Schutte U, Frank NY. et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697-708
28. Perea F, Bernal M, Sanchez-Palencia A. et al. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer. 2017;140:888-99
29. Fernandez L, Valentin J, Zalacain M. et al. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 2015;368:54-63
30. Jewett A, Tseng HC, Arasteh A. et al. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv. 2012;9:5-16
31. Tallerico R, Todaro M, Di Franco S. et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381-90
32. Marincola FM, Jaffee EM, Hicklin DJ. et al. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181-273
33. Zhong A, Xing Y, Pan X. et al. Prognostic value of programmed cell death-ligand 1 expression in patients with non-small-cell lung cancer: evidence from an updated meta-analysis. Onco Targets Ther. 2015;8:3595-601
34. Jin S, Deng Y, Hao JW. et al. NK cell phenotypic modulation in lung cancer environment. PLoS One. 2014;9:e109976
Author contact
![]() Corresponding author: Xiangjiao Meng, Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academic of Medical Science, Jinan, China, No.440 Jiyan Road, Jinan, Shandong, China, 250117. E-mail: mengxiangjiaocom. Tel: +86 531 67626995, Fax: +86 531 67626819
Corresponding author: Xiangjiao Meng, Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academic of Medical Science, Jinan, China, No.440 Jiyan Road, Jinan, Shandong, China, 250117. E-mail: mengxiangjiaocom. Tel: +86 531 67626995, Fax: +86 531 67626819

 Global reach, higher impact
Global reach, higher impact