Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(17):3396-3404. doi:10.7150/jca.21017 This issue Cite
Research Paper
Clinicopathological Characteristics and Prognostic Value of Signet Ring Cells in Gastric Carcinoma: A Meta-Analysis
1. Department of Gastric Surgery, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, China;
2. Department of Gastric Surgery, The 6th Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
# These authors contributed equally to this study.
* These authors also contributed equally to this study.
Received 2017-5-15; Accepted 2017-8-31; Published 2017-9-20
Abstract
Background and Objectives: Previous studies of the prognostic value of the signet ring cell (SRC) type have yielded inconsistent results. Therefore, the aim of the present meta-analysis is to explore the clinicopathological characteristics and prognostic value of SRCs.
Methods: Relevant articles that compared SRC and non-SRC type in PubMed and Web of Science were comprehensively searched. Then, a meta-analysis was performed.
Results: A total of 19 studies including 35947 cases were analyzed. Compared with non-SRC patients, SRC patients tended to be younger (WMD: -3.88, P=0.001) and predominantly female (OR: 1.60, P<0.001). Additionally, SRC patients exhibited less upper third tumor location (OR: 0.62, P<0.001) and less frequent hematogenous metastasis (OR: 0.41, P<0.001). There was no difference in overall survival (OS) between SRC and non-SRC patients in the total population (HR: 1.02, P=0.830). Early gastric cancer with SRCs was associated with better OS (HR: 0.57, P=0.002), while advanced gastric cancer with non-SRCs was associated with a worse prognosis (HR: 1.17, P<0.001).
Conclusions: This meta-analysis revealed that SRC tends to affect young females and tends to be located in the middle and lower third of the stomach. Early SRCs are associated with better prognoses, while advanced SRCs are associated with worse prognoses.
Keywords: signet ring cell, gastric cancer, prognosis, survival.
Introduction
Gastric cancer is the fourth most common malignancy and the third leading cause of cancer-related death worldwide, with an estimated 1.0 million new cases of gastric cancer and 723100 deaths due to gastric cancer in 2012 [1]. The burden caused by gastric cancer is growing worldwide, particularly in developing countries [2].
The incidence of gastric cancer has decreased over the last 50 years. Meanwhile, differential trends have also occurred, such as an increase in the diffuse type, particularly signet ring cell (SRC). Henson et al. reported that SRC has increased on average 6.5% per year from 1973 to 2000 in the United States [3]. SRC is a histological subtype of carcinoma with cells containing large amounts of intracytoplasmic mucins [4].
SRC was classified as one of the undifferentiated gastric carcinomas according to the Japanese Gastric Cancer Association [5]. It was reported that patients with SRC tend to be young females, and SRC patients tend to have more lymph node involvement and even peritoneal seeding [6], which indicates a more aggressive stage of SRC. However, the prognostic value of gastric cancer with SRC remains controversial. Several studies have reported that SRC is a poor prognostic factor for gastric cancer [6-11], while others have argued that gastric cancer patients with SRC have better survival [12-15]. Therefore, we aimed to perform a meta-analysis to evaluate the prognostic value of SRC in gastric cancer.
Methods
Search strategy and study selection
On July 1st, 2017, relevant articles were systematically and independently searched by two authors (RC, Nie and SQ, Yuan) in PubMed and Web of Science using the key words “gastric cancer”, “gastric carcinoma”, “gastric neoplasm”, and “signet ring cell”, limited to Abstract/Title. We also checked the reference lists of the articles retrieved manually to identify additional relevant articles.
According to the WHO criteria, SRC was defined by an adenocarcinoma with more than 50% of the tumor consisting of isolated or small groups of malignant cells containing intracytoplasmic mucins [4]. Studies were included if they compared SRC and non-SRC directly with more than 50 patients involved in the studies. Studies were excluded if they were basic studies about animals or cells, review articles, meta-analyses, abstracts, case reports, case series, letters, conference proceedings or non-English published articles. Studies were also excluded if they did not conform to or mention the WHO criteria. If multiple reports described the same population, the most recent or complete research was included.
Data extraction
The data from the included studies were extracted independently by two authors (RC, Nie and YQ, Yuan). The adjudicating senior authors (S, Chen and YB, Chen) were responsible for resolving any disagreements. The following information was extracted from each study: first author, year of publication, country, stage of disease, sample size and SRC/non-SRC ratio. We also extracted the following clinicopathological characteristics: age, gender, tumor location, tumor size, lymph node involvement, lymphovascular invasion, perineural invasion, early disease ratio, gross type, peritoneal dissemination, hematogenous metastasis, and overall survival (OS).
Statistical analysis
The estimated odds ratio (OR) or weighted mean difference (WMD) was used to summarize the association between SRC pathological type and different clinicopathological characteristics. The hazard ratio (HR) was pooled to analyze the OS results as demonstrated by Parmar et al. [16]. All results were reported with 95% confidence intervals (CIs). Heterogeneity was assessed using the χ² test and quantified using the I2 statistic, with the level of significance set at 10%.
The fixed-effects model was used when P >0.1; otherwise, the random-effects model was used [17]. The publication bias was tested by Begg's linear regression [18]. A P value of < 0.05 was considered significant. Analyses were performed using STATA SE 12.0 (StataCorp, College Station, TX, USA).
Results
Study selection
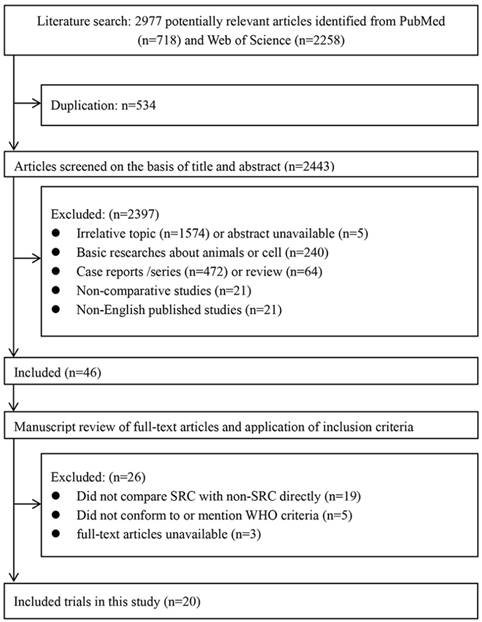
Based on the inclusion and exclusion criteria, a total of 20 studies [6-15, 19-28] including 35947 cases were included in the final analysis (Fig. 1). The characteristics of the included studies are shown in Table 1. These studies were primarily from six countries (Japan, Korea, China, France, America, and Belgium) and were published from 1992 to 2017. The proportion of SRC ranged from 3.4% to 50.0% in the included studies.
Clinicopathological characteristics
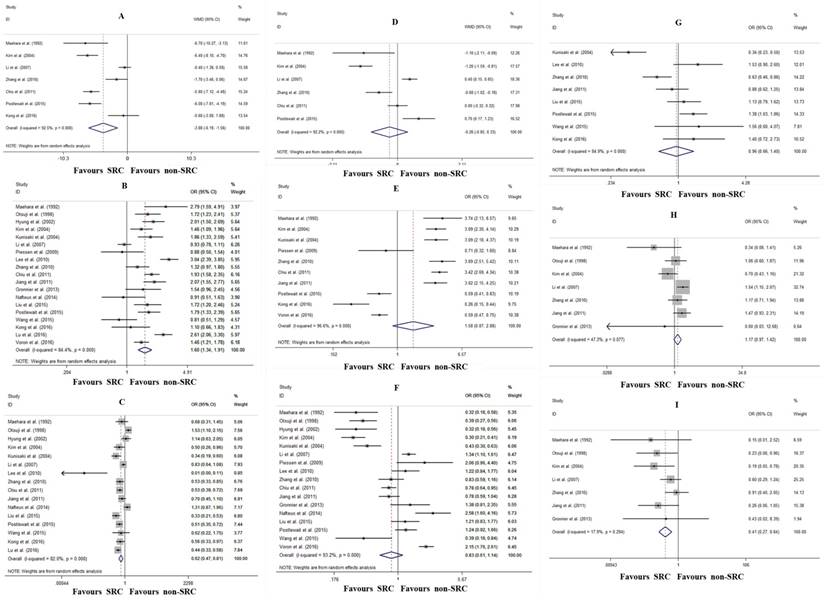
The relationships between the clinicopathological characteristics and SRC were investigated. Compared with non-SRC patients, SRC patients tended to be younger (WMD: -3.88, 95% CI: -6.19- -1.56, P=0.001; I2=92.5%, P<0.001; Fig. 2A) and predominantly female (OR: 1.60, 95% CI: 1.34-1.91, P<0.001; I2=84.4%, P<0.001; Fig. 2B). Additionally, SRC patients exhibited less upper third tumor location (OR: 0.62, 95% CI: 0.47-0.81, P<0.001; I2=82.0%, P<0.001; Fig. 2C). There were no differences between SRC and non-SRC patients with regard to tumor size (WMD: -0.26 cm, 95% CI: -0.86- 0.33, P=0.390; I2=92.2%, P<0.001; Fig. 2D), early gastric cancer ratio (OR: 1.58, 95% CI: 0.87-2.86, P=0.130; I2=96.6%, P<0.001; Fig. 2E), lymph node involvement (OR: 0.83, 95% CI: 0.61-1.14, P=0.247; I2=93.2%, P<0.001; Fig. 2F), lymphovascular invasion (OR: 0.96, 95% CI: 0.66-1.40, P=0.822; I2=84.9%, P<0.001; Fig. 2G), or peritoneal dissemination (OR: 1.17, 95% CI: 0.97-1.42, P=0.092; I2=47.3%, P=0.077; Fig. 2H). However, patients with SRC exhibited less frequent hematogenous metastasis (OR: 0.41, 95% CI: 0.27-0.64, P<0.001; I2=17.9%, P=0.294; Fig. 2I).
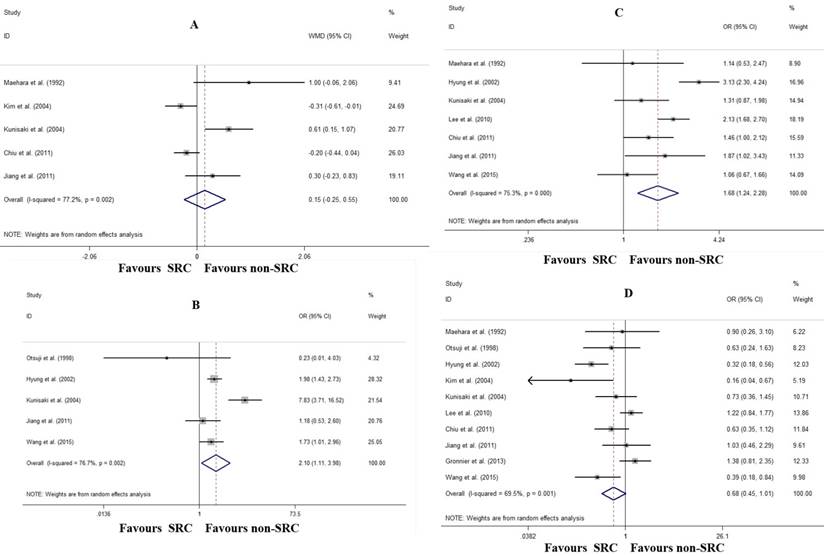
With regard to early gastric cancer, there was no difference in tumor size (WMD: 0.15 cm, 95% CI: -0.25- 0.55, P=0.460; I2=77.2%, P=0.002; Fig. 3A). However, patients with SRC had a higher frequency of the depressed gross type (OR: 2.11, 95% CI: 1.11-3.98, P=0.022; I2=76.7%, P=0.002; Fig. 3B), more mucosal invasion (OR: 1.68, 95% CI: 1.24-2.29, P=0.001; I2=75.3%, P<0.001; Fig. 3C), and marginally less lymph node metastasis (OR: 0.68, 95% CI: 0.46-1.01, P=0.054; I2=69.5%, P=0.001; Fig. 3C).
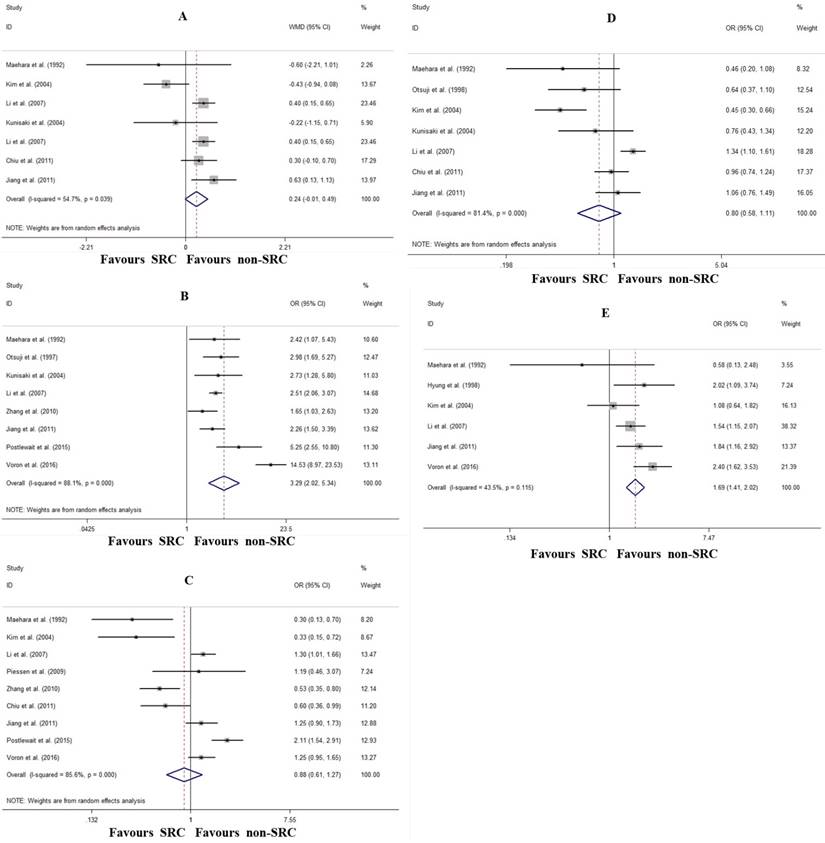
The comparison of the major characteristics of advanced gastric cancer between SRC and non-SRC are shown in Figure 4. Compared with non-SRC, SRC was associated with marginally larger tumor size (WMD: 0.63 cm, 95% CI: -0.01- 1.13, P=0.059; I2=54.7%, P=0.039; Fig. 4A) and increased Borrmann IV type (OR: 3.29, 95% CI: 2.02-5.34, P<0.001; I2=88.1%, P<0.001; Fig. 4B). Although there were no differences in T4 invasion (OR: 0.88, 95% CI: 0.61-1.27, P=0.497; I2=85.6%, P<0.001; Fig. 4C) and lymph node metastasis (OR: 0.80, 95% CI: 0.58-1.11, P=0.183; I2=81.4%, P<0.001; Fig. 4D) between SRC and non-SRC, patients with SRC tended to have more peritoneal dissemination (OR: 1.69, 95% CI: 1.41-2.02, P<0.001; I2=43.5%, P=0.115; Fig. 4E).
Overall survival
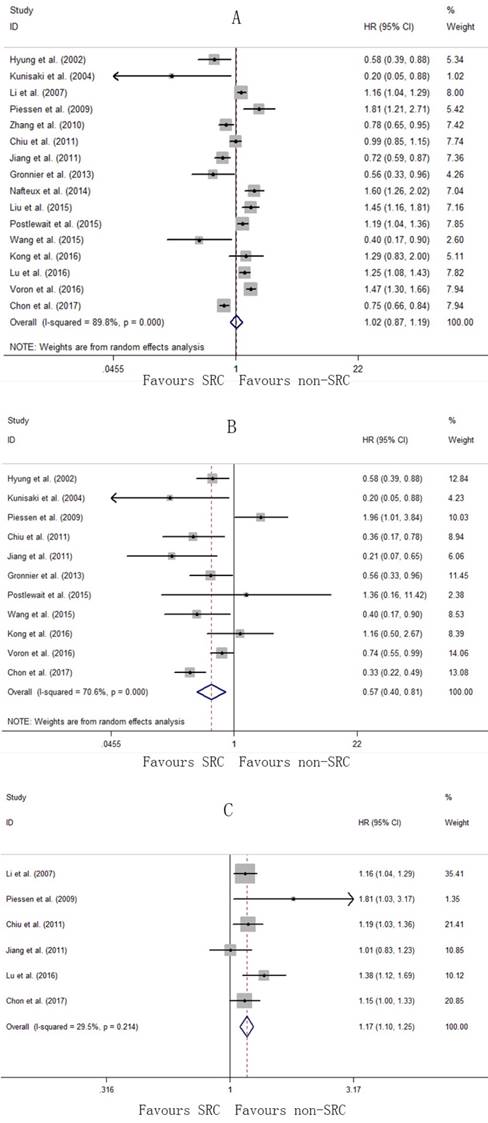
In this meta-analysis, a total of 15 studies [6-15, 22, 24-28] were available for the pooled HR of OS. There was significant heterogeneity among the studies for the pooled HR of OS (I2=89.8%, P<0.001; Fig. 5A); therefore, the random-effects model was used. The pooled HR was 1.02 (95% CI: 0.87-1.19, P=0.830), indicating no difference in OS between the SRC and non-SRC patients. In the subgroup analysis, early gastric cancer with SRC was associated with a better prognosis with a pooled HR of 0.57 (95% CI: 0.40-0.81, P=0.002), with significant heterogeneity (I2=70.6%, P<0.001; Fig. 5B). However, the pooled HR for advanced gastric cancer with SRC was 1.17 (95% CI: 1.10-1.25, P<0.001), with no significant heterogeneity (I2=29.5%, P=0.214; Fig. 5C).
Flow diagram of the included studies.
Characteristics of the included studies
| Author | Year | Country | Stage | No. of patients | SRC/non-SRC (%) | HR for OS (SRC vs. non-SRC) |
|---|---|---|---|---|---|---|
| Maehara et al. | 1992 | Japan | all stage | 1500 | 3.4% vs. 96.6% | NA |
| Otsuji et al. | 1998 | Japan | all stage | 1498 | 10.3% vs. 89.7% | NA |
| Hyung et al. | 2002 | Korea | early stage | 933 | 28.2% vs. 71.8% | 0.58 |
| Kim et al. | 2004 | Korea | all stage | 2358 | 8.7% vs. 91.3% | NA |
| Kunisaki et al. | 2004 | Japan | all stage | 1113 | 15.6% vs. 84.4% | 0.20a |
| Li et al. | 2007 | Korea&China | advanced stage | 4759 | 13.9% vs. 86.1% | 1.16 |
| Piessen et al. | 2009 | France | all stage | 215 | 32.5% vs. 67.5% | 1.81b |
| Lee et al. | 2010 | Korea | early stage | 1326 | 33.8% vs. 66.2% | NA |
| Zhang et al. | 2010 | China | all stage | 1439 | 15.1% vs. 84.9% | 1.28 |
| Chiu et al. | 2011 | China | all stage | 2439 | 20.7% vs. 79.3% | 1.01 |
| Jiang et al. | 2011 | China | all stage | 2315 | 9.1% vs. 90.9% | 0.72 |
| Gronnier et al. | 2013 | France | early stage | 421 | 25% vs. 75% | 0.56 |
| Nafteux et al. | 2014 | Belgium | all stage | 920 | 12.4% vs. 87.6% | 1.60 |
| Liu et al. | 2015 | China | all stage | 1464 | 9.4% vs. 90.6% | 1.45 |
| Postlewait et al. | 2015 | America | all stage | 768 | 40.6% vs. 59.4% | 1.19 |
| Wang et al. | 2015 | China | early stage | 334 | 34.4% vs. 65.6% | 0.40 |
| Kong et al. | 2016 | China | N-stagec | 480 | 18.8% vs. 81.2% | 1.29 |
| Lu et al. | 2016 | China | all stage | 2199 | 16.1% vs. 83.9% | 1.25 |
| Voron et al. | 2016 | France | all stage | 1799 | 50.0% vs. 50.0% | 1.47 |
| Chon et al. | 2017 | Korea | all stage | 7667 | 21.5% vs. 78.5% | 0.75 |
SRC, signet ring cell carcinoma; HR, hazard ratio; OS, overall survival; NA, not available;
a, HR was only available for early stage;
b, HR for resected patients;
c, Study including only node-negative gastric cancer.
Forrest plot assessing different clinicopathological characteristics following SRC and non-SRC gastric cancer in the total population. A, age; B, female; C, tumor location; D, tumor size; E, early gastric cancer ratio; F, lymph node involvement; G, lymphovascular invasion; H, peritoneal dissemination; and I, hematogenous metastasis
Forrest plot assessing different clinicopathological characteristics following SRC and non-SRC in early gastric cancer. A, tumor size; B, depressed gross type; C, mucosal invasion; and D, lymph node involvement
Publication bias
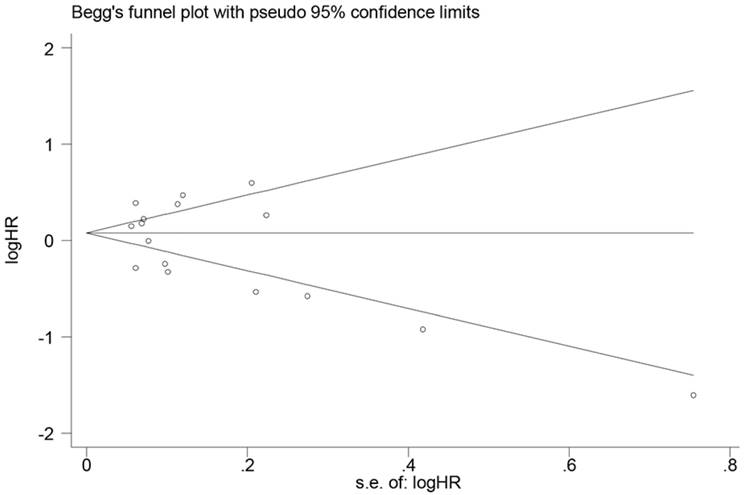
In the present meta-analysis, Begg's linear regression was performed to assess the publication bias of OS. There was no publication bias detected for OS (P=0.374) (Fig. 6).
Discussion
Although the incidence of gastric cancer is decreasing, the proportion of SRC in gastric cancer has tended to increase [3]. The definition of gastric SRC is not based on biological behavior but on microscopic features of the cancer. SRC was classified as a diffuse type by the Lauren classification [29], as an infiltrative type by the Ming classification [30], and as an undifferentiated type by the WHO classification criteria [4]. Previous studies of the characteristics and prognostic value of SRC in gastric cancer have yielded inconsistent results [6-15, 19-28, 31, 32] and, therefore, remain controversial. Therefore, we performed the present meta-analysis to investigate the characteristics and prognostic value of SRC in gastric cancer. The pooled results of OS demonstrated that there was no difference in OS between SRC and non-SRC patients in the total population. However, SRC was associated with a better prognosis in early gastric cancer but worse survival in advanced gastric cancer.
Our results showed that SRC tended to affect younger female patients, and SRC tended to be located in the middle and lower third of the stomach. The association between sex and SRC may be due to sex hormones. It has been reported that levels of the estrogen receptor were higher in young women and in patients with poorly differentiated gastric cancer [33].
Early gastric cancer is defined as a lesion confined to the mucosa or submucosa, regardless of the status of lymph node metastasis [34]. The prognostic value of SRC in early gastric cancer is debatable. Wang et al. reported that early gastric cancer with SRC was associated with a lower incidence of lymph node involvement and a longer 5-year survival (93.9% vs. 85.8%, P=0.027) [15], in accordance with several studies [13, 14, 22, 24, 25, 27]. However, other studies showed that there was no difference in survival between SRC and non-SRC patients [7, 10, 26]. The present meta-analysis revealed that SRC is a better prognostic factor for early gastric cancer (HR: 0.57, P=0.002), which is characterized by a higher proportion of the depressed gross type (OR: 2.11, P=0.022), more mucosal invasion (OR: 1.68, P=0.001), and marginally less lymph node metastasis (OR: 0.68, P=0.054). A higher percentage of the depressed type and the distinct feature of enriched intracytoplasmic mucins with the nucleus compressed peripherally may lead to early detection of the tumor by gastroscopy and biopsy [20]. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been associated with excellent prognoses [35, 36]. EMR and ESD are recommended for early-stage gastric cancer lesions smaller than 2 cm in diameter without relevant ulcer formation [37]. Therefore, considering the lower incidence of lymph node metastasis and better prognosis, less invasive gastric surgery, such as EMR, ESD, or gastrectomy with D1 lymphadenectomy, is highly recommended for patients with early SRC.
Forrest plot assessing different clinicopathological characteristics following SRC and non-SRC in advanced gastric cancer. A, tumor size; B, Borrmann IV type; C, T4 invasion; D, lymph node involvement; and E, peritoneal dissemination
Forrest plot assessing overall survival following SRC and non-SRC in the total population (A), in early gastric cancer (B), and in advanced gastric cancer (C)
Funnel plot to assess publication bias of overall survival in the meta-analysis.
A report by Li et al. showed that advanced gastric SRC had deeper tumor invasion, more lymph node metastasis, and peritoneal dissemination, resulting in a worse prognosis [6]. However, a study by Jiang et al. reported that no significant difference in survival was observed between SRC and non-SRC patients with advanced gastric cancer [25]. Our result showed that SRC was associated with larger tumor size (WMD: 0.63 cm, P=0.059), an increase in Borrmann IV type (OR: 3.29, P<0.001), and more peritoneal dissemination (OR: 1.69, P<0.001). The pooled HR of 1.17 (P<0.001) also indicated a worse prognosis for SRC patients with advanced gastric cancer. Therefore, more radical surgery should be performed for patients with advanced gastric cancer with SRC.
The underlying cause for the opposite prognoses for patients with early and advanced gastric cancer with SRC remains uncertain. One explanation is that early SRC is associated with a low aggressive state because of a CDH1 mutation [38]. When SRC has invaded the muscularis propria, it will accelerate tumor invasion, increase the risk of lymph node metastasis and peritoneal seeding, and worsen the chemosensitivity and prognosis [27].
There are some limitations in the present meta-analysis that must be considered. First, the main limitation is that all the included studies were retrospective studies from different countries, with the proportion of SRC ranging from 3.4% to 50.0%. Second, there is significant between-study heterogeneity in our meta-analysis. However, we included the studies strictly according to the WHO criteria for SRC, and we used a random-effects model to address heterogeneity appropriately.
Conclusions
This meta-analysis indicated that SRC tends to affect young females and tends to be located in the middle and lower third of the stomach. Early SRC patients have better prognoses than non-SRC patients, while advanced SRC patients exhibited the worse prognoses.
Acknowledgements
This work was supported in part by a grant from National Natural Science Foundation of China (81302144) and the Guangdong Science and Technology Department (2012B061700087), the Guangdong Medical Research Foundation (A2015124) and Guangzhou Science and Technology Program key projects (201508020247).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34:502-507
3. Henson DE, Dittus C, Younes M. et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770
4. Flejou JF. WHO Classification of digestive tumors: the fourth edition. Ann Pathol. 2011;31:S27-31
5. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97-100
6. Li C, Kim S, Lai JF. et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64-68
7. Piessen G, Messager M, Leteurtre E. et al. Signet Ring Cell Histology is an Independent Predictor of Poor Prognosis in Gastric Adenocarcinoma Regardless of Tumoral Clinical Presentation. Annals of Surgery. 2009;250:878-887
8. Nafteux PR, Lerut TE, Villeneuve PJ. et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg. 2014;260:1023-1029
9. Liu X, Cai H, Sheng W. et al. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420
10. Postlewait LM, Squires MH 3rd, Kooby DA. et al. The Prognostic Value of Signet-Ring Cell Histology in Resected Gastric Adenocarcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S832-839
11. Lu M, Yang Z, Feng Q. et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore). 2016;95:e4052
12. Zhang M, Zhu G, Zhang H. et al. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601-606
13. Hyung WJ, Noh SH, Lee JH. et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83
14. Gronnier C, Messager M, Robb WB. et al. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093-1099
15. Wang Z, Zhang X, Hu J. et al. Clinicopathological features and outcomes in patients undergoing radical resection for early gastric cancer with signet ring cell histology. J Visc Surg. 2015;152:357-361
16. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834
17. Higgins J, S G. Cochrane handbook for systematic reviews of interventions. New York, NY: Cochrane Collaboration, John Wiley and Sons. 2008
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634
19. Maehara Y, Sakaguchi Y, Moriguchi S. et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645-1650
20. Otsuji E, Yamaguchi T, Sawai K, Takahashi T. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216-220
21. Kim DY, Park YK, Joo JK. et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060-1064
22. Kunisaki C, Shimada H, Nomura M. et al. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319-1324
23. Lee JH, Choi IJ, Kook MC. et al. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg. 2010;97:732-736
24. Chiu CT, Kuo CJ, Yeh TS. et al. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749-1756
25. Jiang CG, Wang ZN, Sun Z. et al. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional study. J Surg Oncol. 2011;103:700-703
26. Kong P, Wu R, Yang C. et al. Prognostic Impact of the Signet Ring Cell Type in Node-Negative Gastric Cancer. Sci Rep. 2016;6:26313
27. Voron T, Messager M, Duhamel A. et al. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19:1027-1040
28. Chon HJ, Hyung WJ, Kim C. et al. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Gastric Cancer. 2017;265:946-953
29. Lauren P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49
30. Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39:2475-2485
31. Hass HG, Smith U, Jaeger C. et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren's) - single center experience of 160 cases. Onkologie. 2010;33:269-269
32. Liu K, Wan J, Bei Y. et al. Prognostic Impact of Different Histological Types on Gastric Adenocarcinoma: a Surveillance, Epidemiology, and End Results Database Analysis. Pathol Oncol Res. 2017
33. Matsui M, Kojima O, Kawakami S. et al. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992;22:421-425
34. The general rules for the gastric cancer study in surgery. Jpn J Surg. 1973;3:61-71
35. Uedo N, Iishi H, Tatsuta M. et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88-92
36. Isomoto H, Shikuwa S, Yamaguchi N. et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336
37. National Comprehensive Cancer Network N. NCCN Clinical Practice Guidelines in Oncology—Gastric Cancer—V.1.2016. National Comprehensive Cancer Network, Inc.; 2016.
38. Fitzgerald RC, Hardwick R, Huntsman D. et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. Journal of Medical Genetics. 2010;47:436-444
Author contact
![]() Corresponding author: Ying-Bo Chen,MD: Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 E Dongfeng Road, Guangzhou, Guangdong, 510060, China Tel: +86-020-87343625 E-mail: chenyborg.cn Shi Chen,PhD: The 6th Affiliated Hospital, Sun Yat-sen University, No. 26, YuanCun ErHeng Raod, TianHe District, 510655, Guangzhou,China. Tel: +86-13828496699 E-mail: chensh47sysu.edu.cn
Corresponding author: Ying-Bo Chen,MD: Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 E Dongfeng Road, Guangzhou, Guangdong, 510060, China Tel: +86-020-87343625 E-mail: chenyborg.cn Shi Chen,PhD: The 6th Affiliated Hospital, Sun Yat-sen University, No. 26, YuanCun ErHeng Raod, TianHe District, 510655, Guangzhou,China. Tel: +86-13828496699 E-mail: chensh47sysu.edu.cn

 Global reach, higher impact
Global reach, higher impact