3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(17):3410-3415. doi:10.7150/jca.21362 This issue Cite
Research Paper
The Effect of CA125 Nadir Level on Survival of Advanced-Stage Epithelial Ovarian Carcinoma after Interval Debulking Surgery
1. Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China;
2. School of Medicine, Tsinghua University, Beijing, China.
* These authors contributed equally to this work
Received 2017-6-6; Accepted 2017-8-31; Published 2017-9-20
Abstract
Purpose. The study aims at investigating the most reliable CA125-related factors in terms of predicting survival outcomes in advanced-stage epithelial ovarian carcinoma (EOC) patients received neoadjuvant chemotherapy (NAC).
Methods. The EOC patients treated with NAC at Peking Union Medical College Hospital by a single gynecological oncology team were enrolled for the retrospective study. The CA125-related variables were categorized into four groups: normalizations, nadirs, half-life and percentage reductions. Associations of these variables with progress-free survival (PFS) and overall survival (OS) were evaluated.
Results. Of the 101 patients included, 81 patients (80.2%) had progressed, and 51 patients (50.5%) had died of the disease progression. Univariate analysis showed that the CA125 nadir, reduction after the first, second and third postoperative chemotherapy cycles, and time to normalization were significantly (P<0.05) associated with PFS. The CA125 nadir, reduction after the first, second and third postoperative chemotherapy cycles were significantly (P<0.05) associated with OS. In the multivariate analysis, the CA125 nadir value was the most significant factor for PFS and OS, using the CA125 median level 13 U/ml as a cutoff value.
Conclusions. Our study suggests that the CA125 nadir value is the most reliable prognostic factor to predict PFS and OS in advanced EOC patients treated with NAC. This information is important in patient counseling and creating individualized follow-up plans.
Keywords: CA125, epithelial ovarian carcinoma, neoadjuvant chemotherapy, tumor growth kinetics, prognosis
Introduction
Epithelial ovarian carcinoma (EOC) is the most lethal genital tract tumor, with more than 150,000 deaths worldwide [1]. In China, approximately 52.1 new patients per 100,000 individuals were diagnosed with ovarian cancer in 2015, with 22.5 annual deaths per 100,000 beings' due to this malignancy alone [2]. Patients with advanced EOC have extremely poor prognoses, with 50%-95% of patients relapsing and 65% of patients dying in the following 5 years [3, 4]. Most patients relapse within the first 2 years after treatment; therefore, follow-up is quite important in detecting the recurrent disease in the early stages.
Serum CA125 detection is considered the standard method of monitoring therapeutic effects and disease recurrence. Previous studies have demonstrated several methods of using the predictive value of CA125 levels after patients received surgery, e.g., normalizations [5, 6], nadirs [7, 8], half-life [8-10] and percentage reductions [11]. While most of these studies were carried out in patients treated with primary debulking surgery (PDS), followed by six to eight cycles of chemotherapy, limited data was published to discuss whether the cutoff was applicable to the patients underwent neoadjuvant chemotherapy (NAC), followed by interval debulking surgery (IDS).
In recent years, two prospective randomized controlled clinical trials have compared NAC-IDS with PDS for the treatment of advanced epithelial ovarian carcinoma [12, 13]. These reports have demonstrated the equal oncologic outcomes between the two groups, but with lower postoperative morbidity in the NAC-IDS group. Though the results remain controversial, the application of neoadjuvant chemotherapy to advanced EOC patients has increased markedly in the past ten years, according to data from the National Cancer Database in the United States [14]. Because most previous research has focused on EOC patients with PDS, it is important to find and validate a powerful CA125-related indicator for patients with NAC-IDS. Therefore, we carried out this research to find out the most reliable CA125-related factors with which to predict survival and the cutoff for advanced EOC patients treated with NAC-IDS.
Material and Methods
Study population
From January 1996 to April 2015, the EOC patients of stage III and IV diagnosed according to International Federation of Gynecology and Obstetrics (FIGO) staging system and treated with NAC-IDS at Peking Union Medical College Hospital were identified. To avoid the researcher bias, all selected patient were diagnosed and treated by the same gynecologic oncologist and gynecological oncology team. The consecutive medical records describing initial and serial serum CA125 levels, demographic data and clinicopathological information were collected. Because of the long time interval of our study, patients received two kinds of platinum-based chemotherapy regimens as the previous study described [15]. All patients received at least six cycles of chemotherapy, including both neoadjuvant and adjuvant chemotherapy.
Following the initial eligibility screening, the inclusion criteria were: (1) patients have had neoadjuvant chemotherapy; (2) patient had an elevated CA125 level at the moment of diagnosis as well as before interval debulking surgery (>35U/ml); (3) patients had complete clinicopathological data with serial serum CA125 levels during primary treatment; (4) disease recurrence determination was based on an increasing CA125 level or the appearance of new lesions upon radiological examination and (5) patients with borderline ovarian tumors were excluded from this study.
Statistical analysis
Variables regarding the characteristics of patients were grouped as following: (1) because the residual disease was recorded as one of four sizes: non-visible, less than 2 cm, more than 2 cm and large mass in early medical records. The patients were divided into two groups, non-visible residual disease (NVRD) and visible residual disease (VRD), as in the previous published study [15]. (2) The continuous variables, e.g., related to CA125 and age, were converted into categorical variables, using the median value as the cutoff. The relative change in serum CA125 was calculated from the date of preoperative serum CA125 measurement to the date after the first, second and third cycles of adjuvant chemotherapy. The CA125 nadir level was defined as the lowest CA125 value when the treatment began. The time to nadir and time to normalization (< 35U/ml) were calculated from the time of the first neoadjuvant chemotherapy cycle to the time on which the CA125 level changed to the nadir or became normal. Half-life was calculated based on the formula Half-life=In2×(t2-t1)/InC1/C2, as reported in earlier studies [8-10]. C1 was the CA125 level before neoadjuvant chemotherapy, and C2 was the first time of CA125 level less than 35U/ml or the lowest antigen level within three months of starting chemotherapy, with t1 and t2 being the corresponding times in days.
Progression-free survival (PFS) was defined as the time of neoadjuvant chemotherapy started until the date of clinically confirmed recurrence or death from any cause. Overall survival (OS) was calculated from the date of neoadjuvant chemotherapy started to the date of death from any cause or the last follow-up date. The Kaplan-Meier method was used to estimate the hazard ratios (HRs) related to PFS and OS. A multiple-regression analysis according to the Cox proportional hazard model was used to identify the relative importance of variables as factors for predicting PFS and OS. All statistical analysis was two-sided and statistical significance was considered when P < 0.05. Analyses of all the data was performed using SPSS V. 20.0 software (IBM Inc., Chicago, IL).
Results
Characteristics of the study population
Following the initial eligibility screening, 118 patients with advanced EOC treated with NAC-IDS were identified. Among them, 13 patients had CA125 <35U/ml before surgery and 4 patients with incomplete data of serum CA125 after surgery were excluded. Therefore, 101 patients were available for the data analysis. The characteristics of the patient for the final analysis are presented in Table 1. The median age was 55 years with a range of 36-76 years-old. Eighty-eight patients (87.1%) had FIGO stage III disease and 85 patients (84.2%) had serous tumor histology upon their initial diagnoses. The results regarding residual disease after IDS were non-visible residual disease in 30 patients (29.7%) and visible residual disease in 71 patients (71.3%). Ninety-eight patients (97.0%) received between one and three cycles of NAC, and only 3 (3.0%) patients received more than four cycles of neoadjuvent chemotherapy. The total cycles of chemotherapy applied were six to eight cycles in 70 patients (69.3%) and more than eight cycles in 31 patients (30.7%).
The median follow-up for the 101 patients was 34 months with a range of 6-111 months. During the follow-up, relapses were recorded in 81 patients (80.2%). There were 51 patients (50.5%) died of the disease. The Kaplan-Meier curve and log-rank test for PFS and OS with regard to age, stage, histology, cytoreduction level and total chemotherapy cycles were shown in Table 2. In the univariate analysis, cytoreduction to non-visible residual disease was significantly correlated with the risk of tumor relapse (P=0.033), and also the risk of OS (P=0.039).
Characteristics of CA125-related parameters
The median CA125 value upon diagnosis in patients with FIGO stage III-IV ovarian cancer was 1952.0 U/ml (56.6-56541.0 U/ml) and the median preoperative CA125 value was 269.0 U/ml (36.8-10971.0 U/ml). The median CA125 level after the first, second and third adjuvant chemotherapy cycles after interval debulking surgery were 44.80 U/ml (7.0-1266.0 U/ml), 27.2 U/ml (3.8-935.1 U/ml) and 19.1 U/ml (0.6-545.1 U/ml), respectively. The median relative changes in CA125 from the preoperatively measurement to the measurement after the first, second, and third adjuvant chemotherapy cycles were 77.2% (-62.6%-99.6%), 89.7% (-27.80%-99.9%) and 91.8% (-160.33%-99.9%), respectively (-62.6%, -27.80% and -160.33% indicate the CA125 levels increased by 62.6%, 27.80% and 160.33%, respectively). The median nadir CA125 value was 12.6U/ml (0.6-545.1 U/ml), the median time to nadir was 161 days (44.0-433.0 days), the median time to normalization was 94.0 days (40.0-811.0 days) and the median half-life for CA125 was 15.9 U/ml (4.8-270.9 U/ml). All the median CA125-related variables are shown in Table 1.
Demographic and clinical characteristics of 101 patients
| Characteristic | Total,n(%)/mean or median(range) |
|---|---|
| Age(years) | 55(36-76) |
| Primary site of disease | |
| Ovary | 94(93.1) |
| Peritoneum | 4(4.0) |
| Fallopian tube | 3(3.0) |
| FIGO Stage | |
| III | 88(87.1) |
| IV | 13(12.9) |
| Tumor Grade | |
| G1 | 3(3.0) |
| G2 | 11(10.9) |
| G3 | 84(83.1) |
| UnKnown | 3(3.0) |
| Histology | |
| Serous | 85(84.2) |
| Endometrioid | 3(3.0) |
| Clear cell | 6(5.9) |
| Mucinous | 1(1.0) |
| Others | 6(5.9) |
| CA125 presentation (U/ml) | 1952.0(56.6-56541.0) |
| CA125 preoperatively (U/ml) | 269.0(36.8-10971.0) |
| CA125 after 1st CT postop (U/ml) | 44.80(7.0-1266.0) |
| CA125 after 2nd CT postop (U/ml) | 27.2(3.8-935.1) |
| CA125 after 3rd CT postop (U/ml) | 19.1(0.6-545.1) |
| CA125 relative change preop to 1st CT postop (%) | 77.2(-62.6-99.6) |
| CA125 relative change preop to 2nd CT postop (%) | 89.7(-27.80-99.9) |
| CA125 relative change preop to 3rd CT postop (%) | 91.8(-160.33-99.9) |
| Nadir (U/ml) | 12.6(0.6-545.1) |
| Time to Normalization (day) | 94.0(40.0-811.0) |
| Time to nadir (day) | 161.0(44.0-433.0) |
| CA125 half-life (day) | 15.9(4.8-270.9) |
| Residual disease | |
| No visible residual | 30(29.7) |
| Residual disease≤2cm | 54(53.5) |
| Residual disease>2cm | 17(16.8) |
| Chemotherapy cycles | |
| 6-8 | 70(69.3) |
| >8 | 31(30.7) |
FIGO: federation of gynecology and obstetrics; CT: chemotherapy; Postop: postoperatively; Preop: preoperatively
Survival analyses for CA125-related parameters
We performed survival analyses for ten CA125-related variables: CA125 level after the (1) first, (2) second and (3) third adjuvant chemotherapy cycles postoperatively; the relative percentage reduction from the preoperative measurement to the measurement after the (4) first, (5) second and (6) third postoperative chemotherapy cycles; (7) nadir level; (8) time to normalization; (9) time to nadir and (10) half-life. The cutoff used to divide these parameters into two groups was described in the Method section.
Univariate analyses of associated clinicopathological characteristics with progression free survival and overall survival
| Variables | Categories | P value of PFS | P value of OS |
|---|---|---|---|
| Age(years) | ≤55 | 0.968 | 0.653 |
| >55 | |||
| FIGO Stage | III | 0.583 | 0.454 |
| IV | |||
| Histology | High-grade serous | 0.310 | 0.314 |
| Others | |||
| Cytoreduction to no visible disease | Yes | 0.033* | 0.039* |
| No | |||
| Chemotherapy cycles >8 | Yes | 0.286 | 0.148 |
| No |
* Statistically significant (P < 0.05); FIGO: federation of gynecology and obstetrics; PFS: progression free survival; OS: overall survival
In the univariate Kaplan-Meier analysis, the factors significantly (P<0.05) associated with PFS were CA125 level after the first, second, and third adjuvant chemotherapy cycles postoperatively; the nadir value and the time to normalization. The CA125 level after the first, second and third postoperative chemotherapy cycles, and the nadir were significantly (P<0.05) associated with OS (Table 3).
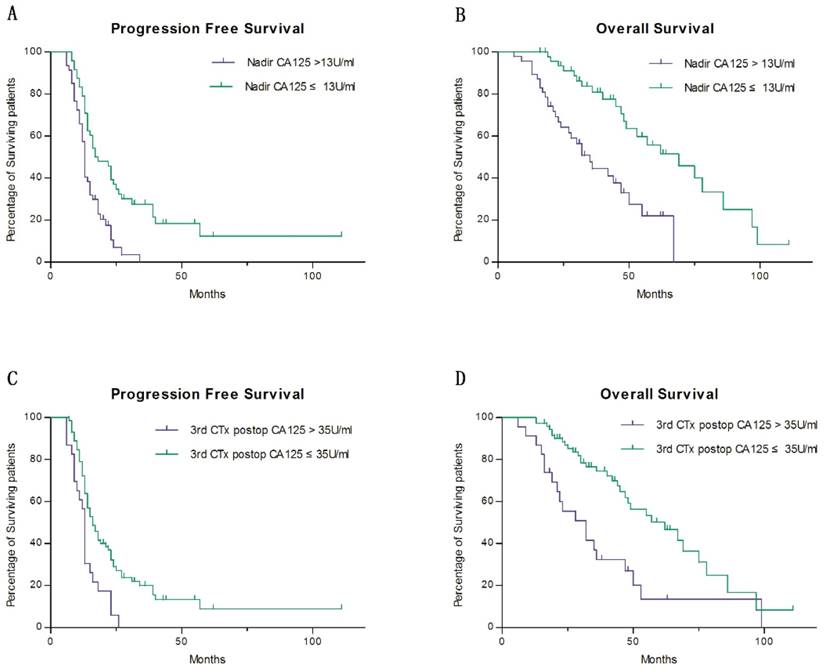
When considering the most significant prognostic variables for PFS and OS, we used a multivariate Cox regression to analyze the factors which have showed significant differences in previous univariate analysis. As a consequence, the nadir was selected as the most significant variable to predict PFS and OS, and was divided into two groups at a cutoff value of 13 U/ml. The patients whose CA125 nadir levels ≤13 U/ml had a two-year PFS rates of 39.2%, as compared with 10.5% for patients whose CA125 nadir levels were >13 U/ml (HR 1.796, 95% CI 1.002-3.221, P=0.049) (Fig. 1 a). The 5-year OS rates in patients with CA125 nadir values ≤13 U/ml and >13 U/ml were 55.8% and 21.9%, respectively (HR 2.207, 95% CI 1.416-5.969, P=0.004) (Fig. 1 b). Meanwhile, the CA125 value after the third postoperative chemotherapy cycle was identified as the second most useful variable in predicting OS, though not PFS (P=0.210) (Fig. 1 c). Patients with CA125 values ≤35 U/ml after the third postoperative chemotherapy cycle had 5-year OS rates of 50.2%, as compared to 13.5% for patients whose CA125 levels were >35 U/ml (HR 2.279, 95% CI 1.052-4.935, P=0.037) (Fig. 1 d).
Discussion
In our presented study, the power of various CA125-related variables of predicting the survival of advanced EOC patients treated with NAC-IDS was evaluated. Our data suggested that CA125 nadir after interval debulking surgery is the most significant prognostic factor for predicting tumor recurrence and OS. And for all we know, this is the first study to evaluate the various parameters associated with CA125 serum level in advanced EOC patients received NAC-IDS and treated by a single gynecological oncologist.
Univariate and multivariate analyses of associated CA125 related prognostic factors with progression free survival and overall survival
| Variables | Categories | PFS | OS | ||||
|---|---|---|---|---|---|---|---|
| Univariate P value | Multivariate P value | Multivariate HR(95%CI) | Univariate P value | Multivariate P value | Multivariate HR(95%CI) | ||
| CA125 after 1st CT postop (U/ml) | ≤35 | 0.013* | 0.645 | 1.162(0.613-2.203) | 0.006* | 0.309 | 1.492(0.691-3.224) |
| >35 | |||||||
| CA125 after 2nd CT postop (U/ml) | ≤35 | 0.006* | 0.650 | 0.846(0.412-1.739) | 0.008* | 0.356 | 0.664(0.279-1.583) |
| >35 | |||||||
| CA125 after 3rd CT postop (U/ml) | ≤35 | 0.002* | 0.210 | 1.551(0.781-3.083) | 0.001* | 0.037* | 2.279(1.052-4.935) |
| >35 | |||||||
| CA125 relative change preop to 1st CT postop (%) | ≤77 | 0.765 | 0.135 | ||||
| >77 | |||||||
| CA125 relative change preop to 2nd CT postop (%) | ≤90 | 0.542 | 0.234 | ||||
| >90 | |||||||
| CA125 relative change preop to 3rd CT postop (%) | ≤92 | 0.913 | 0.191 | ||||
| >92 | |||||||
| Nadir (U/ml) | ≤13 | 0.000* | 0.049* | 1.796(1.002-3.221) | 0.000* | 0.004* | 2.207(1.416-5.969) |
| >13 | |||||||
| Time to Normalization (day) | ≤94 | 0.017* | 0.576 | 1.223(0.604-2.476) | 0.066 | ||
| >94 | |||||||
| Time to nadir (day) | ≤160 | 0.180 | 0.318 | ||||
| >160 | |||||||
| CA125 half-life (day) | ≤16 | 0.094 | 0.083 | ||||
| >16 | |||||||
* Statistically significant (P < 0.05); PFS: progression free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; CT: chemotherapy; Postop: postoperatively; Preop: preoperatively
Survival estimates stratified by CA125-related factors a Progress free survival comparing between groups of CA125 nadir levels ≤13 U/ml and CA125 nadir levels >13 U/ml b Overall survival comparing between groups of CA125 nadir levels ≤13 U/ml and CA125 nadir levels >13 U/ml c Progress free survival comparing between groups of CA125 levels ≤35 U/ml after the third postoperative chemotherapy cycle and that of CA125 levels >35 U/ml d Overall survival comparing between groups of CA125 levels ≤35 U/ml after the third postoperative chemotherapy cycle and that of CA125 levels >35 U/ml
The post-surgery levels and changes in serum CA125 have been extensively investigated for epithelial ovarian cancer response monitoring. CA125 normalization is the most studied target in predicting survival of advanced ovarian cancer [5, 6, 16-18]. The vast majority of research reported that the normalization of CA125 values after the third adjuvant chemotherapy cycle was the best variable to predict survival [6, 16-18]. Another response value regarding CA125 levels is the nadir. The nadir is reported using various cut-offs, and various time points are used for nadir determination [7, 8, 19-20]. CA125-related time-to-event variables included the time to CA125 normalization and the time to nadir, both of which have been shown to correlate with PFS [20] and OS [21]. Of the kinetic variables regarding CA125, the most reported is CA125 half-life. Several studies have shown a strong correlation between CA125 half-life and the survival [8-10, 22]. However, patients treated with PDS or both PDS and NAC-IDS were enrolled in those studies. The cut-offs and conclusion may not apply to patients with NAC. Therefore, we carried out this research to figure out the most powerful and reliable CA125-related prognostic predictor using the reported calculation method and to find a cut-off value that might apply to patients with NAC.
In our cohort, we found that the nadir of CA125 was the most powerful predictor of survival among the evaluated CA125-related measures. Crawford et al. observed that among 79 EOC patients with FIGO stage IC-IV disease and underwent PDS following chemotherapy, CA125 nadir after PDS was predictive of PFS and OS with a cut-off value at 10U/ml [19]. Prat et al. examined 96 EOC patients with FIGO stage III-IV disease treated with PDS or IDS followed by chemotherapy and drew the same conclusion. Patients whose CA125 nadir values less than 10U/ml had significantly higher rates of PFS and OS than those of patients whose CA125 nadir values more than 10U/ml [7]. Unlike previous studies, our study focused on the stage IIIC-IV patients with NAC operand treated by a single surgeon, since this advanced stage of patients accounted for 70% of all ovarian cancer cases. Patients with CA125 nadir values ≤ 13 U/ml have a better prognosis, with less recurrence and longer OS. This finding reminds gynaecological oncologist that patients whose CA125 values arrived at normal but were more than 13 U/ml at the end of chemotherapy should be followed up more closely and more frequently than others. Also, we should consider whether patients should be given maintenance therapy when nadir CA125 is higher than 13 U/ml, and significant progress have been made in using PARP inhibitors as a maintenance therapy for EOC patients [23]. However, the retrospective nature of our study limits the conclusions, and a prospective study designed to validate the various reported CA125-related measures is strongly suggested.
CA125 is not the best prognostic variable, but it remains an important support for gynecological oncologists in managing the diagnoses, treatment and follow-up of EOC patients. Our study suggested that CA125 nadir value was the most significant prognostic factor for predicting progress-free survival and overall survival in advanced EOC patients treated with NAC-IDS. It is important for patient counseling and decision-making for individual follow-up plans.
Compliance with ethical standards
Research involving human participants
All procedures performed in our studies involving human participants have been approved by the ethics committee of Peking Union Medical College Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Competing Interests
The authors have declared that no competing interest exists.
References
1. WHO. Global health observatory data repository. Number of deaths: world by cause. 2014
2. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-32
3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30
4. Gadducci A, Cosio S, Zola P. et al. Surveillance procedures for patients treated for epithelial ovarian cancer: a review of the literature. Int J Gynecol Cancer. 2007;17(1):21-31
5. Markman M, Federico M, Liu PY. et al. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol. 2006;103(1):195-8
6. Rocconi RP, Matthews KS, Kemper MK. et al. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2009;114(2):242-5
7. Prat A, Parera M, Peralta S. et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008;19(2):327-31
8. Riedinger JM, Eche N, Basuyau JP. et al. Prognostic value of serum CA 125 bi-exponential decrease during first line paclitaxel/platinum chemotherapy: a French multicentric study. Gynecol Oncol. 2008;109(2):194-8
9. Gadducci A, Zola P, Landoni F. et al. Serum half-life of CA 125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: results of a multicentric Italian study. Gynecol Oncol. 1995;58(1):42-7
10. Gadducci A, Cosio S, Fanucchi A. et al. The predictive and prognostic value of serum CA 125 half-life during paclitaxel/platinum-based chemotherapy in patients with advanced ovarian carcinoma. Gynecol Oncol. 2004;93(1):131-6
11. Lee CK, Friedlander M, Brown C. et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. J Natl Cancer Inst. 2011;103(17):1338-42
12. Vergote I, Tropé CG, Amant F. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine. N Engl J Med. 2010;363(10):943-53
13. Kehoe S, Hook J, Nankivell M. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249-57
14. Melamed A, Hinchcliff EM, Clemmer JT. et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143(2):236-40
15. Zeng J, Yin J, Song X. et al. Reduction of CA125 Levels During Neoadjuvant Chemotherapy Can Predict Cytoreduction to No Visible Residual Disease in Patients with Advanced Epithelial Ovarian Cancer, Primary Carcinoma of Fallopian tube and Peritoneal Carcinoma. J Cancer. 2016;7(15):2327-32
16. Riedinger JM, Bonnetain F, Basuyau JP. et al. Change in CA 125 levels after the first cycle of induction chemotherapy is an independent predictor of epithelial ovarian tumour outcome. Ann Oncol. 2007;18(5):881-5
17. Fayers PM, Rustin G, Wood R. et al. The prognostic value of serum CA 125 in patients with advanced ovarian carcinoma: an analysis of 573 patients by the Medical Research Council Working Party on Gynaecological Cancer. Int J Gynecol Cancer. 1993;3(5):285-92
18. Pelissier A, Bonneau C, Chéreau E. et al. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2014;135(3):542-6
19. Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol. 2005;16(1):47-50
20. Riedinger JM, Wafflart J, Ricolleau G. et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006;17(8):1234-8
21. Frasci G, Conforti S, Zullo F. et al. A risk model for ovarian carcinoma patients using CA 125: Time to normalization renders second-look laparotomy redundant. Cancer. 1996;77(6):1122-30
22. Mano A, Godinho I, Falcao AC. CA 125 half-life breakpoint between a “good” and “poor” prognosis in patients with ovarian cancer. Int J Gynaecol Obstet. 2005;88(3):333-5
23. Spriggs DR, Longo DL. PARP Inhibitors in Ovarian Cancer Treatment. N Engl J Med. 2016;375(22):2197-8
Author contact
![]() Corresponding authors: Lingya Pan, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China Tel: +86-10-65296203, Fax: +86-10-65124875 Email: panlycn and Ying Jin, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China Tel: +86-10-69155731, Fax: +86-10-65124875 Email: jinyingcn
Corresponding authors: Lingya Pan, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China Tel: +86-10-65296203, Fax: +86-10-65124875 Email: panlycn and Ying Jin, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 1 Shuai Fu Yuan, Wang Fu Jing Street, Beijing 100730, China Tel: +86-10-69155731, Fax: +86-10-65124875 Email: jinyingcn

 Global reach, higher impact
Global reach, higher impact