3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(17):3480-3489. doi:10.7150/jca.21241 This issue Cite
Research Paper
Restoration of KLF4 Inhibits Invasion and Metastases of Lung Adenocarcinoma through Suppressing MMP2
1. Department of Respiratory Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
2. Center of Organ Transplantation, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Received 2017-5-30; Accepted 2017-8-6; Published 2017-9-27
Abstract
Background: KLF4 is a zin-finger transcription factor that plays roles in differentiation, development, and proliferation. Recent studies show that KLF4 is involved in tumorigenesis and somatic cells reprogramming. Metastasis is the primary cause of death in patients with lung cancer, and its biological mechanisms are poorly understood.
Goals: In this study, we aim to explore the expression pattern and biological function of KLF4 in lung adenocarcinoma.
Methods: We determined KLF4 in lung adenocarcinoma tissue and cell lines, using immunohistochemistry and western blotting. And we further analyzed the correlation between KLF4 expression and clinicopathologic parameters. We restored KLF4 expression and studied its effect on lung adenocarcinoma cells in vivo and in vitro. Luciferase assay was used to study impact of KLF4 on activity of MMP2 promoter.
Results: KLF4 is dramatically down-regulated in lung adenocarcinoma tissue and cell lines. Promoter methylation contributes to the down-regulation of KLF4. Down-regulation of KLF4 in lung adenocarcinoma tissue is significantly associated with reduced survival time. Restoration of KLF4 inhibits migration and invasion of lung adenocarcinoma cells in vitro. Metastases to lungs significantly decrease in mice intravenously injected with tumor cells overexpressing KLF4. KLF4 inhibits invasion and metastasis via suppressing MMP2 promoter activity.
Conclusion: The ability of KLF4 to inhibit migration, invasion, and metastasis of lung tumor cells indicates a potential role of KLF4 as therapeutic target in lung adenocarcinoma. KLF4 might be utilized as a favorable biomarker for prognosis of lung adenocarcinoma patients.
Keywords: Krüppel-like factor, GKLF, Invasion, Metastasis, MMP2
Background
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related mortality worldwide [1]. Lung adenocarcinoma is the most common histologic subtype of lung cancer, accounting for more than 40% of incidence [2, 3]. Moreover, lung adenocarcinoma has a high risk of distant metastasis at each disease stage and the primary cause of lung cancer‑associated mortality is metastasis [4]. However, the mechanisms underlying the metastasis of lung adenocarcinoma remain incompletely understood.
Krüppel-like factor 4, formerly known as gut-enriched Krüppel-like factor (GKLF), is a member of the Krüppel-like factor (KLF) transcription factor family. The KLFs play important roles in proliferation, differentiation, development, and apoptosis [5-7]. KLF4 mRNA expression is found primarily in postmitotic, terminally differentiated epithelial cells of the skin and gastrointestinal tract [8]. It is also detected in the organs including lung [9], testis [9], skin [10] and thymus [11], and in vascular endothelial cells [9]. KLF4 plays important roles in cell-cycle regulation and differentiation [12]. In vitro, KLF4 inhibits proliferation by blocking cell cycle progression at the G1/S boundary [13] and mediates the cell cycle checkpoint function of the tumor suppressor p53 following DNA damage [14, 15]. Recently, KLF4 is identified as one of four transcription factors required for the induction of pluripotent stem cells (iPS) [16] and is indispensable for the maintenance of stemness [17].
Interestingly, multiple lines of evidence show that KLF4 can function as an oncogene or a tumor suppressor in a context-dependent manner [18]. Its role is likely determined by expression patterns of other genes and the chromatin environment of tumor cells. However, the exact mechanisms underlying these contradictions remain undefined.
High KLF4 expression has been demonstrated in primary breast ductal carcinoma, head and neck squamous cell carcinoma, and skin squamous cell carcinoma, suggesting the role of oncogene in these tumors [19-21]. Ectopic expression of KLF4 in mice induced squamous epithelial dysplasia [20]. In a spontaneously metastatic 4T1 breast cancer mouse model and an immunodeficient NOD/SCID mouse model, KLF4 knockdown delayed tumor development and inhibited pulmonary metastasis [22]. Consistently, high KLF4 expression has been revealed to be a poor prognostic factor in skin cancer and early stage breast cancer[23, 24].
By contrast, KLF4 was frequently decreased or lost in medulloblastoma, lung, esophageal, gastrointestinal, pancreatic, hepatic, bladder, renal, and cervical cancers [25-35]. Low expression of KLF4 is an indicator for poor prognosis in gastric and colon cancer, suggesting KLF4 as a tumor suppressor [27, 36]. Promoter methylation and loss of heterozygosity (LOH) contribute to the reduced KLF4 expression in human gastrointestinal cancer and medulloblastoma [25, 27, 30, 37]. Hypermethylation of KLF4 promoter was also demonstrated in hepatocellular, cervical, renal cell carcinoma [31, 33, 34]. Restoration of KLF4 inhibited tumorigenecity of colonic and gastric cancer cells both in vitro and in vivo [27, 38]. Deletion of KLF4 in mouse models leads to abnormal differentiation, increased proliferation in the colon and gastric epithelia [39, 40].
In human lung cancer, Hu et al. reported that enforced expression of KLF4 in cancer cells induced cell cycle arrest at G1-S checkpoint and inhibited cell growth, via up-regulating p21 and down-regulating cyclin D1 [29] and human telomerase reverse transcriptase (hTERT) [41]. Consistently, enforced expression of KLF4 in lung cancer cells suppressed tumor growth in vivo.
However, the clinical relevance and effect on invasion and metastasis of KLF4 in lung adenocarcinoma is still elusive.
Material and methods
Patient information and samples
Formalin-fixed and paraffin-embedded tissues of 60 lung adenocarcinoma cases diagnosed from January 2006 to December 2007 were retrieved from the archives of our hospital. All of the cases were classified according to the cancer staging criteria of American joint committee [42]. Complete clinical information, including the five-year survival outcome, was obtained for this study. This study was carried out with patients' consent and approval of Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. Tissue microarray (TMA) with 33 cases of matched lung adenocarcinoma, tumor-adjacent tissue (≥ 3cm from the tumor) and normal lung tissues (≥ 5cm from the tumor) was brought from Auragene Bioscience, China.
Immunohistochemistry
Immunohistochemistry was performed as described previously [43] with antibody for human KLF4 (1:100; Abcam, USA). Negative controls had the primary antibody replaced by PBS. The method for scoring took into account the intensity of staining (intensity score) and proportion of positively staining tumor cells (extent score). The extent of staining, defined as the percentage of positively staining tumor cells in relation to the whole tissue area, was scored as follows: 0,<10%; 1, 10-25%; 2, 26-50%;3, 51-75%; and 4,>75%. The intensity of staining was scored as follows: 0, negative; 1, light yellow; 2, yellow brown; 3, brown. The total IHC score equals to multiply the intensity score by extent score. And total score of ≥ 5 and < 4 were regarded as high and low KLF4 expression levels, respectively. Scoring of sections was completed by two independent observer blinded to the clinical information. Conflicting scores were resolved by discussion.
Cell lines and culture conditions
The human lung adenocarcinoma cell lines A549 and H322 were purchased from Cell Bank, Chinese Academy of Sciences (Shanghai, China). 293T cells and the immortal human bronchial epithelial cell line 16HBE was gifted from the Cancer Center of Sun Yat-sen University. All of the cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum(FBS), 100 U/ml penicillin G sodium and 100 μg/ml streptomycin sulfate at 37°C in a humidified atmosphere containing 5% CO2.
5'-aza-2'-deoxycytidine treatment
Cell lines were cultured in fresh medium containing 10% FBS for 24 hours, then the medium was replaced by complete medium with 0.01, 0.1, 1, 5, or 10 mM 5'-aza-2'-deoxycytidine (5-Aza-dC; Sigma, USA) or equal volume of vehicle (phosphate buffer solution, PBS). The medium containing drug or vehicle was replaced every 24 hours.
Plasmid Construction, transfection and luciferase reporter gene assays
The eukaryotic expression plasmid of pcDNA3.1+ was routinely stored in our laboratory, and pGL3 and the pRL-TK plasmids were purchased from Promega, USA. The full-length KLF4 cDNA was subcloned into vector pcDNA3.1+, and MMP2 proximal promoter (-1659 ~ +57, transcription initiation site as +1) was amplified from human genomic DNA and cloned into the reporter plasmids of pGL3. The inserts were verified by sequencing. For transfection, A549 or H322 cells were plated into 6-well plates and allowed to adhere for 12 hours. Lipofectamine 2000 (Invitrogen, USA) was used for transfecting the cells with either pcDNA3.1-KLF4 or pCDNA3.1 according to the manufacturer's instruction. Cells were cultured for 72 additional hours after transfection. G418 (MERCK, Germany) were used to generate stably transfected cell lines. For luciferase assay, 293T cells were co-transfected with expression plasmids, promoter reporter plasmids and the pRL-TK plasmids using Lipofectamine 2000. 12 hours later, cells were washed with PBS twice and then incubated in fresh medium supplemented with 10% fetal bovine serum for additional 36 hours. Luciferase activity was detected with the Dual Luciferase Reporter Assay System (Promega, USA) according to the manufacturer's instructions. The relative luciferase activity was determined using a ModulusTM Laboratory Luminometer (Turner Biosystems, USA). Transfection efficiency was normalized to Renilla luciferase activity.
Real time-PCR analysis
Total RNA was extracted using Trizol (Invitrogen, USA) according to the manufacturer's protocol. Complementary DNA was then synthesized using PrimeScript RT reagent Kit (Invitrogen, USA). Quantitative RT-PCR (qRT-PCR) was performed using SYBR Premix Ex Taq (TaKaRa, Japan) on ABI 7900PRISM PCR System (Applied Biosystems, USA). Specific primers used are as followed: KLF4: 5´-TCGTCCACCGCAAATGCTTCTAG-3' (forward), 5'-ACTGCTGTCACCTTCACCGTTCC-3' (reverse); MMP2: 5'-ATGGATCCTGGCTTTCCC-3' (forward), 5'-GCTTCCAAACTTCACGCTC-3' (reverse); β-actin: 5´-TCGTCCACCGCAAATGCTTCTAG-3' (forward), 5'-ACTGCTGTCACCTTCACCGTTCC-3' (reverse).
Western blot analysis
Western blotting was performed as previously described [34] with antibody against KLF4 (1:500 dilution; Abcam, USA), MMP2 (1:1000 dilution; CST, USA), or GAPDH (1:5000, Bioworld, USA), and detection was carried out using biotinylated goat anti-rabbit antibody (1:5000, Sigma, USA), followed by incubation with peroxidase-linked avidin-biotin complex.
Migration and invasion assay
Transwell chambers (24-well insert, 8 micron; BD, USA) with or without precoated matrigel were used to analyze the ability of cell invasion and migration respectively. 48h after transfection, cells were resuspended in fresh medium with 1% FBS and added to the upper chambers. At the end point of incubation (migration assay, 48 hours and invasion, 72 hours), cells on the upper membrane surface were removed. The lower membrane surface was fixed by 4% formaldehyde, and then stained with crystal violet and counted under a microscope.
Animal Experiments
Six-week-old female athymic BALB/c nude mice were purchased from Laboratory Animal Center of Guangdong Province (Guangdong, China), and housed in laminar flow cabinets under specific pathogen-free conditions. To produce experimental lung metastases, control or KLF4 overexpressing H322 cells (1×106/100ul) were injected into tail vein. Mice were killed 30 to 40 days after tumor injection or when they had become moribund. Lungs were separated and weighted. Metastases on the surface of lung were counted (double blinded) with the aid of a dissecting microscope. Then the lungs were fixed by formalin, embedded with paraffin and cut into 4 μm-thick slices. Slices were stained with hematoxylin-eosin and examined under microscope. This study was approved by the Animal Care and Use Committee of The First Affiliated Hospital of Sun Yat-sen University.
Statistical analysis
All statistical analyses were carried out using the SPSS 17.0 software. Expression of KLF4 was compared between clinicopathological characteristics using Chi-square test. Kaplan-Meier method was used to plot the survival curves, and the differences between curves were validated by log-rank test. The significance of various clinical risk factors for survival were initially analyzed using univariate analysis and the significant variables were then analyzed using multivariate analysis in the Cox proportional hazards regression model. For in vitro and in vivo studies, each experiment was done independently at least thrice with similar results; one representative experiment was presented. The in vitro and in vivo data were expressed as the mean ± standard deviation (SD), and analyzed for significance using the Student's t test or one-way analysis of variance. All statistical tests were two-tailed. P < 0.05 was considered statistically significant.
Results
Promoter hypermethylation contributed to reduced expression of KLF4 in lung adenocarcinoma cells and tissue
Promoter hypermethylation contributed to reduced expression of KLF4 in lung adenocarcinoma cells and tissue. Expression of KLF4 was detected in lung adenocarcinoma cell lines (A549 and H322) and human bronchial epithelial cells (16HBE) using western blotting (A) and real-time PCR (B). GAPDH and β-actin were used as control. C, Immunohistochemistry showed that KLF4 protein was significantly reduced in lung adenocarcinoma (C1), compared with matched tumor adjacent tissue (C2) and normal lung tissue (C3). D, Lung adenocarcinoma cell lines were treated with 5'-aza-2'-deoxycytidine (5-Aza-dC), a methyltransferase inhibitor. Results showed that 5-Aza-dC restored KLF4 expression in a concentration- and time-dependent manner. *p<0.05.
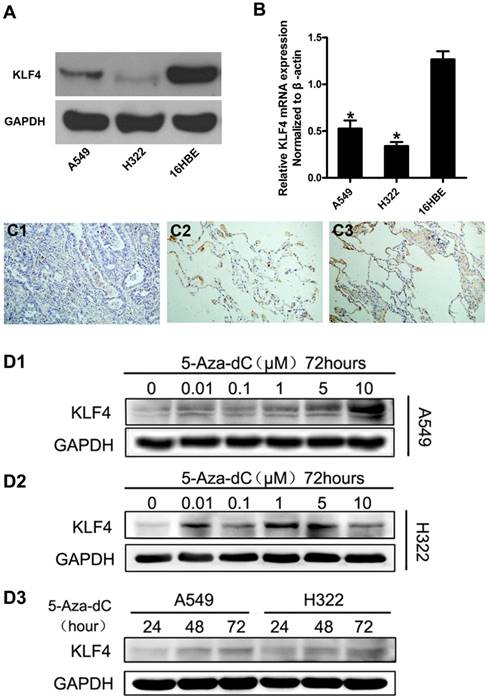
We firstly investigated the expression of KLF4 in lung adenocarcinoma cell lines and immortal human bronchial cell line using western blot and quantitative real-time PCR. The results showed that KLF4 was significantly decreased in lung adenocarcinoma cell lines at both mRNA and protein levels, compared with the normal bronchial cell line (Fig. 1A, 1B). To further verify this finding, we detected KLF4 protein in 33 matched lung adenocarcinoma, tumor-adjacent tissue and normal lung tissue in a tissue microarray (TMA) using immunohistochemistry. We found moderate to strong KLF4 expression in 100% (33/33) of the normal lung tissue and tumor-adjacent tissue. However, only 5 out of 33 (15.2%) cases of lung adenocarcinoma was KLF4-positive (Fig. 1C). Since the promoter region of KLF4 contains typical CpG islands, we treated the lung adenocarcinoma cells with 5'-aza-2'-deoxycytidine, an inhibitor of DNA methyltransferase, to determine whether promoter hypermethylation contributes to the downregulation. Compared with control, 5'-aza-2'-deoxycytidine significantly restored KLF4 protein expression in A549 in a time- and concentration-dependent manner, and in H322 in a time-dependent manner. Even though not in a strict concentration-dependent manner, addition of 5'-aza-2'-deoxycytidine did restore the expression of KLF4 in H322 to various degree (Fig. 1D). These results indicate that promoter hypermethylation may contribute to the reduced KLF4 expression and KLF4 may play a role of tumor suppressor in lung adenocarcinoma.
Reduced KLF4 expression was related to metastatic progress and poor survival in lung adenocarcinoma
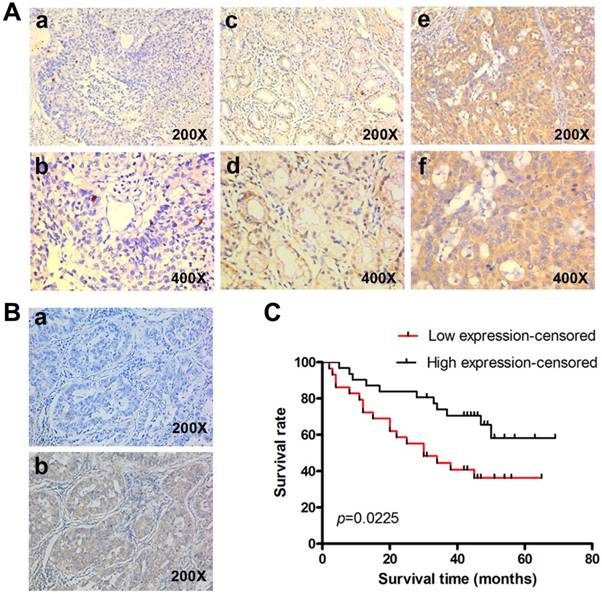
To determine the clinical significance of KLF4, we performed IHC in 60 lung adenocarcinoma tissue with archived clinical data. Representative images of different staining were shown in Fig. 2A. Negative control had the KLF4 antibody replaced by PBS (Fig. 2B). Results showed that patients with low KLF4 were more likely to have advanced tumor staging and to be classified as M1 (Table 1). There was no difference in KLF4 expression when stratified by age, gender and smoking status. Kaplan-Meier curve showed that patients in low KLF4 expression group had shorter survival time than those in high expression group (Fig. 2C, log-rank test p=0.022). Other variables that affected survival in univariate analyses included T classification, N classification, M classification and staging (Table 2). The patients' age at diagnosis, sex, and smoking status had no significant effect on survival. Cox proportional hazards model was further utilized for multivariate analysis. When the effect of covariates was adjusted, the loss of KLF4 expression was an independent predictor for poor survival (hazard ratio (HR), 2.382; 95% confidence interval (CI), 1.130-5.021).
In addition, the advanced staging (stage IIIB-IV) was also an independent predictor for poor survival (HR, 2.326; 95% CI, 1.038-5.213). These results suggest that KLF4 may play a role in the progression and may be a prognostic biomarker in lung adenocarcinoma.
Restoration of KLF4 inhibited invasion and lung colonization
Reduced KLF4 expression predicts poorer survival in patients with lung adenocarcinoma. (A) KLF4 was detected in 60 lung adenocarcinoma tissues with archived clinical data using immunohistochemistry. Shown were representative images of negative (a, b), weak-positive (c, d), and strong-positive (e, f) staining. (B) Negative control had the KLF4 antibody replaced by PBS (a). (C) Kaplan-Meier curve showed that patients with low KLF4 expression had shorter survival time than those with high expression (log-rank test, p=0.022).
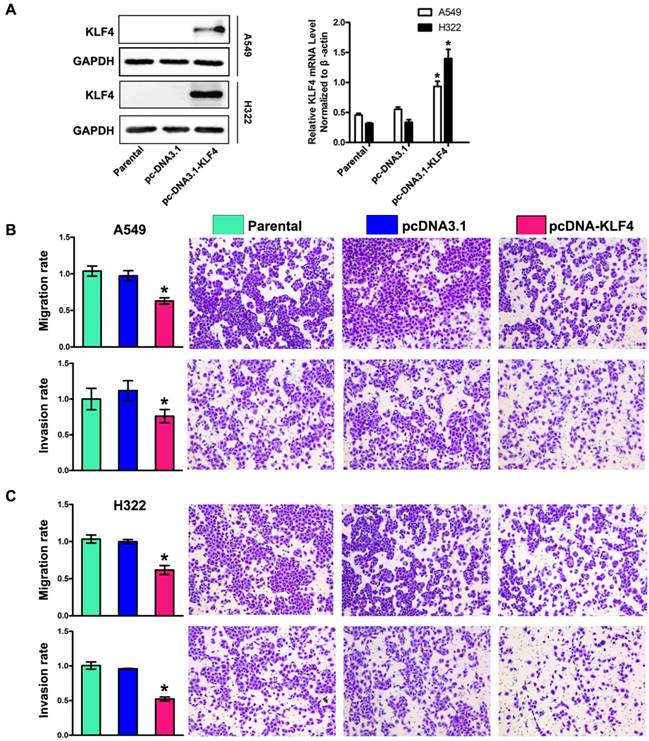
To determine the role of KLF4 in invasion and metastasis of lung adenocarcinoma, we chose A549 and H322 cells with low KLF4 expression, and then restored KLF4 expression by transfecting cells with plasmids expressing KLF4 (pcDNA3.1-KLF4) or empty vector respectively. Effect of transfection was verified by Western blotting and Real-time PCR (Fig. 3A). The ability of tumor cells to migrate through the membranes coated with and without the matrigel was detected 72 and 48 hours after cell seeding, respectively. Results showed that ectopic expression of KLF4 drastically suppressed migratory and invasive ability of both cell lines, compared with that of parental or empty vector-transfected cells (Fig. 3B, 3C). This effect was unlikely due to KLF4-mediated cell growth inhibition, because we previously found that ectopic expression of KLF4 had no effect on lung tumor cell growth within 72 hours after cell seeding [44].
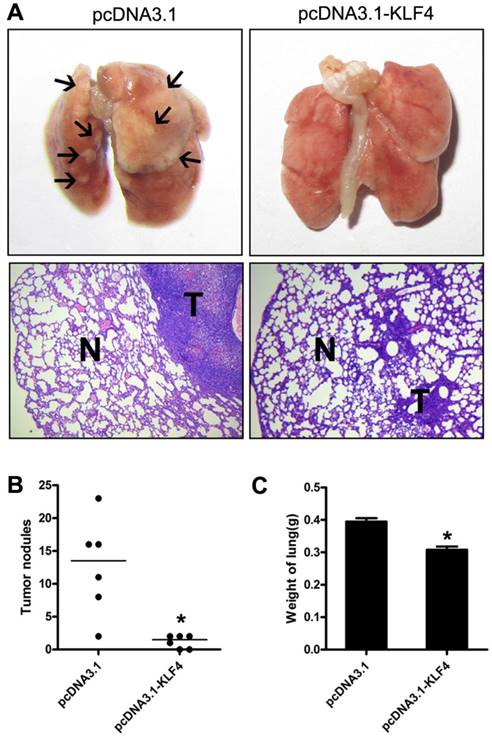
To further investigate potential effect of KLF4 on tumor metastasis in vivo, we injected H322 cells stably transfected with pcDNA3.1-KLF4 or empty vector through tail vein into BALB/c-nu mice, respectively. Mice were sacrificed 30 to 40 days after injection or when they had become moribund. Lungs were separated and weighted. Macroscopic and microscopic analyses revealed that enforced KLF4 significantly reduced lung colonization (Fig. 4A), numbers of visible nodules (Fig. 4B) and total lung weight (0.308 g for KLF4 group vs. 0.395 g for controls, n = 6) (Fig. 4C). Taken together, the ability of KLF4 to inhibit migration and invasion, as well as metastasis of lung tumor cells indicates a potential role of KLF4 as therapeutic target in NSCLC.
Correlation of KLF4 expression with clinicopathological characteristics of patients with lung adenocarcinoma
| Variables | KLF4 expression | Chi square test p value | |
|---|---|---|---|
| High | Low | ||
| Age(y) | 0.570 | ||
| ≥ 60 | 14 | 11 | |
| < 60 | 17 | 18 | |
| Sex | 0.833 | ||
| Male | 19 | 17 | |
| Female | 12 | 12 | |
| Clinical stage | 0.016 | ||
| I | 19 | 11 | |
| II | 4 | 2 | |
| III | 6 | 8 | |
| IV | 2 | 8 | |
| T classification | 0.021 | ||
| T1 | 11 | 4 | |
| T2 | 14 | 13 | |
| T3 | 3 | 4 | |
| T4 | 3 | 8 | |
| N classification | 0.044 | ||
| N0 | 21 | 13 | |
| N1 | 5 | 5 | |
| N2 | 4 | 8 | |
| N3 | 1 | 3 | |
| M classification | 0.028 | ||
| M0 | 29 | 21 | |
| M1 | 2 | 8 | |
| Smoking history | 0.611 | ||
| Smokers | 14 | 15 | |
| Non smokers | 17 | 14 | |
Univariate and multivariate cox regression analysis of potential prognostic variables in patients with lung adenocarcinoma
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Patients (n) | Regression coefficient (SE) | p | Hazard ratio (95% CI) | p | |
| Clinical stage | |||||
| I-IIIA | 32 | 0.926 (0.357) | 0.009 | 2.326 (1.038-5.213) | 0.029 |
| IIIB-IV | 18 | ||||
| T classification | |||||
| T1-2 | 42 | 0.880 (0.362) | 0.015 | NA | |
| T3-4 | 18 | ||||
| N classification | |||||
| N0 | 34 | 0.798 (0.358) | 0.026 | NA | |
| N1-3 | 26 | ||||
| M classification | |||||
| M0 | 50 | 0.97 (0.413) | 0.019 | NA | |
| M1 | 10 | ||||
| KLF4 level | |||||
| High | 31 | -0.89 (0.367) | 0.015 | 2.382 (1.130-5.021) | 0.043 |
| Low | 29 | ||||
Note: NA, not available; SE, standard error; CI, confidence intervals
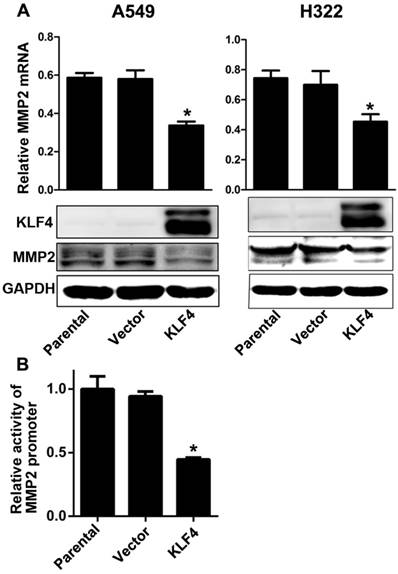
Ectopic expression of KLF4 decreased MMP2 expression by inhibiting promoter activity
Matrix metalloproteinase 2 (MMP2) is a well known type IV collagenase which promotes tumor metastasis by degrading the basement membrane. Tang et al. has reported that KLF4 expression is inversely associated with MMP2 expression in colorectal cancer, and MMP2 is the direct target of KLF4 [45]. We found that enforced KLF4 could suppress the expression of MMP2 at both mRNA and protein levels (Fig. 5A). Since KLF4 could activate or inactivate transcription of many genes, we performed a dual luciferase assay to explore effect of KLF4 on the activity on MMP2 promoter. As shown in Fig. 5B, enforced KLF4 significantly depressed activity of MMP2 promoter. These results indicate that KLF4 may inhibit invasion and metastases through suppressing the transcription of MMP2 gene.
Discussion
KLF4 has been shown to play the role of oncogene or tumor suppressor in a context-dependent manner. Several lines of evidence have indicated that KLF4 is a putative tumor suppressor in lung cancer. Enforced expression of KLF4 in lung cancer cells induces G1/S cell cycle arrest and inhibits tumor growth in vitro and in vivo [29, 41]. Our previous study shows that KLF4 inhibits invasion of lung cancer cells via suppression of secreted protein acidic and rich in cysteine (SPARC) [44]. In this study, we investigated the role of KLF4 in lung adenocarcinoma using cell lines, animal models and human specimen. Firstly, we explored the expression pattern of KLF4 protein in tissue microarrays immunohistochemically, which showed that KLF4 was frequently lost in lung adenocarcinoma compared with normal lung tissue and tumor adjacent tissue. Hypermethylation of KLF4 promoter contributed at least partly to its downregulation. We further analyzed relationship between KLF4 expression and clinical parameters in 60 lung adenocarcinoma tissues with survival data, and found that KLF4 expression inversely correlated with T, N and M classification, and poor prognosis. Effects of KLF4 on lung adenocarcinoma cells were determined in vivo and in vitro. Results showed that enforced KLF4 expression in lung cancer cells significantly inhibited migration, invasion and metastases to lung. Moreover, ectopic KLF4 expression suppressed expression of MMP2 via inhibiting its promoter activity. In conclusion, our study provided clinical evidence and mechanism of action that KLF4 inhibited progression of lung adenocarcinoma in vivo and in vitro by suppressing MMP2 transcription. KLF4 may be a new target for patients with lung cancer.
Restoration of KLF4 inhibited migration, invasion. (A) Expression of KLF4 was significantly restored by stable transfection of pcDNA3.1-KLF4 vector at both protein and mRNA levels, compared with untreated parental group and mock plasmid group. Migration and invasion (B, C) of tumor cells were determined as described in Materials and Methods section. The untreated cells were given arbitrary migration and invasiveness percentages of 100%. *p<0.05.
Though 2 studies have shown that KLF4 is reduced in non-small cell lung cancer, its expression pattern in lung adenocarcinoma and correlation with prognosis is still unclear. Hu et al [29]. found that KLF4 protein was dramatically down-regulated in 21 of 25 (84%) primary lung tumors, eight out of 25 were lung adenocarcinoma. Another study shows that KLF4 is significantly downregulated in lung adenocarcinoma, compared with normal control [46]. However, neither of them studied the prognostic role of KLF4 in lung cancer. Consistently, we found that KLF4 was significantly down-regulated in lung adenocarcinoma, compared with matched normal lung tissue and tumor adjacent tissue. Moreover, we found that reduced expression of KLF4 correlated with poor prognosis, suggesting its role as a prognostic biomarker in lung adenocarcinoma. Similar expression pattern and correlation with poor prognosis of reduced KLF4 have been shown in gastric and colon cancer [27, 36]. These results indicate that KLF4 may function as a tumor suppressor and could become a prognostic biomarker in lung adenocarcinoma.
Moreover, promoter hypermethylation contributes at least partly to downregulation of KLF4 since treatment with methyltransferase inhibitor restored its expression in lung cancer cell lines. Expression of KLF4 is regulated at both transcriptional and post-transcriptional levels. Transcriptional regulation includes methylation of KLF4 promoter and histones, and micro-RNAs (miR). Consistent with our finding, promoter hypermethylation is the most frequently-reported mechanism in tumors including colorectal, gastric, and medulloblastoma tissues [25, 27, and 30]. Whether other mechanisms like loss of heterozygosity and point mutation involve in the loss of KLF4 in lung cancer still needs further study.
Restoration of KLF4 inhibited pulmonary metastases of lung adenocarcinoma cells. 1×106 H322 cells stably transfected with pc-DNA3.1 or pc-DNA3.1-KLF4 vector were intravenously injected into nude mice through tail vein (n=6). 30 to 40 days after injection, mice were sacrificed. Lungs were separated and examined carefully with macroscope and microscope. Representative images were shown (A). Metastases on the surface of lung were counted (B). Lungs were weighed (C). N: normal lung tissue; T: tumor. *p<0.05.
Previous studies have shown that KLF4 inhibits migratory and metastatic capacity of gastric, esophageal cancer [47, 48]. Our previous study shows that KLF4 inhibits invasion of lung cancer cells via suppression of SPARC [49]. Moreover, KLF4 reverses the epithelial-mesenchymal transition, one important mechanism allowing tumor metastases, via activating epithelial markers of E-Cadherin while suppressing mesenchymal markers of SNAIL and SLUG [50-52]. Consistently, we found that enforced expression of KLF4 inhibited migration, invasion and pulmonary metastases of lung adenocarcinoma cells.
Degradation of the basement membrane is an essential step for the metastases of most cancers. MMP2 is capable of degrading type IV collagen, the most abundant component of the basement membrane, to allow cancer cells to migrate out of the primary tumor to form distant metastases. MMP2 has been proved to promote tumor metastasis and to correlate with adverse prognosis in various tumors including lung cancer [53, 54]. Previous studies have shown that KLF4 inhibited Snail expression [55], and Snail significantly increased MMP2 expression [56], suggesting indirect regulation of MMP2 by KLF4. Besides, there are three putative KLF4-binding sites in MMP2 promoter region. KLF4 has been shown to suppress MMP2 transcription by direct binding to its promoter and MMP2 expression is negatively correlated with KLF4 expression in colorectal cancer tissue [57]. Consistent with these studies, we found that enforced KLF4 expression remarkably suppressed expression of MMP2 at both mRNA and protein levels. Dual-luciferase assay showed that enforced KLF4 remarkably reduced the activity of MMP2 promoter. We assume that KLF4 suppresses the transcription of MMP2 in direct and/or indirect way to suppress invasion of lung cancer cells. However, further study is needed to clarify the underlying mechanisms. Before incorporating into clinical usage, mechanism via which KLF4 inhibits metastasis still requires further investigation.
Enforced KLF4 downregulated MMP2 expression by suppressing its promoter activity. (A) Enforced KLF4 reduced MMP2 protein and mRNA expression. (B) Promoter activity of MMP2 was determined by dual luciferase assay. *p<0.05.
Conclusion
Taken together, our present study found that KLF4 expression was frequently lost in lung adenocarcinoma and hypermethylation of KLF4 promoter contributed at least partly to its downregulation. Downregulation of KLF4 expression predicted poor prognosis of patients with lung adenocarcinoma. Enforced expression of KLF4 inhibited migratory and metastatic capacity of lung cancer cells in vitro and in vivo. Potential mechanism of action included suppression of tumor invasion-associated genes of MMP2. Our study shed light on the role of KLF4 in lung adenocarcinoma and paved the way for its utility as prognostic biomarker and treatment target in the future.
Acknowledgements
We thank Dr. Li-hua Xu (Cancer Prevention Center of Sun Yat-sen University, China) for the gift of 293T and 16HBE immortal human bronchial epithelial cells.
Abbreviations
GKLF: gut-enriched Krüppel-like factor; KLF: Krüppel-like factor; iPS: induced pluripotent stem cells; LOH: loss of heterozygosity; hTERT: human telomerase reverse transcriptase; TMA: Tissue microarray; DMEM: Dulbecco's modified Eagle's medium; FBS: fetal bovine serum; 5-Aza-dC: 5'-aza-2'-deoxycytidine; PBS: phosphate buffer solution; HR: hazard ratio; CI: confidence interval; MMP2: Matrix metalloproteinase 2; SPARC: secreted protein acidic and rich in cysteine; miR: micro-RNAs.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5-29
2. Travis WD, Brambilla E, Nicholson AG. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260
3. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992-1001
4. Consonni D, Pierobon M, Gail MH. et al. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015;107:v59
5. Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103-1121
6. Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143-160
7. Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206
8. Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27:23-31
9. Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009-20017
10. Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384-31390
11. Panigada M, Porcellini S, Sutti F. et al. GKLF in thymus epithelium as a developmentally regulated element of thymocyte-stroma cross-talk. Mech Dev. 1999;81:103-113
12. Ghaleb AM, Yang VW. Kruppel-like factor 4 (KLF4): What we currently know. Gene. 2017;611:27-37
13. Chen X, Johns DC, Geiman DE. et al. Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423-30428
14. Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101-2105
15. Yoon HS, Ghaleb AM, Nandan MO. et al. Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene. 2005;24:4017-4025
16. Takahashi K, Tanabe K, Ohnuki M. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872
17. Polo JM, Anderssen E, Walsh RM. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617-1632
18. Ghaleb AM, Yang VW. Kruppel-like factor 4 (KLF4): What we currently know. Gene. 2017;611:27-37
19. Foster KW, Frost AR, McKie-Bell P. et al. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488-6495
20. Foster KW, Liu Z, Nail CD. et al. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24:1491-1500
21. Tai SK, Yang MH, Chang SY. et al. Persistent Kruppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011;102:895-902
22. Yu F, Shi Y, Wang J. et al. Deficiency of Kruppel-like factor KLF4 in mammary tumor cells inhibits tumor growth and pulmonary metastasis and is accompanied by compromised recruitment of myeloid-derived suppressor cells. Int J Cancer. 2013;133:2872-2883
23. Chen YJ, Wu CY, Chang CC. et al. Nuclear Kruppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7:777-782
24. Pandya AY, Talley LI, Frost AR. et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709-2719
25. Zhao W, Hisamuddin IM, Nandan MO. et al. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395-402
26. Yang Y, Katz JP. KLF4 is downregulated but not mutated during human esophageal squamous cell carcinogenesis and has tumor stage-specific functions. Cancer Biol Ther. 2016;17:422-429
27. Wei D, Gong W, Kanai M. et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746-2754
28. Zammarchi F, Morelli M, Menicagli M. et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am J Pathol. 2011;178:361-372
29. Hu W, Hofstetter WL, Li H. et al. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin Cancer Res. 2009;15:5688-5695
30. Nakahara Y, Northcott PA, Li M. et al. Genetic and epigenetic inactivation of Kruppel-like factor 4 in medulloblastoma. Neoplasia. 2010;12:20-27
31. Yang WT, Zheng PS. Promoter hypermethylation of KLF4 inactivates its tumor suppressor function in cervical carcinogenesis. PLoS One. 2014;9:e88827
32. Li H, Wang J, Xiao W. et al. Epigenetic inactivation of KLF4 is associated with urothelial cancer progression and early recurrence. J Urol. 2014;191:493-501
33. Li H, Wang J, Xiao W. et al. Epigenetic alterations of Kruppel-like factor 4 and its tumor suppressor function in renal cell carcinoma. Carcinogenesis. 2013;34:2262-2270
34. Li Q, Gao Y, Jia Z. et al. Dysregulated Kruppel-like factor 4 and vitamin D receptor signaling contribute to progression of hepatocellular carcinoma. Gastroenterology. 2012;143:799-810
35. Cho YG, Song JH, Kim CJ. et al. Genetic and epigenetic analysis of the KLF4 gene in gastric cancer. APMIS. 2007;115:802-808
36. Patel NV, Ghaleb AM, Nandan MO. et al. Expression of the tumor suppressor Kruppel-like factor 4 as a prognostic predictor for colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2631-2638
37. Cho YG, Song JH, Kim CJ. et al. Genetic and epigenetic analysis of the KLF4 gene in gastric cancer. APMIS. 2007;115:802-808
38. Dang DT, Chen X, Feng J. et al. Overexpression of Kruppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene. 2003;22:3424-3430
39. Ghaleb AM, McConnell BB, Kaestner KH. et al. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011;349:310-320
40. Katz JP, Perreault N, Goldstein BG. et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935-945
41. Hu W, Jia Y, Yu Z. et al. KLF4 downregulates hTERT expression and telomerase activity to inhibit lung carcinoma growth. Oncotarget. 2016;7(33):52870-52887
42. Greene FL, Page DL, Fleming ID. et al. American joint committee on cancer staging manual. 6th ed. New York: Springer. 2009
43. Li J, Zhang N, Song LB. et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319-3326
44. Zhou Y, Hofstetter WL, He Y. et al. KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol Ther. 2010;9:507-513
45. Tang W, Zhu Y, Gao J. et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer. 2014;110:450-458
46. Fadous-Khalife MC, Aloulou N, Jalbout M. et al. Kruppel-like factor 4: A new potential biomarker of lung cancer. Mol Clin Oncol. 2016;5:35-40
47. Zhang N, Zhang J, Shuai L. et al. Kruppel-like factor 4 negatively regulates beta-catenin expression and inhibits the proliferation, invasion and metastasis of gastric cancer. Int J Oncol. 2012;40:2038-2048
48. Yang Y, Goldstein BG, Chao HH, Katz JP. KLF4 and KLF5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther. 2005;4:1216-1221
49. Zhou Y, Hofstetter WL, He Y. et al. KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol Ther. 2010;9:507-513
50. Chen Z, Wang Y, Liu W. et al. Doxycycline Inducible Kruppel-Like Factor 4 Lentiviral Vector Mediates Mesenchymal to Epithelial Transition in Ovarian Cancer Cells. PLoS ONE. 2014;9:e105331
51. Lin ZS, Chu HC, Yen YC. et al. Kruppel-like factor 4, a tumor suppressor in hepatocellular carcinoma cells reverts epithelial mesenchymal transition by suppressing slug expression. PLoS One. 2012;7:e43593
52. Liu YN, Abou-Kheir W, Yin JJ. et al. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol. 2012;32:941-953
53. Giannelli G, Falk-Marzillier J, Schiraldi O. et al. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225-228
54. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9-34
55. Yori JL, Seachrist DD, Johnson E. et al. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia. 2011;13:601-610
56. Miyoshi A, Kitajima Y, Sumi K. et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265-1273
57. Tang W, Zhu Y, Gao J. et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer. 2014;110:450-458
Author contact
![]() Corresponding author: Yanbin Zhou, e-mail: sysuzybcn, Fax: +86 20 8775 0632
Corresponding author: Yanbin Zhou, e-mail: sysuzybcn, Fax: +86 20 8775 0632

 Global reach, higher impact
Global reach, higher impact