Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(18):3849-3855. doi:10.7150/jca.21217 This issue Cite
Research Paper
Comparable Survival between Additional Radiotherapy and Local Surgery in Occult Breast Cancer after Axillary Lymph Node Dissection: A Population-based Analysis
1. Department of Radiation Oncology, Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Xiamen 361003, People's Republic of China
2. Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou 510060, People's Republic of China
3. Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Medical College, Xiamen University, Xiamen 361005, People's Republic of China
San-Gang Wu and Wen-Wen Zhang contributed equally to this work.
Received 2017-5-28; Accepted 2017-8-14; Published 2017-10-17
Abstract
Purpose: To investigate the clinical value of additional local treatment strategies in occult breast cancer (OBC) after axillary lymph node dissection (ALND).
Methods: Patients diagnosed with OBC between 1990 and 2013 were included from the Surveillance, Epidemiology, and End Results registry database. The significant risk factors of cause-specific survival (CSS) and overall survival (OS) were identified using univariate and multivariate Cox regression analyses.
Results: We identified 980 patients, including 219 (22.3%), 252 (25.7%), 263 (26.8%), and 246 (25.1%) of patients underwent ALND, ALND + radiotherapy (RT), ALND + surgery (S) (mastectomy or breast-conserving surgery), and ALND + S + RT, respectively. Patients with younger age, diagnosed before 2000, advanced nodal stage, ER-negative disease, and PR-negative disease were more likely to undergo additional local treatment compared with ALND only. The 10-year rate CSS of the ALND only group was 57.2%, while that of the ALND + RT, ALND + S, and ALND + S + RT groups was 78.0%, 81.0%, and 71.5%, respectively (p < 0.001). The 10-year OS rate in the ALND only, ALND + RT, ALND + S, and ALND + S + RT groups was 46.0%, 69.5%, 66.1%, and 67.0%, respectively (p < 0.001). Multivariate analysis indicated that older age, advanced nodal stage, and ALND only were independent risk factors for decreased CSS and OS. CSS and OS among the groups including ALND + RT, ALND + S, and ALND + S + RT were not significantly different.
Conclusions: Additional local treatment (local surgery or RT) improves survival outcomes compared with ALND only in OBC after ALND. ALND + RT may be the optimal local treatment for OBC due to no different in survival outcomes and cosmesis is better.
Keywords: Breast neoplasms, Surgery, Radiotherapy.
Introduction
Although the breast is the most common primary site of tumor origin (1), occult breast cancer (OBC) presenting with axillary lymphadenopathy is a rare pathologic subtype, representing less than 1% of all breast cancers (2-5). OBC was first reported in 1907 by Halsted (6); patients with OBC had axillary lymphadenopathy but clinical, radiological, and pathological evaluation could not identify the primary breast tumor (2-5). The survival outcomes of patients with OBC remain unclear due to the limited number of patients included in previous studies (3, 7-11). However, several matched studies have reported that OBC has similar survival to palpable breast cancer (2, 5, 12).
The current National Comprehensive Cancer Network (NCCN) guidelines recommend axillary lymph node dissection (ALND) + mastectomy or ALND + whole breast irradiation ± nodal irradiation for OBC [13]. However, an American Society of Breast Surgeons survey found that 43%, 37%, and 6% of respondents chose to undergo mastectomy, whole breast irradiation, and observation, respectively (14). Therefore, the optimal management of the breast in OBC is unresolved. A prospective randomized clinical trial would be the ideal means of resolving this issue but is almost impossible to undertake, given the rarity of the disease (3, 7-11). In the present study, we performed a large population-based study using data from the Surveillance, Epidemiology, and End Results (SEER) registry database to evaluate the clinical value of additional local treatment strategies in patients with OBC after ALND.
Materials and Methods
Patients
Patients diagnosed with OBC from 1990 to 2013 were included using the SEER database (15). The SEER program currently includes and publishes cancer incidence mortality and survival rates from population-based cancer registries covering 28% of the US population, and is maintained by the National Cancer Institute (15). Patients who met the following inclusion criteria were included in the analysis: 1) female OBC who had undergone ALND; 2) axillary lymph node metastasis confirmed by pathology; 3) information on the number of positive lymph nodes and additional local treatment strategies, i.e., radiotherapy (RT) or local surgery (S) (mastectomy or breast-conserving surgery [BCS]) were available. Patients with SEER distant-stage disease were excluded. The present study was based on publicly available data from the SEER program and we accessed the database with the permission number 10269-Nov2015. The the First Affiliated Hospital of Xiamen University, and Sun Yat-sen University Cancer Center ethics committees approved this study.
Clinicopathological Features
The following demographical and clinicopathological factors were evaluated: year of diagnosis, age, ethnicity, tumor grade, pathological lymph node staging, estrogen receptor (ER) status, progesterone receptor (PR) status, and local treatment strategies. The primary endpoints of the study were cause-specific survival (CSS) and overall survival (OS).
Statistical Analysis
Differences between the classification variables were assessed using the Pearson chi-square test. Survival curves were calculated by the Kaplan-Meier method and were compared using the log-rank test to assess significant differences for CSS and OS. Significant and independent risk factors of CSS and OS were identified by Cox proportional hazard models. All statistical analyses were performed using the SPSS software package (version 22.0; IBM Corporation, Armonk, NY, USA). p < 0.05 was considered statistically significant in all analyses.
Results
Patient Characteristics and Treatment
A total of 980 patients were identified. Table 1 lists the patient characteristics. In total, 219 (22.3%) patients underwent ALND, 252 (25.7%) had ALND + RT, 263 (26.8%) had ALND + RT, and 246 (25.1%) received ALND + S + RT. The median number of total lymph nodes examined was 12 (range, 1-85), and the median number of positive lymph nodes was 2 (range, 1-40). Patients with younger age (p < 0.001), diagnosed before 2000 (p < 0.001), advanced nodal stage (N2-N3) (p < 0.001), ER-negative disease (p = 0.021), and PR-negative disease (p = 0.003) were more likely to undergo additional local treatment compared with ALND only.
Survival
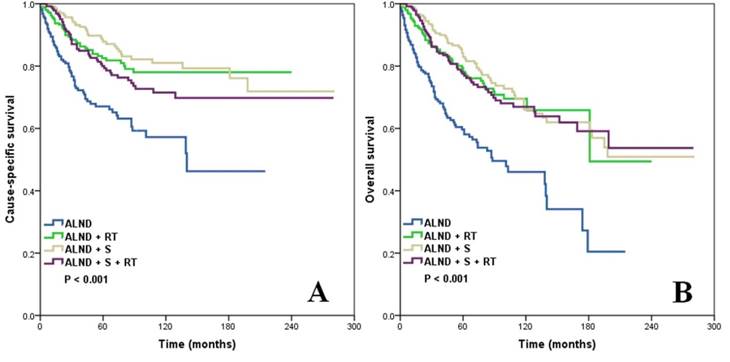
In the entire cohort, the median follow-up was 53 months, and the 5- and 10-year CSS rate was 79.9% and 72.6%, respectively, while the 5- and 10-year OS rate was 75.2% and 62.3%, respectively. The 10-year CSS rate of patients who had undergone ALND only was 57.2%, while the 10-year CSS rate of the ALND + RT, ALND + S, and ALND + S + RT groups was 78.0%, 81.0%, and 71.5%, respectively (log-rank test, p < 0.001) (Figure 1a). Patients who had undergone ALND and additional local treatments also had significantly better OS: the 10-year OS rate in the ALND only, ALND + RT, ALND + S, and ALND + S + RT groups was 46.0%, 69.5%, 66.1%, and 67.0%, respectively (log-rank test, p < 0.001) (Figure 1b).
Prognostic Analysis
Univariate analysis showed that older age (≥70 years), advanced nodal stage (N3), and having undergone ALND only were risk factors for decreased CSS and OS (Table 2). The variables with statistical significance at p < 0.05 in the univariate analysis were examined using multivariate Cox regression analysis. Older age, advanced nodal stage, and having undergone ALND only were stall the independent risk factors for decreased CSS and OS. However, the CSS and OS among the ALND + RT, ALND + S, and ALND + S + RT groups were not significantly different (Table 3).
Patient Characteristics
| Variable | n | ALND (%) | ALND + RT (%) | ALND + S (%) | ALND + S+ RT (%) | p |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≤49 | 210 | 30 (13.7) | 40 (15.9) | 73 (27.8) | 67 (27.2) | < 0.001 |
| 50-69 | 555 | 115 (52.5) | 146 (57.9) | 147 (55.9) | 147 (59.8) | |
| ≥70 | 215 | 74 (33.8) | 66 (26.2) | 43 (16.3) | 32 (13.0) | |
| Year of diagnosis | ||||||
| 1990-1999 | 96 | 11 (5.0) | 11 (4.4) | 41 (15.6) | 33 (13.4) | < 0.001 |
| 2000-2013 | 884 | 208 (95.0) | 241 (95.6) | 222 (84.4) | 213 (86.6) | |
| Ethnicity | ||||||
| White | 795 | 172 (78.5) | 208 (82.5) | 210 (79.8) | 205 (83.3) | 0.840 |
| Black | 116 | 28 (12.8) | 28 (11.1) | 33 (12.5) | 27 (11.0) | |
| Other and Unknown | 69 | 19 (8.7) | 16 (6.3) | 20 (7.6) | 14 (5.7) | |
| Grade | ||||||
| G1-2 | 59 | 7 (3.2) | 9 (3.6) | 24 (9.1) | 19 (7.7) | 0.002 |
| G3-4 | 218 | 44 (20.1) | 46 (18.3) | 59 (22.4) | 69 (28.0) | |
| Unknown | 703 | 168 (76.7) | 197 (78.2) | 180 (68.4) | 158 (64.2) | |
| Nodal stage | ||||||
| N1 | 631 | 171 (78.1) | 159 (63.1) | 176 (66.9) | 125 (50.8) | < 0.001 |
| N2 | 189 | 26 (11.9) | 51 (20.2) | 55 (20.9) | 57 (23.2) | |
| N3 | 160 | 22 (10.0) | 42 (16.7) | 32 (12.2) | 64 (26.0) | |
| ER status | ||||||
| Negative* | 332 | 58 (26.5) | 91 (36.1) | 86 (32.7) | 97 (39.4) | 0.021 |
| Positive | 515 | 121 (55.3) | 135 (53.6) | 136 (51.7) | 123 (50.0) | |
| Unknown | 133 | 40 (18.3) | 26 (10.3) | 41 (15.6) | 26 (10.6) | |
| PR status | ||||||
| Negative* | 458 | 82 (37.4) | 118 (46.8) | 122 (46.4) | 136 (55.3) | 0.003 |
| Positive | 366 | 90 (41.1) | 102 (40.5) | 94 (35.7) | 80 (32.5) | |
| Unknown | 156 | 47 (21.5) | 32 (12.7) | 47 (17.9) | 30 (12.2) |
* Includes patients with borderline status.
ALND, axillary lymph node dissection; ER, estrogen receptor; G1, well-differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated; PR, progesterone receptor; RT, radiotherapy; S, surgery.
Kaplan-Meier analysis and log-rank test showing the CSS (a) and OS (b) of patients with OBC according to the local treatment strategy.
Univariate Analysis of Prognostic Factors
| Variable | CSS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Age (years) | ||||||
| ≤49 | 1 | 1 | ||||
| 50-69 | 1.285 | 0.879-1.881 | 0.196 | 1.462 | 1.029-2.079 | 0.034 |
| ≥70 | 1.858 | 1.204-2.866 | 0.005 | 3.216 | 2.212-4.676 | < 0.001 |
| Year of diagnosis | ||||||
| 1990-1999 | 1 | 1 | ||||
| 2000-2013 | 1.267 | 0.818-1.961 | 0.289 | 1.355 | 0.936-1.959 | 0.107 |
| Ethnicity | ||||||
| White | 1 | 1 | ||||
| Black | 0.964 | 0.618-1.505 | 0.872 | 1.047 | 0.723-1.517 | 0.807 |
| Other and Unknown | 0.709 | 0.374-1.343 | 0.292 | 0.814 | 0.490-1.352 | 0.426 |
| Grade | ||||||
| G1-2 | 1 | 1 | ||||
| G3-4 | 0.513 | 0.240-1.096 | 0.085 | 0.653 | 0.372-1.147 | 0.139 |
| Unknown | 0.875 | 0.626-1.222 | 0.433 | 0.792 | 0.594-1.057 | 0.114 |
| Nodal stage | ||||||
| N1 | 1 | 1 | ||||
| N2 | 1.301 | 0.904-1.873 | 0.156 | 0.965 | 0.698-1.334 | 0.829 |
| N3 | 2.195 | 1.578-3.052 | < 0.001 | 1.783 | 1.342-2.368 | < 0.001 |
| ER status | ||||||
| Negative* | 1 | 1 | ||||
| Positive | 0.889 | 0.647-1.222 | 0.467 | 0.948 | 0.724-1.243 | 0.700 |
| Unknown | 1.414 | 0.955-2.093 | 0.083 | 1.370 | 0.979-1.916 | 0.066 |
| PR status | ||||||
| Negative* | 1 | 1 | ||||
| Positive | 0.853 | 0.617-1.180 | 0.337 | 0.950 | 0.721-1.251 | 0.713 |
| Unknown | 1.284 | 0.894-1.845 | 0.175 | 1.381 | 1.018-1.873 | 0.038 |
| Local treatment strategies | ||||||
| ALND | 1 | 1 | ||||
| ALND + RT | 0.427 | 0.290-0.628 | < 0.001 | 0.439 | 0.316-0.612 | < 0.001 |
| ALND + S | 0.320 | 0.213-0.482 | < 0.001 | 0.379 | 0.273-0.526 | < 0.001 |
| ALND + S + RT | 0.501 | 0.348-0.723 | < 0.001 | 0.434 | 0.314-0.604 | < 0.001 |
* Includes patients with borderline status.
ALND, axillary lymph node dissection; CI, confidence interval; CSS, cause-specific survival; ER, estrogen receptor; G1, well-differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated; HR, hazard ratio; OS, overall survival; PR, progesterone receptor; RT, radiotherapy; S, surgery.
Multivariate Analysis of Prognostic Factors
| Variable | CSS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Age (years) | ||||||
| ≤49 | 1 | 1 | ||||
| 50-69 | 1.157 | 0.789-1.697 | 0.454 | 1.354 | 0.951-1.928 | 0.093 |
| ≥70 | 1.469 | 0.942-2.291 | 0.090 | 2.645 | 1.803-3.879 | < 0.001 |
| Nodal stage | ||||||
| N1 | 1 | 1 | ||||
| N2 | 1.534 | 1.060-2.220 | 0.023 | 1.147 | 0.826-1.594 | 0.413 |
| N3 | 2.537 | 1.805-3.566 | < 0.001 | 2.020 | 1.506-2.710 | < 0.001 |
| Local treatment strategies | ||||||
| ALND | 1 | 1 | ||||
| ALND + RT | 0.379 | 0.256-0.560 | < 0.001 | 0.442 | 0.316-0.617 | < 0.001 |
| ALND + S | 0.295 | 0.195-0.445 | < 0.001 | 0.429 | 0.307-0.601 | < 0.001 |
| ALND + S + RT | 0.395 | 0.271-0.577 | < 0.001 | 0.442 | 0.314-0.622 | < 0.001 |
| Local treatment strategies | ||||||
| ALND + RT | 1 | 1 | ||||
| ALND | 2.530 | 1.733-3.694 | < 0.001 | 2.264 | 1.607-3.189 | < 0.001 |
| ALND + S | 0.958 | 0.632-1.454 | 0.842 | 1.000 | 0.693-1.443 | 0.999 |
| ALND + S + RT | 0.746 | 0.482-1.155 | 0.19 | 0.972 | 0.678-1.394 | 0.877 |
ALND, axillary lymph node dissection; CI, confidence interval; CSS, cause-specific survival; HR, hazard ratio; OS, overall survival; RT, radiotherapy; S, surgery.
Discussion
We investigated the clinical value of additional local treatment strategies in patients with OBC with axillary lymph node metastases after ALND. Our results show that additional local treatment strategies including RT or mastectomy/BCS, improved survival compared to ALND only.
Although the size of the tumor is relatively small, OBC often has involvement of regional lymph nodes as a presenting finding. The primary breast tumors in OBC may be too small to be detected from conventional pathological sections. Several studies have found that primary breast tumors could be determined in approximately 30-76% of patients with OBC who had undergone mastectomy (3, 11, 12, 16, 17). Breast magnetic resonance imaging (MRI) is often the first choice for detecting primary tumors in OBC, and approximately two-thirds of primary tumors can be identified with high sensitivity (96%). However, the specificity is much lower (63%) (18, 19). Therefore, lesions found on MRI require further histological confirmation by biopsy. In addition, routine use of breast MRI in OBC may alter locoregional therapy for one-third of patients by offering the choice of BCS (18).
ALND is an important part of surgical treatment of OBC. However, additional local treatment strategies that include RT or local surgery in OBC remain controversial. Several studies before 2003 found that local control following RT was similar to mastectomy (3, 7-10). However, a meta-analysis by the American Society of Breast Surgeons found that 43%, 37%, and 6% of respondents chose to undergo mastectomy, whole breast radiation, and observation, respectively (14).
Several recent studies have confirmed the clinical value of additional local treatment strategies in OBC. He et al. studied 95 patients with OBC, and found that patients who had undergone ALND + mastectomy or ALND + RT had significantly improved locoregional and distant recurrence rates compared to patients who had undergone ALND only (p < 0.05), and the survival outcomes between the ALND + mastectomy and ALND + RT groups were similar (11). Wang et al. also showed that patients who had undergone mastectomy (n = 38) had better survival outcomes compared to those who had undergone ALND only (n = 13) (12). In the present study, we also found that patients who had undergone mastectomy or BCS had significantly improved CSS and OS compared to patients who had undergone ALND only. However, in patients who had undergone additional local surgery, adjuvant RT, for which indications may differ from that of palpable breast cancer, did not further improve survival. In the current NCCN guidelines, adjuvant RT improves the survival of patients with positive lymph nodes who undergo mastectomy or BCS (13). Therefore, the RT indications may differ between OBC and non-OBC.
The introduction of more advanced techniques for breast imaging may decrease the incidence of OBC. Breast MRI can help one-third of this patient population avoid unnecessary mastectomy (18). Masinghe et al. found that primary breast RT may reduce ipsilateral breast tumor recurrence and increase survival in patients with OBC with axillary lymph node metastases (n = 53) (20). Barton et al. also found that patients who had undergone breast RT (n = 35) had better local and distant control compared to patients who had undergone observation (n = 13), but found that there was no difference in OS (84% vs. 85%, p = 0.2) (21). Vlastos et al. studied 45 patients diagnosed from 1951 to 1998: 29% had undergone mastectomy and 71% had undergone treatment with intent to preserve the breast; they found that breast-preserving treatment did not have a negative effect on local control or survival (3). Varadarajan et al. also showed that breast-preserving treatment with RT alone can be considered a treatment choice for OBC presenting with axillary lymph node metastasis (22). Therefore, the current literature supports the use of breast-preserving treatment involving BCS or breast RT as an alternative to mastectomy.
In a meta-analysis that included 241 patients with OBC, 94 had undergone ALND + RT, 112 had undergone mastectomy, and 35 had undergone ALND only. RT improved the locoregional control (p = 0.01) and possibly mortality rates (p = 0.09) of patients who had undergone ALND + RT compared to that of patients who had undergone ALND only. Survival outcomes between ALND + mastectomy and ALND + RT were not significantly different (23). In addition, Walker et al. used the SEER database and found that survival after mastectomy + ALND ± RT and BCS + ALND + RT was similar (p = 0.79) and was associated with better survival compared to ALND alone (p = 0.04) (24). The current NCCN guidelines also recommend ALND + mastectomy or ALND + whole breast irradiation ± nodal irradiation in OBC (13). In the present study, we also found no significant differences in CSS and OS among the ALND + RT, ALND + S, and ALND + S + RT groups. Ideally, a prospective randomized clinical trial would resolve this controversial issue, but is almost impossible to undertake given the rarity of the disease. Therefore, given the cosmetic advantage of breast conservation, ALND + RT may be the optimal local treatment for OBC.
Due to its rarity, the prognostic factors of OBC are not well-established. Axillary nodal status is an important factor in the risk stratification of non-OBC (25). Our results indicate that advanced nodal stage is an independent adverse risk factor of OBC, which is similar to previously suggested conclusions (2, 3, 9, 11, 24, 26). Walker et al. found that ER-negative disease was associated with poor survival outcome (24). However, the multivariate analysis by He et al. did not find that ER-negative disease affected survival (11). Montagna et al. also found that ER and PR status were not associated with survival outcomes, while patients with triple-negative OBC had a significantly higher risk of disease recurrence and death (2). In the present large-sample study, we also found no significant difference for ER and PR status in terms of CSS and OS. As the SEER program included human epidermal growth factor receptor 2 (HER2) data only after 2010, we were unable to assess the prognosis of OBC based on breast cancer subtype. According to the above studies, more studies with large sample sizes are needed to better understand the prognostic value of hormone receptors and HER2 status in OBC.
There are several limitations in our study. First, a retrospective study has inherent bias and weakness. However, the primary strength of our study is that we performed population-based analysis of a rare disease. Second, the SEER database lacks information on systemic therapy, including chemotherapy, endocrine therapy, and targeted therapy, which could have affected our results. In addition, the SEER database also lacks the definition of target volume, RT dose, and recurrence after RT. Barton et al. found no difference in locoregional recurrence between patients who received 50 Gy versus 60 Gy RT (p = 0.3) (21). Lastly, we could not determine whether there was a potential lesion in patients who had undergone local surgery, and the final pathological results after local surgery were also unknown, as primary breast tumors are detected postoperatively in 30-76% of patients with OBC (3, 11, 12). However, several matched studies found no significant differences in survival outcomes between OBC and non-OBC (2, 5, 12).
Conclusion
In conclusion, our results show that additional local treatment that includes local surgery or RT improves survival outcomes compared to ALND alone in patients with OBC after ALND. However, RT did not have an inferior outcome in comparison with local surgery. ALND + RT may be the optimal local treatment for OBC due to no different in CSS/OS and cosmesis is better. Further prospective studies are needed to confirm our findings.
Abbreviations
ALND, axillary lymph node dissection; BCS, breast-conserving surgery; CSS, cause-specific survival; ER, estrogen receptor; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; OBC, occult breast cancer; OS, overall survival; PR, progesterone receptor; SEER, Surveillance, Epidemiology, and End Results.
Acknowledgements
This work was partly supported by the Natural Science Foundation of Fujian Province (No. 2016J01635), the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070), and Guangdong Medical Research Foundation (No. A2017023).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30
2. Montagna E, Bagnardi V, Rotmensz N. et al. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat. 2011;129(3):867-75
3. Vlastos G, Jean ME, Mirza AN. et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8(5):425-31
4. Patel J, Nemoto T, Rosner D. et al. Axillary lymph node metastasis from an occult breast cancer. Cancer. 1981;47(12):2923-7
5. Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. 1990;21(5):518-23
6. Halsted WS. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg. 1907;46(1):1-19
7. Baron PL, Moore MP, Kinne DW. et al. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125(2):210-4
8. Ellerbroek N, Holmes F, Singletary E. et al. Treatment of patients with isolated axillary nodal metastases from an occult primary carcinoma consistent with breast origin. Cancer. 1990;66(7):1461-7
9. Campana F, Fourquet A, Ashby MA. et al. Presentation of axillary lymphadenopathy without detectable breast primary (T0 N1b breast cancer): experience at Institut Curie. Radiother Oncol. 1989;15(4):321-5
10. Shannon C, Walsh G, Sapunar F. et al. Occult primary breast carcinoma presenting as axillary lymphadenopathy. Breast. 2002;11(5):414-8
11. He M, Tang LC, Yu KD. et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38(11):1022-8
12. Wang X, Zhao Y, Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010;16(1):32-7
13. National Comprehensive Cancer Network. Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
14. Khandelwal AK, Garguilo GA. Therapeutic options for occult breast cancer: a survey of the American Society of Breast Surgeons and review of the literature. Am J Surg. 2005;190(4):609-13
15. SEER. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973-2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S, 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.
16. Matsuoka K, Ohsumi S, Takashima S. et al. Occult breast carcinoma presenting with axillary lymph node metastases: follow-up of eleven patients. Breast Cancer. 2003;10(4):330-4
17. Ashikari R, Rosen PP, Urban JA, Senoo T. Breast cancer presenting as an axillary mass. Ann Surg. 1976;183(4):415-7
18. de Bresser J, de Vos B, van der Ent F. et al. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: a systematic review. Eur J Surg Oncol. 2010;36(2):114-9
19. Fayanju OM, Stoll CR, Fowler S. et al. Geographic and temporal trends in the management of occult primary breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20(10):3308-16
20. Masinghe SP, Faluyi OO, Kerr GR. et al. Breast radiotherapy for occult breast cancer with axillary nodal metastases-does it reduce the local recurrence rate and increase overall survival? Clin Oncol (R Coll Radiol). 2011;23(2):95-100
21. Barton SR, Smith IE, Kirby AM. et al. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer. 2011;47(14):2099-106
22. Varadarajan R, Edge SB, Yu J. et al. Prognosis of occult breast carcinoma presenting as isolated axillary nodal metastasis. Oncology. 2006;71(5-6):456-9
23. Macedo FI, Eid JJ, Flynn J. et al. Optimal Surgical Management for Occult Breast Carcinoma: A Meta-analysis. Ann Surg Oncol. 2016;23(6):1838-44
24. Walker GV, Smith GL, Perkins GH. et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010;116(17):4000-6
25. Coates AS, Winer EP, Goldhirsch A. et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533-46
26. Merson M, Andreola S, Galimberti V. et al. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992;70(2):504-8
Author contact
![]() Corresponding author: Yong-Xiong Chen, Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Medical College of Xiamen University, Xiamen 361000, China Tel. +86 592 2183761 Fax. +86 592 2186786 E-mail. yxchen1962edu.cn Zhen-Yu He, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China; Tel: +86 20 87343543; Fax: +86 20 87343392 E-mail. hezhyorg.cn
Corresponding author: Yong-Xiong Chen, Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Medical College of Xiamen University, Xiamen 361000, China Tel. +86 592 2183761 Fax. +86 592 2186786 E-mail. yxchen1962edu.cn Zhen-Yu He, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China; Tel: +86 20 87343543; Fax: +86 20 87343392 E-mail. hezhyorg.cn

 Global reach, higher impact
Global reach, higher impact