3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(19):4098-4105. doi:10.7150/jca.21030 This issue Cite
Research Paper
Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in patients with breast cancer: a meta-analysis
1. Breast Disease Center, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, P.R. China;
2. Department of Cancer Prevention Research, Sun Yat-sen University Cancer Center, Guangzhou 510080, P.R. China.
* Contributed equally to this work
Received 2017-5-15; Accepted 2017-10-16; Published 2017-11-1
Abstract
Purpose: The prognostic value of tumor-infiltrating Foxp3+ regulatory T cells (Tregs) in breast cancer remains controversial. Therefore, we performed this meta-analysis to determine the impact of Foxp3+ Tregs infiltration on survival outcomes.
Methods: Relevant literature was retrieved from Pubmed, Web of science and Cocohrane until May 30, 2016. Meta-analysis was performed using hazard ratios (HRs), odds ratio (OR) and 95 % confidence intervals (CI) as effect measures.
Results: Fourteen studies (10,259 patients) were included. Meta-analysis showed that high Foxp3+ Tregs infiltration was correlated with high histological grade (OR= 2.96, 95%CI [2.03-4.31]), estrogen receptor (ER) negativity (OR= 0.38, 95%CI [0.23-0.60]), human epidermal growth factor receptor type 2 (HER2) positivity (OR=2.43, 95%CI [1.69-3.51]). The detection of FOXP3+ Tregs was significantly associated with recurrence-free survival (RFS) of patients (HR = 1.58, 95 % CI [1.03-2.44]).
Conclusion: Our meta-analysis suggests that high Foxp3+ Tregs infiltration is associated with poor RFS in breast cancer patients and predicts histological grade, estrogen receptor and HER-2 status.
Keywords: Foxp3, Regulatory T cells, Breast cancer, Prognosis, Meta-analysis.
Introduction
Breast cancer is the most commonly diagnosed cancer in women and the second leading cause of cancer-related mortality among women worldwide [1, 2]. Despite recent progress in breast cancer diagnosis and therapy, the prognosis of breast cancer patients is still poor. Local tumor microenvironment, including tumor cells, extracellular substrates, cytokines and tumor-infiltrating immune cells play an essential role in tumor formation, growth, invasion, and metastasis [3-5]. Regulatory T cells (Tregs) are a subgroup of CD4 + T helper cells that suppress T cell immunity. Forkhead box protein P3 (FoxP3) is a transcription factor imperative and sufficient for the induction of immunosuppressive function of Tregs and could be the most specific marker for Tregs in tumors [6].
Due to the ability to inhibit anti-tumor immunity, high level of tumor-infiltrating Foxp3+ Tregs is expected to be associated with poor prognosis. However, recent studies have challenged this idea and shown that Foxp3+Tregs could improve survival in some tumors [7-9]. To resolve the contradiction, in this study we performed a meta-analysis to investigate the correlation of Foxp3+Tregs and the survival of breast cancer patients.
Methods
Search strategy
Pubmed, Web of science, Cocohrane (last search updated in May 30, 2016) were searched by using the following keywords (Foxp3 OR Forkhead transcription Factors), (Breast cancer OR breast neoplasm OR breast tumor), (Survival OR prognosis OR outcome OR mortality). The identified studies were selected based on following criteria:
Inclusion criteria
(1) Histological type of cancer was breast cancer. (2) Samples were collected by core-needle biopsy or surgery. (3) FOXP3 expression was detected by immunohistochemistry (IHC). (4) Hazard ratio (HR), odds ratio (OR) and their 95 % confidence interval (CI) were reported; if not, outcomes RFS and OS were reported. (5) Published in English. (6) When duplicate data were reported, only the most recent data were included.
Exclusion criteria
(1) Reviews, meta-analysis and conference abstracts were excluded. (2) Animal or cell line studies. (3) Peripheral blood samples or Foxp3 expressed on tumor cells.
Data extraction
Two investigators independently extracted the data from each study, including the first author's surname, geographical location, sample size, publication date, the source of the subjects, protein detection method, median follow-up time and survival data, the discrepancy was resolved by discussion.
Methodological assessment
To evaluate the study methodology, two investigators scored each study independently using Newcastle-Ottawa Scale (NOS) criteria [10]: (1) subject selection: 0-4; (2) comparability of subject: 0-2; (3) clinical outcome: 0-3. NOS scores ranged from 0 to 9; and a score≥7 indicated good quality.
Statistical analysis
The heterogeneity was assessed by X2 test and the I2 statistic of inconsistency [11, 12]. Statistically significant heterogeneity was defined as X2 P < 0.1 or I2 > 50%. We conducted subgroup analysis by stratifying Tregs location and geographical location. For the pooled analysis of the correlation between FoxP3+ Tregs infiltration and clinicopathological features (Tumor size, histological grade, Lymphnodes metastasis, ER and HER-2 status), odds ratios (ORs) and their 95% CIs were combined to estimate the effect. We performed sensitivity analysis to evaluate the influence of single study on the overall outcome. In addition, we performed funnel plots and Begg's linear regression test to examine publication bias [13]. All analysis was performed using STATA version 12.0.
Results
Study selection and characteristic
As shown in Figure 1, we identified 778 articles. 235 duplicated articles were excluded. We reviewed the titles and abstracts of 543 articles and excluded 503 irrelevant articles. After we reviewed the full texts 26 articles were further excluded. After the selection, 14 articles were finally enrolled for meta-analysis of the prognostic value of tumor-infiltrating Foxp3+ T cells in breast cancer [14-27].
We extracted RFS data from 8 of the 14 articles and OS data from 11 of the 14 articles. These studies evaluated patients from East Asia, Europe, Middle East and North America, including 3 from China, 2 from Japan, 2 from Korea, 2 from Netherlands, 2 from UK, 1 from Turkey and 2 from Canada. All the included studies evaluated FOXP3+ Tregs with immunohistochemical method. The 14 studies included 10,259 patients, the sample sizes ranged from 86 to 3,277. Six of these studies enrolled less than 200 patients and eight studies included more than 200 patients. The NOS scores of these studies ranged from 5 to 8, thus the quality of all included studies was good. Table 1 listed clinical features of 14 included studies.
Main results of meta-analysis
Correlation of FOXP3+Tregs with clinicopathological parameters
Our meta-analysis showed statistically significant association between Foxp3+Tregs and histological grade (III vs. І, П OR=2.96, 95%CI 2.03-4.31, Random effect). In addition, the presence of FOXP3+Tregs showed significant difference between ER positive and ER negative groups (OR=0.38, 95%CI 0.23-0.60, Random effect). There was also a significant difference in the incidence of FOXP3+Tregs detection between HER2 positive group and HER2 negative group (OR=2.43, 95%CI 1.69-3.51, Random effect). Other factors such as tumor size (>2 cm vs. ≤2 cm OR=1.08, 95%CI 0.86-1.35, Random effect) and lymph node metastasis (Yes vs. No OR=1.08, 95% CI 0.85-1.39, Random effect) had no significant association with Foxp3+ Tregs infiltration (Table 2).
Flow chart shows study selection procedure
Main features and methodological assessment of the included studies
| Study | Publication year | Patients source | No. of patients | NOS score | Tregs Location | Methods | Outcome | Follow-up (M) |
|---|---|---|---|---|---|---|---|---|
| Engels | 2015 | Netherland | 2426 | 7 | Total | IHC | OS/RFS | 70.8 |
| Kim | 2014 | Korea | 143 | 5 | peritumoral | IHC | OS/RFS | 69 |
| Sun | 2014 | China | 218 | 8 | Total | IHC | OS/RFS | 72 |
| Demir | 2013 | Turkey | 101 | 6 | Intratumoral | IHC | OS | 27,7 |
| Lee | 2013 | Korea | 86 | 6 | peritumoral | IHC | OS | 73.5 |
| Miki | 2013 | Japan | 100 | 6 | Total | IHC | OS | NR |
| Liu | 2011 | China | 1270 | 7 | Peritumoral Intratumoral | IHC | OS | 66 |
| Bates | 2006 | UK | 237 | 6 | Total | IHC | OS/RFS | 87.6 |
| Mahmoud | 2011 | UK | 1448 | 6 | Peritumoral Intratumoral | IHC | OS | 128 |
| Liu | 2012 | China | 132 | 6 | Peritumoral Intratumoral | IHC | OS/RFS | 62 |
| De krui | 2010 | Netherland | 556 | 7 | Intratumoral | IHC | RFS | 228 |
| West | 2013 | Canada | 175 | 6 | Total | IHC | RFS | 83 |
| Maeda | 2014 | Japan | 90 | 6 | Total | IHC | RFS | 67 |
| Liu S | 2014 | Canada | 3277 | 6 | Intratumoral | IHC | OS | 151 |
IHC: Immunohistochemistry; OS: Overall survival; NR: Not reported; M: Months
Meta-analysis of high Tregs infiltration and clinicopathological features of breast cancer patients
| Stratification of breast cancer (Foxp3+ High vs Low) | No. of studies | No. of patients | Pooled OR (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Fixed | Random | I2 | P-value | |||
| Tumor size (>2cm vs ≤2cm) | 7 | 5,748 | 0.99(0.89-1.11) | 1.08(0.86-1.35) | 63.5% | 0.012 |
| LN metastasis (Yes vs No) | 9 | 5,842 | 1.02(0.92-1.13) | 1.08(0.85-1.39) | 73.3% | <0.0001 |
| Histological grade (ш vs І, П) | 9 | 6,078 | 2.33(2.09-2.60) | 2.96(2.03-4.31) | 85.6% | <0.0001 |
| ER status (Positive vs Negative) | 9 | 6,247 | 0.32(0.28-0.38) | 0.38(0.23-0.60) | 87.8% | <0.0001 |
| HER-2 status (Positive vs Negative) | 9 | 3,789 | 2.26(1.88-2.71) | 2.43(169-3.51) | 54.1% | 0.026 |
Meta-analysis of the effects of Foxp3+ Tregs infiltration on RFS of patients with breast cancer.
Impact of FOXP3+ Tregs on survival outcomes
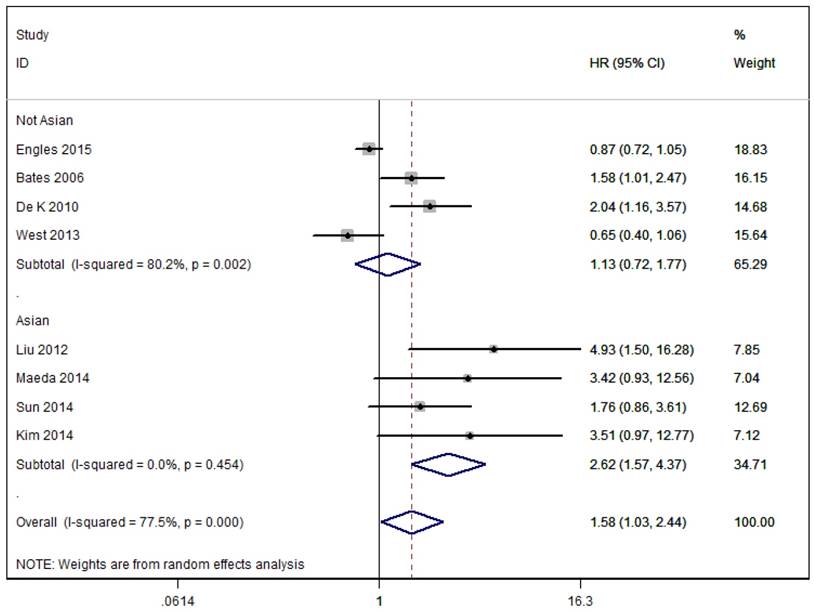
As shown in Figure 2, the pooled results of eight studies that assessed RFS showed that patients with high overall Foxp3+ T cells infiltration had significantly decreased RFS compared to patients with low/negative Foxp3+ Tregs infiltration in the random effects model (combined HR 1.58, 95% CI 1.03-2.44) and presented a significant degree of heterogeneity (I²=77.5%, p<0.0001). Subgroup analysis based on geographical location showed similar results to overall analysis in Asian group patients (Asia: HR = 2.62, 95 % CI [1.57, 4.37], I2=0.0%), but not in No-Asian group patients (Europe and North America: HR = 1.13, 95 % CI [0.72, 1.77], I2=80.2%).
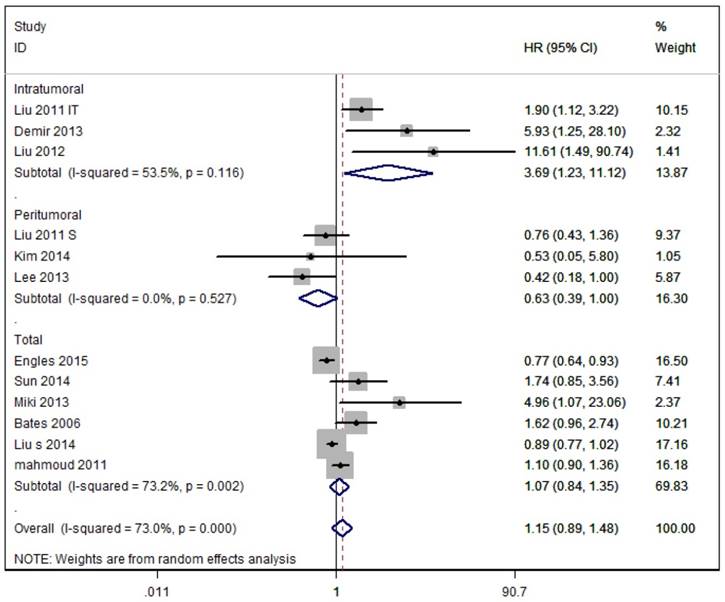
As shown in Figure 3, eleven studies assessed OS with the pooled random HR of 1.15 (95%CI 0.89-1.48) and there was evidence for heterogeneity (I2=73.0%, P<0.0001). Subgroup analysis based on Tregs site showed poor OS in breast cancer patients with intratumoural detection of FOXP3+ Tregs, but not in those with peritumoral or Total detection of FOXP3+ Tregs (Intratumoural: HR = 3.69, 95 % CI [1.23-11.12], I2= 53.5 %; Peritumoral: HR = 0.63, 95% CI [0.39-1.004], I 2= 0.0 %; Total: HR = 1.07, 95% CI [0.84-1.35], I2 = 73.2 %).
Sensitivity analysis
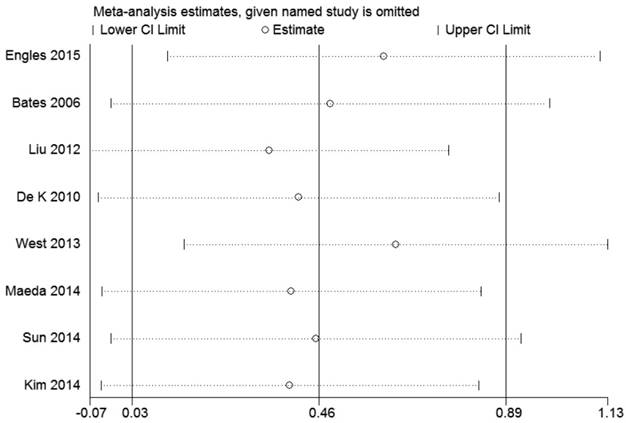
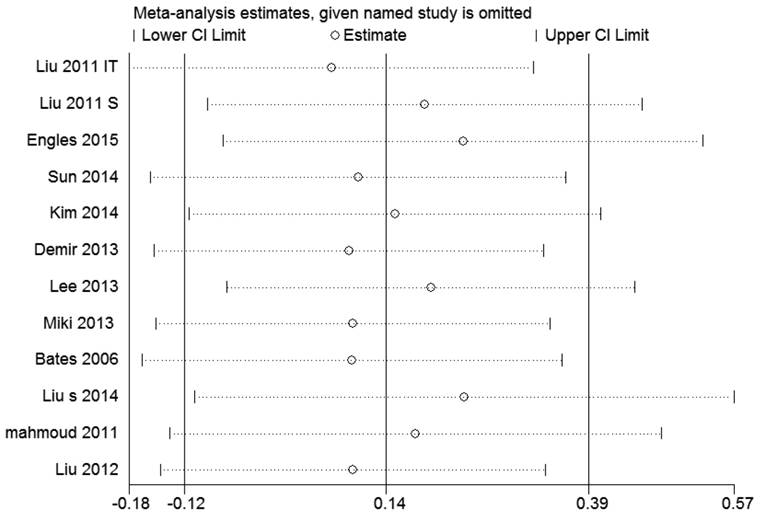
By sensitivity analysis we found similar HRs and 95% CIs if we omitted any single study, indicating that the results of RFS and OS were relatively stable (Figure 4 and 5).
Publication bias
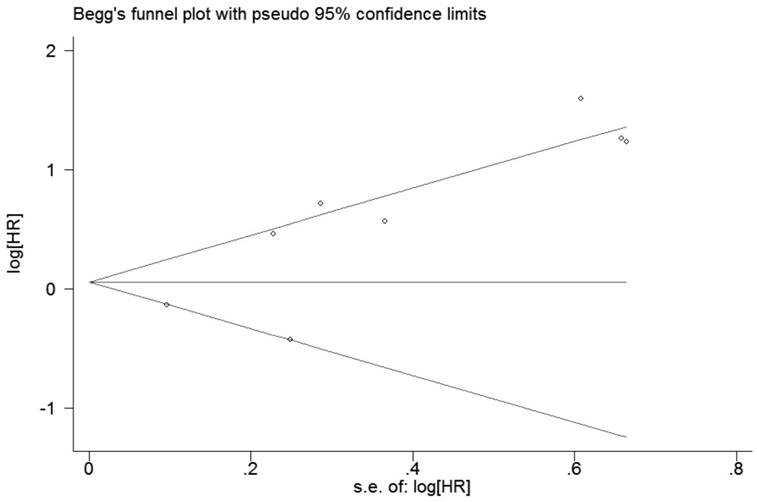
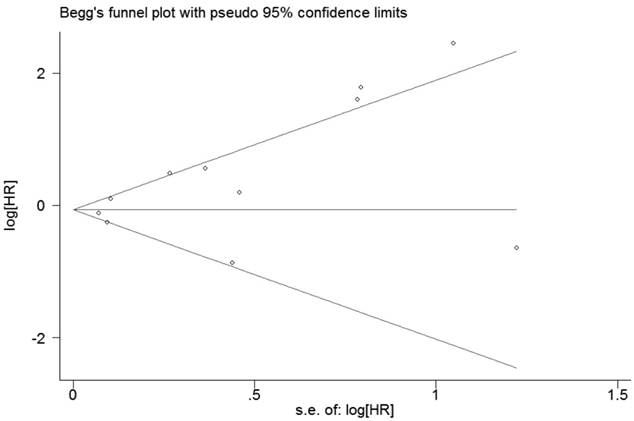
For RFS, the evaluation of publication bias by Bgger tests (p=0.386, Figure 6) showed no publication bias for all included studies. Egger's linear regression did not reveal obvious publication bias (P=0.057). For OS, Bgger's funnel plot did not show obvious asymmetry (P = 0.119, Figure 7), and Egger's linear regression did not reveal obvious publication bias (P = 0.069).
Meta-analysis of the effects of Foxp3+ Tregs infiltration on OS of patients with breast cancer. As Liu et al. 2012 reported two HRs based on the location of Foxp3+ Tregs, we used their data separately.
Sensitivity analysis of the pooled hazard ratios coefficients on the relationships between Foxp3+ Tregs and RFS of patients with breast cancer.
Sensitivity analysis of the pooled hazard ratios coefficients on the relationships between Foxp3+ Tregs and OS of patients with breast cancer.
Funnel plot of publication biases on the relationships between Foxp3+ Tregs infiltration and RFS of patients with breast cancer.
Funnel plot of publication biases on the relationships between Foxp3+ Tregs infiltration and OS of patients with breast cancer.
Discussion
Tumor immunity has big impact on clinical outcome of cancer patients [28]. Tregs play an important role of immunosuppression by regulating the balance between tolerance and rejection of self and altered self and secreting cytokines such as TGF-b, IL-4 and IL-10 [29]. Tregs markers such as GITR, OX-4, CTLA-4, CD127 and transcription factor FoxP3 have been reported, and FoxP3 is regarded as the most specific marker [30, 31]. Emerging evidence suggests that Tregs play an essential role in immune evasion mechanisms of cancer [32-35]. However, prognostic significance of FOXP3+ Tregs in breast cancer patients is still unclear. A study showed that Foxp3+Tregs were associated with good outcome of breast cancer patients [12]. Nevertheless, other studies suggested that Foxp3+Tregs were correlated with poor prognosis of breast cancer [17, 20]. Insignificant association between Foxp3+Tregs and prognosis of breast cancer patients was also reported [15, 16]. The inconsistency may be caused by different genetic backgrounds, chemotherapy agents and surgical procedures. A recent review suggested that Tregs are equipped with cytotoxic potential which may directly kill effector T cell [36]. This may explain the relationship between infiltration of Foxp3+ Tregs and poor RFS of breast cancer patients. In addition, our data showed that high infiltrated Foxp3+ Tregs was associated with a worse OS. However, the results were not statistically significant. As we know, many kinds of factors may affect overall survival rate of patients. Thus Foxp3+ Treg alone may be not enough to predict OS of breast cancer patients.
In this meta-analysis, we investigated the association between Foxp3+Tregs and clinical outcome of breast cancer patients. Combining the data of 10,259 patients from 14 studies, our analysis revealed that high overall Foxp3+Tregs infiltration was positively correlated with high histological grades, ER negativity and Her-2 overexpression, and short RFS. However, FOXP3+Tregs infiltration was not associated with OS of breast cancer patients. Subsequently, sensitivity analysis confirmed that our results were stable. Funnel plot analysis revealed no publication bias. The relationship between Foxp3+Tregs and poor RFS may be explained by the immunosuppressive function of Foxp3+Tregs in breast cancer. On the other hand, the relationship between Foxp3+Tregs and OS may be explained by that Foxp3+Tregs infiltration alone is not enough to predict the OS of breast cancer patients. The ratio between effector T cells and Tregs may serve as a better independent prognostic factor for OS of breast cancer patients [37].
We acknowledge that our meta-analysis has several limitations. First, we only selected the studies published in English. Second, the test for heterogeneity of included studies was significant (I2 = 77.5%, p<0. 0001 and I2=73%, p<0.001). We employed subgroup analysis and sensitivity analysis to find out the source, but the results were negative. Despite these limitations, the selection process of the eligible articles was strict and all included studies had high quality. Moreover, 10,259 patients were included in our meta-analysis, which made our results convincible.
In conclusion, our meta-analysis indicates that the presence of high levels of FOXP3+ Tregs is associated with poor RFS for breast cancer patients and predicts histological grade, estrogen receptor and HER-2 status.
Acknowledgements
This work was supported by Sun Yat-Sen University Clinical Research 5010 Program (#2016007) to Y. L.; The Natural Science Foundation of Guangdong Province(#2016A030310134) to N. S.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. (). Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90
3. Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375-379
4. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101(4):805-815
5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883-899
6. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151-1164
7. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467-476
8. DeLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022-3029
9. Yeong J, Thike AA, Lim JC. et al. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2017;163(1):21-35
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605
11. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560
13. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676-680
14. Engels CC, Charehbili A, van de Velde CJ. et al. The prognostic and predictive value of Tregs and tumor immune subtypes in postmenopausal, hormone receptor-positive breast cancer patients treated with adjuvant endocrine therapy: a Dutch TEAM study analysis. Breast Cancer Res Treat. 2015;149(3):587-596
15. Sun S, Fei X, Mao Y. et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer ImmunolImmunother. 2014;63(4):395-406
16. Kim S, Lee A, Lim W. et al. Zonal difference and prognostic significance of foxp3 regulatory T cell infiltration in breast cancer. J Breast Cancer. 2014;17(1):8-17
17. Demir L, Yigit S, Ellidokuz H. et al. Predictive and prognostic factors in locally advanced breast cancer: effect of intratumoral FOXP3+ Tregs. Clin Exp Metastasis. 2013;30(8):1047-1062
18. Lee S, Cho EY, Park YH, Ahn JS, Im YH. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol. 2013;52(1):73-81
19. Liu F, Lang R, Zhao J. et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645-655
20. Takenaka M, Seki N, Toh U. et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1(4):625-632
21. Bates GJ, Fox SB, Han C. et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373-5380
22. Liu S, Foulkes WD, Leung S. et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16(5):432
23. Maeda N, Yoshimura K, Yamamoto S. et al. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann Surg Oncol. 2014;21(Suppl 4):S546-S554
24. de Kruijf E M, van Nes JG, Sajet A. et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010;16(4):1272-1280
25. West NR, Kost SE, Martin SD. et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108(1):155-162
26. Liu F, Li Y, Ren M. et al. PeritumoralFOXP3(+) regulatory T cell is sensitive to chemotherapy while intratumoral FOXP3(+) regulatory T cell is prognostic predictor of breast cancer patients. Breast Cancer Res Treat. 2012;135(2):459-467
27. Mahmoud SM, Paish EC, Powe DG. et al. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127(1):99-108
28. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3(11):999-1005
29. Chang KM. Regulatory T cells and the liver: a new piece of the puzzle. Hepatology. 2005;41(4):700-702
30. Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz). 2008;56(3):181-191
31. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345-352
32. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193(11):1295-1302
33. Ng WF, Duggan PJ, Ponchel F. et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98(9):2736-2744
34. Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389-400
35. Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3(3):189-198
36. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017 Jul 31. doi: 10.1038/nri.2017.75. [Epub ahead of print] Review
37. DeLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022-3029
Author contact
![]() Corresponding author: Ying Lin M.D., Ph.D., Breast Disease Center, The First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan 2nd Road, Guangzhou 510080, P.R. China. Phone: 86 20 87755766-8198 Fax: 86 20 28823235 E-mail: linying3sysu.edu.cn
Corresponding author: Ying Lin M.D., Ph.D., Breast Disease Center, The First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan 2nd Road, Guangzhou 510080, P.R. China. Phone: 86 20 87755766-8198 Fax: 86 20 28823235 E-mail: linying3sysu.edu.cn

 Global reach, higher impact
Global reach, higher impact