3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(2):232-238. doi:10.7150/jca.22962 This issue Cite
Review
Interferon-γ and Colorectal Cancer: an up-to date
1. 3rd Department of Surgery, AHEPA University Hospital, Aristotle University of Thessaloniki, Medical School;
2. Pathology Department, Faculty of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece;
3. 1st Internal Medicine Division, University Hospital of Ioannina, University of Ioaninna, Medical School;
4. Surgery Department, “Interbalkan” European Medical Center, Thessaloniki, Greece;
5. Oncology Department, “Interbalkan” European Medical Center, Thessaloniki, Greece;
6. Pulmonary - Oncology Department, “Theageneio” Anticancer Hospital, Thessaloniki, Greece;
7. Research Laboratory and International Collaboration, Bon Secours Cancer Institute, VA, USA.
Received 2017-9-24; Accepted 2017-10-25; Published 2018-1-1
Abstract
Colorectal cancer still remains the third cause of cancer death among cancer patients. Early diagnosis is crucial and they can be either endoscopic or with blood biomarkers. Endoscopic methods consist of gastroscopy and colonoscopy, however; in recent years, endoscopic ultrasound is being used. The microenvironment is very important for the successful delivery of the treatment. Several proteins and hormones play a crucial role in the efficiency of the treatment. In the current mini review we will focus on interferon-γ.
Keywords: colorectal cancer, microenvironment, interferon-γ.
Introduction
Over the past decade a slight decline has been observed in colorectal cancer (CRC), however; it still remains the third most common cancer among cancer patients worldwide. It has been observed that CRC is associated with old age, lifestyle factors and in some cases due to underlying genetic disorders [1]. The well-known risk factors include obesity, diet, lack of physical activity and smoking [1]. Moreover; there are several dietary factors that have been identified to increase the risk of CRC such as; red and processed meat, and alcohol. Other risk factors identified are inflammatory bowel diseases, which include ulcerative colitis and Crohn's disease. There are also on the other hand inherited genetic disorders that have been identified to cause CRC and these include familial adenomatous polyposis and hereditary non-polyposis colon cancer; < 5% of the CRC cases [1]. Usually a benign tumor, a polyp, occurs and which over time becomes cancerous [1]. It may be diagnosed by obtaining a sample of the colon during sigmoidoscopy or colonoscopy. Staging follows with imaging techniques to determine if the disease has spread [2]. Currently we have effective screening methods which prevent and decrease deaths from CRC [3]. We have a number of methods which are recommended and can start from the age of 50 to 75 [3]. Furthermore, during colonoscopy, small polyps may be removed if found. On the other hand when a large polyp or tumor is found, then a biopsy may be performed to check if it is cancerous. However; it has been observed that aspirin and other non-steroidal anti-inflammatory drugs decrease the risk [4]. They are not used in general for this purpose, due to side effects [5].
There are currently several treatments used for colorectal cancer and these are: combination of surgery, chemotherapy, radiation therapy and targeted therapy [6]. Colorectal cancers that are confined within the wall of the colon may be curable with surgery while cancer that has spread widely is usually not curable. The CRCs that cannot be removed then management is being directed towards improving quality of life and symptoms [6]. Until now, five year survival rates in the United States are around 65% [7]. Survival rate however depends on how advanced the cancer is, and whether or not all the cancer bulk can be removed with surgery. All these factors of course are well associated with the person's overall health. Again, CRC is the third most common type of cancer making up about 10% of all cases [7]. In 2012, there were 1.4 million new cases and 694,000 deaths from the disease [7]. It is more common in developed countries, where more than 65% of cases are found [7]. It is less common in women than men [7]. In this current mini-review we will concentrate on the tumor microenvironment (ME) and the interferon-γ as factor associated with the disease outcome.
Microenvironment
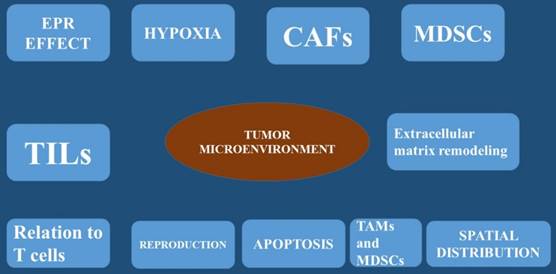
The tumor microenvironment (TME) is considered the physiological order within a tissue. This tissue space consists of tumor cells and an array of non-malignant cells such as; fibroblasts, immune cells, blood and lymphatic vessel networks, adipose tissue, and of course the extracellular matrix [8]. The non-cancerous cells can be more than 50% of the overall tumor mass [9]. Inside the TME, complex communication pathways have been observed [10], allowing the tumor and the TME to independently influence one another. The TME is responsible for the tumor phenotype and targeted therapy [11]. A healthy local microenvironment surrounding a tumor provides a barrier with anticancer effects. This observation was firstly made in melanoma where the number of tumor-infiltrating lymphocytes was shown to correlate with better clinical outcomes. Moreover; the same was also observed in colorectal cancer, where an active TME and signs of an immune response were associated with the absence of early metastasis and increased survival [12]. Local immune cells were observed to act as guards against nascent transformed cells over 60 years ago [13]. However, as tumors develop, their TME becomes disrupted, protection is lost, and tumor progression continues. The association between a disrupted microenvironment and malignancy is well established and is correlated with chronic inflammation [14] where local dysfunction acts to induce oncogenic mutations, angiogenesis, genomic instability, and early tumor promotion [14]. Cancer cells are known to directly disrupt and manipulate their local microenvironment by hijacking local cells and coercing them to provide varying services to help avoid the immune response or aid in other processes of tumor development. This occurs by supplying cytokines, growth factors, proteinases through angiogenesis [15]. To date the best studied of these commandeered cells are the tumor-associated macrophage (TAM), the myeloid-derived suppressor cells (MDSCs) and the cancer-associated fibroblast (CAF). The TAM cell is known to provide local support to the TME [16]. Usually macrophages act as a main defense against pathogens and form a bridge between the innate and adaptive immune systems. However; when they are exposed to hypoxic conditions and tumor-derived factors, macrophages begin to change phenotype and functionality [17]. To date clinical data indicate that the accumulation of TAMs within a tumor has been associated with poorer prognosis [18]. The production of TAMs was initially described as a phenotypic change of the M1 macrophage, which in turn produces pro-inflammatory cytokines. The M2 macrophage are known to produce anti-inflammatory and pro-tumorigenic functions [19]. This observation was best described in a mouse mammary cancer model that suggested TAMs are phenotypically and functionally different from M2 macrophages. Furthermore; this reported that TAMs are more likely to originate from CCR2+ inflammatory monocytes that depend on the notch signaling pathway for differentiation [20]. There are also cancer-associated fibroblasts that are fibroblasts that have become activated by local factors within the TME [21]. These have similar morphological properties to myofibroblasts [22] and are known to assume the phenotype of a facilitator of tissue repair. The mechanism starts by generating and releasing growth factors and regulating inflammation and immunity [23]. On the other hand cancer-associated fibroblasts (CAFs), do not revert to a normal phenotype nor undergo apoptosis [24]. These cells have been implicated in many aspects of tumor progression through varying mechanisms [25]. It has been observed CAFs isolated from human breast carcinoma were subcutaneously co-injected with a breast cancer cell line into an immunodeficient murine host, then tumor proliferation was significantly increased. Additional experiments attributed this to the CAF's ability to secrete stromal cell-derived factor-1 (SDF-1; also known as CXCL12) [26]. In another experiment; mice orthotopically co-xenografted with human pancreatic cancer cells and CAFs developed pancreatic tumors and metastases. Cancer-associated fibroblasts (CAFs) secretome stimulates the epithelial-mesenchymal transition (EMT) [27]. Moreover; cancer-associated fibroblasts also assist in metastasis by supplying transforming growth factor beta (TGF-β) to tumor cells which is a multifunctional cytokine known to mediate the epithelial mesenchymal transition (EMT) [28]. Moreover; tumor necrosis growth factor (TNF-β) is also pro-angiogenic, platelet-derived growth factor (PDGF), and along with vascular endothelial growth factor (VEGF), fibroblast growth factor, and SDF-1 is released by CAFs to enhance endothelial cell proliferation and migration [25]. The myeloid-derived suppressor cells (MDSC) is activated in pathological states and it has been known to have immunosuppressive capacity [29]. The myeloid-derived suppressor cells are known to protect the host organ from the harmful effects of excessive immune stimulation (such as; chronic inflammation). It has been observed that myeloid-derived suppressor cells were elevated in mice with antigen-induced autoimmune enterocolitis [30]. On the other hand in cancer patients, activation of myeloid-derived suppressor cells is an effective method of protection from immune- mediated killing [29] by inhibiting antigen-presenting dendritic cells, T and B cell proliferation, and natural killer cell cytotoxicity [31]. Furthermore, myeloid-derived suppressor cells (MDSCs) have also been implicated directly in tumor metastasis through the epithelial-mesenchymal transition (EMT) [22]. U373 glioma cells that expressed a highly oncogenic form of epidermal growth factor receptor (EGFR), called EGFRvIII, were observed to have the ability to transmit this protein to non-EGFRvIII expressing cells via extracellular vesicles (EVs) [32]. Moreover; on the study it was confirmed that that the cells expressing the malignant form of EGFR also increased their rate of extracellular vesicles production to levels that were easily detected in the blood of its murine host [33]. Tumor-derived extracellular vesicles were also observed to interact with and influence cells of the surrounding stroma. It has been also observed that breast cancer-derived extracellular vesicles can carry oncogenic proteins to surrounding fibroblasts inducing transformation and the acquisition of malignant features [34]. Furthermore; the following proteins heat shock proteins (HSP70 and HSP90) and survivin, were observed to inhibit apoptosis and increase cellular proliferation. These have been isolated from tumor-derived extracellular vesicles and have shown to promote a more aggressive cancer phenotype [35] [36]. Moreover; it has been observed that cancer-associated fibroblasts CAF-derived extracellular vesicles containing increased levels of miRNA-21 suppress apoptosis and profoundly promote tumor growth in ovarian cancer [37]. Increased pancreatic cancer aggressiveness has been associated with tumor cell-mediated uptake of CAF-derived Annexin A6+ (ANXA6) EVs [38]. Human mesenchymal stem cells release extracellular vesicles that lead to the phosphorylation of protein kinase B in gastric cancer cell lines which are associated with cancer cell proliferation [39]. It is known that tumorigenesis is associated with increased uptake amounts of oxygen and nutrients to support the enlarging cellular population and angiogenesis which are necessary mechanisms for tumor survival [40]. Extracellular vesicles are implicated in many of the sophisticated processes of angiogenesis. Extracellular vesicles carry pro-angiogenic factors as cargo including vascular endothelial growth factor (VEGF), fibrotic growth factor-2 (FGF2), which leads to a global increase in extracellular vesicles release from cancer cells [41]. It is known that glioblastoma is characterized by severe hypoxia [42], and therefore large areas of necrosis are observed within the tumor. Glioblastoma cells have been demonstrated not only to secrete extracellular vesicles that contain tissue factor but also activate hypoxic endothelial cells [43, 44]. Furthermore, RNAs carried by extracellular vesicles EVs have also been implicated in neo-angiogenic processes. In specific in CRC, tumor-derived extracellular vesicles contain cell cycle M-phase-related mRNAs including centromere protein E, and cyclin-dependent kinase 8 which promote endothelial proliferation [45]. CRC cell-derived extracellular vesicles also carry miRNAs, including miRNA-9, which causes strong angiogenic [46] (Figure 1-3).
Interferon-γ
Interferon-γ, is also known as type II interferon, is a cytokine that is critical for innate and adaptive immunity against viral infections and some bacterial protozoal. Interferon-γ is known to be an important activator of macrophages and also inducer of Class II major histocompatibility complex (MHC) molecule expression. In the case of aberrant interferon-γ expression a number of auto-inflammatory and autoimmune diseases have been observed. Interferon-γ has the ability to inhibit viral replication directly, and also has immune-stimulatory and immunomodulatory effects. Interferon-γ is known to be produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 Th1 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once the antigen-specific immunity complex develops [47]. Moreover; interferon-γ is also known to be produced by non-cytotoxic innate lymphoid cells (ILC) which is a family of immune cells first discovered in the early 2010's [48].
It has been observed that interferon-γ is secreted by T helper cells (specifically, Th1 cells), cytotoxic T cells (TC cells), mucosal epithelial cells, macrophages, and NK cells. Interferon-γ is the only Type II interferon and it is serologically distinct from Type I interferons. The properties of interferon-γ can be summarized to: a) immunoregulatory, b) antiviral, and c) anti-tumor properties [49]. Interferon-γ is responsible for the transcription of 30 genes which produce a variety of physiological and cellular responses. The most important effects are: a) Promotion of Natural Killer cell activity, b) Increase of antigen presentation and lysosome activity of macrophages, c) Activation of inducible nitric oxide synthase (iNOS), d) Induction of the production of IgG2a and IgG3 from activated plasma B cells, e) Cause normal cells to increase expression of class I MHC molecules as well as class II MHC on antigen-presenting cells—to be specific. This occurs through induction of antigen processing genes, including subunits of the immunoproteasome (MECL1, LMP2, LMP7), f) Promotion of adhesion and binding required for leukocyte migration, h) Induction of the expression of intrinsic defense factors—for example, with respect to retroviruses, relevant genes include tripartite motif-containing protein 5(TRIM5alpha), apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC), and tetherin, representing directly antiviral effects.
T helper cells1 cells are known to interferon-γ, which in turn causes more undifferentiated CD4+cells (Th0 cells) to differentiate into Th1 cells [47], and in turn represent a positive feedback loop—which suppress Th2 cell differentiation.
The natural killer cells and CD8+ cytotoxic T cells also known to produce interferon-γ. Interferon-γ is known to suppress osteoclast formation by rapidly degrading the nuclear factor-kappa B (RANKadaptor) protein TNF receptor associated factor (TRAF6) in the RANK-RANK-L (ligand) signaling pathway, which otherwise stimulates the production of NF-κB (Table 1).
Recent published studies on interferon-γ
| Author | Patients | Animals | Cell Lines | Interferon-γ |
|---|---|---|---|---|
| Wang L. et. al. | - | √ | - | Deficiency |
| Liu S. et. al. | - | - | √ | Specific expression |
| Ragaghi A. et. al. | - | - | √ | Specific expression |
| Kim A. et. al. | - | √ | - | Specific expression |
| Djaafar S. et. al. | - | √ | - | Interferon-γ-inducible chemokines |
The enhanced permeability and retention effect (EPR effect), hypoxia, carcinoma associated fibroblasts (CAFs), Myeloid-derived suppressor cells (MDSCs), Tumor infiltrating lymphocytes (TILs), extracellular matrix remodeling, relation to T cells, apoptosis, reproduction
Treatment options and algorithm
Chemical structure of interferon-γ and sequence

In the study by Wang L. et al. [50] it was observed that deficiency of interferon-gamma or its receptor promotes colorectal cancer development. In the study by Liu S. et al. [51] it was observed that specific expression of interferon-γ induced by synergistic activation mediator-derived (SAM) systems activates innate immunity and inhibits tumorigenesis. In the study by Razaghi A. et al. [52] improved therapeutic efficacy of mammalian expressed-recombinant interferon gamma against ovarian cancer cells was observed. In the study by Li Z. et al. [53] it was observed that anti-VEGFR-interferon-α2 regulates the tumor microenvironment and exhibits potent antitumor efficacy against colorectal cancer. In the study by Katlinsky K.V. et al. [54] it was observed that inactivation of interferon receptor promotes the establishment of immune privileged tumor microenvironment. In the study by Djaafar S. et al. [55] it was observed that enoxaparin attenuates mouse colon cancer liver metastases by inhibiting heparanase and interferon-γ-inducible chemokines. In the study by Radice E. et al. [56] it was observed that low-doses of sequential-kinetic-activated interferon-γ enhance the ex vivo cytotoxicity of peripheral blood natural killer cells from patients with early-stage colorectal cancer.
Discussion
Interferon-γ is known to have a potential use in immunotherapy. To date interferon-γ is not approved yet for the treatment in any cancer immunotherapy. However, improved survival has been observed when it was administrated to patients with bladder carcinoma and melanoma cancers. The most promising result were obsreved in patients with stage 2 and 3 of ovarian carcinoma. It is known that the in vitro study of interferon-γ in cancer cells is more extensive. Moreover; results indicate an anti-proliferative activity of IFN-gamma leading to the growth inhibition or cell death, and apoptosis through autophagy [57]. New evidence suggests that interferon-γ expression is regulated by a pseudoknotted element in its 5' UTR [58]. There is also evidence that interferon-γ is regulated either directly or indirectly by the microRNAs: miR-29 [59]. Furthermore, it has been observed that interferon-γ expression is regulated via glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in T-cells. This interaction takes place in the 3'UTR, where binding of glyceraldehyde 3-phosphate dehydrogenase prevents the translation of the mRNA sequence [60]. The local microenvironment in the surgical site is affected by the surgical technique as it was previously observed. [61] Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study [61]. In the study by Zhao X. et al. [62] it was observed that the location of the CRC plays a crucial role in the inflammatory cytokines that are being secreted locally. In the study by Wang H. et al. [63] a new prognostic factor and treatment target was suggested and this was the programmed death-ligand-2 (PD-L2) expression. In the study by Chen X. et al. [64] it was suggested that PD-L1 expression could be a possible future treatment target. In the study by Van Der Kraak L. et al. [65] it was observed that pretreatment with immunotherapy can upregulate the programmed death pathway making it a new treatment option. In any case there should be a balance between the inflammatory cytokines, they can either up-regulate the local environment in favor of tumorigenesis or inhibit tumorigenesis. The authors suggest a measurement of interferon-γ levels before admission of therapy (any therapy) and based on these levels additionally possibly low doses of interferon-γ could be administered.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Carini F, Mazzola M, Rappa F, Jurjus A, Geagea AG, Al Kattar S. et al. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer research. 2017;37:4759-66 doi:10.21873/anticanres.11882
2. Gill AA, Enewold L, Zahm SH, Shriver CD, Stojadinovic A, McGlynn KA. et al. Colon cancer treatment: are there racial disparities in an equal-access healthcare system? Diseases of the colon and rectum. 2014;57:1059-65 doi:10.1097/DCR.0000000000000177
3. Aedo KP, Conde LF, Pereyra-Elias R. Colorectal cancer screening in Latin America: Are we still in the Stone Age? Acta gastroenterologica Latinoamericana. 2016;46:104-5
4. Thorat MA, Cuzick J. Role of aspirin in cancer prevention. Current oncology reports. 2013;15:533-40 doi:10.1007/s11912-013-0351-3
5. Force USPST. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2007;146:361-4
6. Aoyagi T, Terracina KP, Raza A, Takabe K. Current treatment options for colon cancer peritoneal carcinomatosis. World journal of gastroenterology. 2014;20:12493-500 doi:10.3748/wjg.v20.i35.12493
7. Altekruse SF, Rosenfeld GE, Carrick DM, Pressman EJ, Schully SD, Mechanic LE. et al. SEER cancer registry biospecimen research: yesterday and tomorrow. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:2681-7 doi:10.1158/1055-9965.EPI-14-0490
8. Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y. et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC medicine. 2015;13:45. doi:10.1186/s12916-015-0278-7
9. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. Journal of cell science. 2012;125:5591-6 doi:10.1242/jcs.116392
10. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239-52 doi:10.1038/nrc2618
11. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature medicine. 2013;19:1423-37 doi:10.1038/nm.3394
12. Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654-66 doi:10.1056/NEJMoa051424
13. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3:991-8 doi:10.1038/ni1102-991
14. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-99 doi:10.1016/j.cell.2010.01.025
15. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346-54 doi:10.1038/nature12626
16. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51 doi:10.1016/j.cell.2010.03.014
17. Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ breast cancer. 2016:2 doi:10.1038/npjbcancer.2015.25
18. Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cellular & molecular immunology. 2015;12:1-4 doi:10.1038/cmi.2014.83
19. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889-96 doi:10.1038/ni.1937
20. Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K. et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921-5 doi:10.1126/science.1252510
21. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6:392-401 doi:10.1038/nrc1877
22. De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. International journal of cancer. 2008;123:2229-38 doi:10.1002/ijc.23925
23. Kalluri R. The biology and function of fibroblasts in cancer. Nature reviews Cancer. 2016;16:582-98 doi:10.1038/nrc.2016.73
24. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. Journal of cellular biochemistry. 2007;101:805-15 doi:10.1002/jcb.21159
25. Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers. 2015;7:2443-58 doi:10.3390/cancers7040902
26. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-48 doi:10.1016/j.cell.2005.02.034
27. Moatassim-Billah S, Duluc C, Samain R, Jean C, Perraud A, Decaup E. et al. Anti-metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancer-associated fibroblasts. Oncotarget. 2016;7:41584-98 doi:10.18632/oncotarget.9296
28. Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells, tissues, organs. 2007;185:7-19 doi:10.1159/000101298
29. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125:3356-64 doi:10.1172/JCI80005
30. Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM. et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871-81 81 e1-5. doi:10.1053/j.gastro.2008.06.032
31. Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of immunology. 2001;166:678-89
32. Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619-24 doi:10.1038/ncb1725
33. Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10:1470-6 doi:10.1038/ncb1800
34. Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL. et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4852-7 doi:10.1073/pnas.1017667108
35. Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis: an international journal on programmed cell death. 2011;16:1-12 doi:10.1007/s10495-010-0534-4
36. Graner MW, Cumming RI, Bigner DD. The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11214-27 doi:10.1523/JNEUROSCI.3588-07.2007
37. Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS. et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nature communications. 2016;7:11150. doi:10.1038/ncomms11150
38. Leca J, Martinez S, Lac S, Nigri J, Secq V, Rubis M. et al. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. The Journal of clinical investigation. 2016;126:4140-56 doi:10.1172/JCI87734
39. Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H. et al. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Molecular medicine reports. 2016;14:3452-8 doi:10.3892/mmr.2016.5625
40. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-57 doi:10.1038/35025220
41. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC cancer. 2012;12:421. doi:10.1186/1471-2407-12-421
42. Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT. et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:8177-84 doi:10.1158/1078-0432.CCR-04-1081
43. Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC. et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13147-52 doi:10.1073/pnas.1104261108
44. Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M. et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7312-7 doi:10.1073/pnas.1220998110
45. Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P. et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796-804
46. Wendler F, Favicchio R, Simon T, Alifrangis C, Stebbing J, Giamas G. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 2017;36:877-84 doi:10.1038/onc.2016.253
47. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Advances in immunology. 2007;96:41-101 doi:10.1016/S0065-2776(07)96002-2
48. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293-301 doi:10.1038/nature14189
49. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of leukocyte biology. 2004;75:163-89 doi:10.1189/jlb.0603252
50. Wang L, Wang Y, Song Z, Chu J, Qu X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2015;35:273-80 doi:10.1089/jir.2014.0132
51. Liu S, Yu X, Wang Q, Liu Z, Xiao Q, Hou P. et al. Specific expression of interferon-gamma induced by synergistic activation mediator-derived (SAM) systems activates innate immunity and inhibits tumorigenesis. Journal of microbiology and biotechnology. 2017 doi:10.4014/jmb.1705.05081
52. Razaghi A, Villacres C, Jung V, Mashkour N, Butler M, Owens L. et al. Improved therapeutic efficacy of mammalian expressed-recombinant interferon gamma against ovarian cancer cells. Experimental cell research. 2017 doi:10.1016/j.yexcr.2017.08.014
53. Li Z, Zhu Y, Li C, Trinh R, Ren X, Sun F. et al. Anti-VEGFR2-interferon-alpha2 regulates the tumor microenvironment and exhibits potent antitumor efficacy against colorectal cancer. Oncoimmunology. 2017;6:e1290038. doi:10.1080/2162402X.2017.1290038
54. Katlinski KV, Gui J, Katlinskaya YV, Ortiz A, Chakraborty R, Bhattacharya S. et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer cell. 2017;31:194-207 doi:10.1016/j.ccell.2017.01.004
55. Djaafar S, Dunand-Sautier I, Gonelle-Gispert C, Lacotte S, A DEA, Petro M. et al. Enoxaparin Attenuates Mouse Colon Cancer Liver Metastases by Inhibiting Heparanase and Interferon-gamma-inducible Chemokines. Anticancer research. 2016;36:4019-32
56. Radice E, Miranda V, Bellone G. Low-doses of sequential-kinetic-activated interferon-gamma enhance the ex vivo cytotoxicity of peripheral blood natural killer cells from patients with early-stage colorectal cancer. A preliminary study. International immunopharmacology. 2014;19:66-73 doi:10.1016/j.intimp.2013.12.011
57. Razaghi A, Owens L, Heimann K. Review of the recombinant human interferon gamma as an immunotherapeutic: Impacts of production platforms and glycosylation. Journal of biotechnology. 2016;240:48-60 doi:10.1016/j.jbiotec.2016.10.022
58. Sadir R, Forest E, Lortat-Jacob H. The heparan sulfate binding sequence of interferon-gamma increased the on rate of the interferon-gamma-interferon-gamma receptor complex formation. The Journal of biological chemistry. 1998;273:10919-25
59. Asirvatham AJ, Gregorie CJ, Hu Z, Magner WJ, Tomasi TB. MicroRNA targets in immune genes and the Dicer/Argonaute and ARE machinery components. Molecular immunology. 2008;45:1995-2006 doi:10.1016/j.molimm.2007.10.035
60. Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O'Sullivan D. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239-51 doi:10.1016/j.cell.2013.05.016
61. Siekmann W, Eintrei C, Magnuson A, Sjolander A, Matthiessen P, Myrelid P. et al. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2017;19:O186-O95 doi:10.1111/codi.13643
62. Zhao X, Li L, Starr TK, Subramanian S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget. 2017;8:54775-87 doi:10.18632/oncotarget.18423
63. Wang H, Yao H, Li C, Liang L, Zhang Y, Shi H. et al. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology. 2017;6:e1327494. doi:10.1080/2162402X.2017.1327494
64. Chen X, Xu J, Guo Q, Wang L, Yang Y, Guo H. et al. Therapeutic efficacy of an anti-PD-L1 antibody based immunocytokine in a metastatic mouse model of colorectal cancer. Biochemical and biophysical research communications. 2016;480:160-5 doi:10.1016/j.bbrc.2016.10.011
65. Van Der Kraak L, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP. et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. Journal for immunotherapy of cancer. 2016;4:65. doi:10.1186/s40425-016-0163-8
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary - Oncology Department, “Theageneio” Anticancer Hospital, Thessaloniki, Greece Mobile: 00306977271974 E-mail: pzarogcom
Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary - Oncology Department, “Theageneio” Anticancer Hospital, Thessaloniki, Greece Mobile: 00306977271974 E-mail: pzarogcom

 Global reach, higher impact
Global reach, higher impact