3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(2):415-423. doi:10.7150/jca.19185 This issue Cite
Research Paper
GP73 level determines chemotherapeutic resistance in human hepatocellular carcinoma cells
1. Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China
2. Department of Pathology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, P. R. China
3. Department of First Chemotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China
4. School of Public Health, Sun Yat-Sen University, Guangdong Guangzhou, 510060, P. R. China
Received 2017-1-14; Accepted 2017-11-29; Published 2018-1-1
Abstract
Objective GP73 is a new hepatocellular carcinoma (HCC) marker, which is highly expressed in hepatocellular carcinoma and closely relates to prognosis. This study was to investigate the effects of GP73 on cellular proliferation, apoptosis, oxaliplatin (OXA) resistance and secretory clusterin (sCLU) of HCC cells.
Materials and Methods Western blot and immunofluorescence was used to detect the expression of GP73 in 8 types of commonly used HCC cell lines. Drug resistance was induced by increasing concentration gradient method. The drug-resistant human HCC cell lines underwent GP73 overexpression or inhibition. Flow cytometry were used to detect the proliferation and apoptosis of HCC cell lines. The changes of sCLU were detected by enzyme-linked immunosorbent assay (ELISA).
Results The expression of GP73 in MHC-97H cells was the highest and in Hep3B cells the lowest. The expression of GP73 was found further elevated in OXA-resistant MHC-97H cells. After the knockdown of GP73 in OXA-resistant 97H cells, the IC50 of OXA decreased and the ability of cell proliferation decreased significantly. After over-expression of GP73 in OXA-resistant Hep3B cells, the IC50 of OXA increased and the cell proliferation ability increased, showing that GP73 is critical for OXA resistant in HCC cell lines; No significant change of sCLU level in GP73 overexpressed Hep3B and GP73 blocked MHCC-97H were identified.
Conclusion The expression level of GP73 is critical for the resistance of OXA in HCC cell lines.
Keywords: hepatocellular carcinoma, GP73, chemotherapy, drug resistance, OXA
Introduction
Primary hepatocellular carcinoma (PHC) is the sixth most commonly occurring cancer in the world and the third largest cause of cancer mortality [1]. The incidence of PHC is 37.5% in China [2]. Most PHC patients are hepatitis B carrier and have liver cirrhosis background. They are often diagnosed at late stage, lacking operation opportunities. Thus, ablation or TACE treatment of PHC patients with systemic chemotherapy is commonly used in palliative treatment.
Oxaliplatin (OXA) is the third-generation platinum analog, which is cell cycle non-specific drug and has been shown efficacy against many tumor cell lines, including some that are resistant to cisplatin and carboplatin. It is currently considered as the most effective chemotherapy drugs for PHC patients. Like other platinum-based compounds, OXA exerts cytotoxic effect mainly through DNA damage. OXA induces DNA lesion crosslinks formation, blocks DNA and mRNA synthesis [3]. In the treatment of advance PHC patients, OXA has achieved a certain effect. However, the efficacy of chemotherapy contained OXA to liver cancer are only 8.15% to 15.6% [3, 4]. The efficacy of OXA on PHC did not show encouraging results and chemotherapeutic resistance has become a huge obstacle in cancer treatment. Therefore, the mechanism of chemotherapeutic resistance in HCC requires further studies.
Golgi protein-73 (GP73) was originally detected in hepatocytes of patients with hepatocellular carcinoma caused by adenovirus. It also expresses in epithelial cells such as stomach, spleen and colon. In the normal hepatobiliary system, GP73 only expresses in adult bile duct epithelium while liver cells do not express it [5]. However, GP73 is highly expressed in HCC cells, regardless of whether viruses' infection exist or not [6]. Secretory GP73 is also found in serum of patients with HCC. Some studies suggested that GP73 serum levels can assist HCC diagnosis and prediction, showing even better efficacy and accuracy than AFP [7, 8]. Additionally, the increase of GP73 after treatment predicts a higher tumor recurrence rates in HCC [9]. Moreover, the expression level of GP73 in hepatocellular carcinoma is related to tumor size, tumor invasiveness, and tumor differentiation [10]. Using cDNA microarray, Takeyasu katada et al found that GP73 gene highly expressed in high-resistant TE4 esophageal cancer cells and the sensitivity of TE4 cells to cisplatin was significantly increased after GP73 inhibition [11]. Thus, we hypothesize that GP73 may be involved in the chemotherapeutic resistance of HCC. However, no report about GP73 and chemo-resistance of HCC is found. The aim of this study was to investigate the effects of the manipulation of GP73 on OXA resistance of HCC cell lines.
Methods
Cell culture
Human normal liver cell line and human hepatic carcinoma cell line (HepG2, Bel7402, Bel7404, SMMC7721, SNU739) were purchased from the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences (CAS). After being thawed, resuscitated and passaged, the cells were kept in RPMI 1640 medium containing 10% fetal bovine serum and cultured in 37 ℃, 5% CO2 saturated humidity cell incubator.
Antibodies, Western blots and Immunofluorescence
The antibodies used in this study were as follows: mouse-derived anti-GP73 antibody (Proteintech); the secondary antibodies in this study were derived from Jackson ImmunoResearch. The expression of GP73 was detected by Western blotting (1: 800 dilution). The secondary antibody was either mouse anti-IgG or rabbit anti-IgG (1: 8000 dilution). An enhanced chemiluminescent agent, supplied by Millipore Corporation, is used for immunoreaction detection. For immunofluorescence, Alexa Fluor® 488 Donkey Anti-Rabbit IgG (Invitrogen, No: A21206, 1:1000) and Alexa Fluor® 555 Donkey Anti-Goat IgG (Invitrogen, No: A21432, 1:1000) were applied as secondary antibodies. Experiments were conducted based on standard protocols.
Establishment of OXA-resistant Hepatocellular Carcinoma Cell Line
The drug resistance was induced by increasing the concentration gradient. First, the IC50 of the cells was detected by CCK method, and the highest concentration of OXA was the concentration determined by IC50. After 2 μM of OXA was selected as the starting concentration, the culture medium was changed to a culture medium containing OXA for 48 hours and the culture was continued until cell death was observed. After the first round incubation, the drug concentration was gradually increased to 4, 6, 8, 10, 30 and 60μM. After rounds of induction, drug-resistant cell lines were frozen for the following experiments.
Establishment of stable cell lines in which GP73 was overexpressed or knocked down
The siRNA sequence was the following: Si RNA 1: 5'-agggaaacgtgcttggtaa-3'; Si RNA 2:5'-gaatagaagaggtcaccaa-3'. Lentiviral vectors encoding shRNA were designed based on the sequences of siRNA to knock down GP73 expression (GP73-KD). These vectors were constructed by Hanyin Co. (Shanghai, China). The recombinant lentiviruses (KD) and negative control (NC) lentivirus were prepared and titered to 109 transfection units (TU)/mL. To obtain stable cell lines, cells were seeded in six-well plates and infected with virus and polybrene on the following day. Positive clones were selected with puromycin for 14 days to establish the stable cell lines. The lentiviruses expressing the GP73 sequence (OE) and the negative control lentivirus (Vector) were also constructed by Hanyin Co. (Shanghai, China). GP73-OE and control stable cell lines were then established in a similar way. The efficiency of GP73 knockdown and overexpression were confirmed by qRT-PCR.
CCK method
5 × 103 cells in 200μl culture medium were inoculated into each well of 96-well plate. After cell adherence, the medium was changed to different concentrations of OXA (8-256 μM) contained RPA1640 and further incubation for 72 hours. Then, 10 μl of MTT solution was added to each well. After further incubation for 1 hour, the absorbance at 450 nm was measured by a microplate reader. IC50 was calculated by software, and the multiple of drug resistance and reversal of drug resistance after GP73 manipulation were calculated (drug-resistant multiples = IC50 of drug-resistant cells/IC50 of parent cells; drug-resistant reversal multiple = IC50 before reversal drug resistance/ IC50 after reversal drug resistance).
Fluorescence-activated cell sorting (FACS)
The reagents used were as follows: Annexin V-FITC, Propidium Iodide (BD Biosciences, USA). The cells were digested with 0.25% trypsin without EDTA and washed with PBS. After resuspending the cells, Annexin V-FITC and propidium iodide were added and mixed well. Cells were incubated in dark room for 15 minutes and analyzed by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
The reagents used were as follows: human CLU elisa kit (MLBIO Co, Shanghai, China). CLU expression test was performed strictly according to the instructions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism7 software (standard version, California, USA). A two-sided probability value less than 0.05 was considered statistically significant. Unless stated, data are presented as mean ± SD in the figures. A Student t test was performed to compare the data.
Results
The expression of GP73 in HCC cell lines
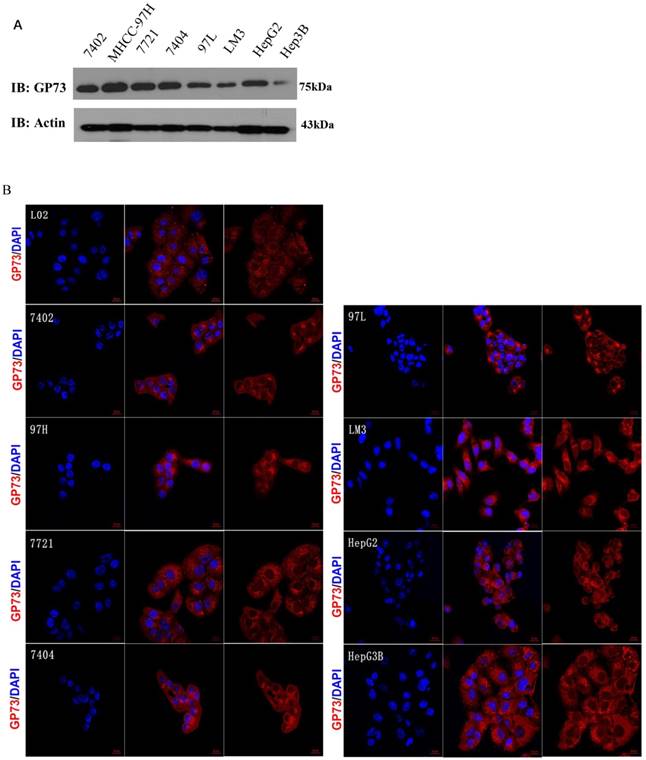
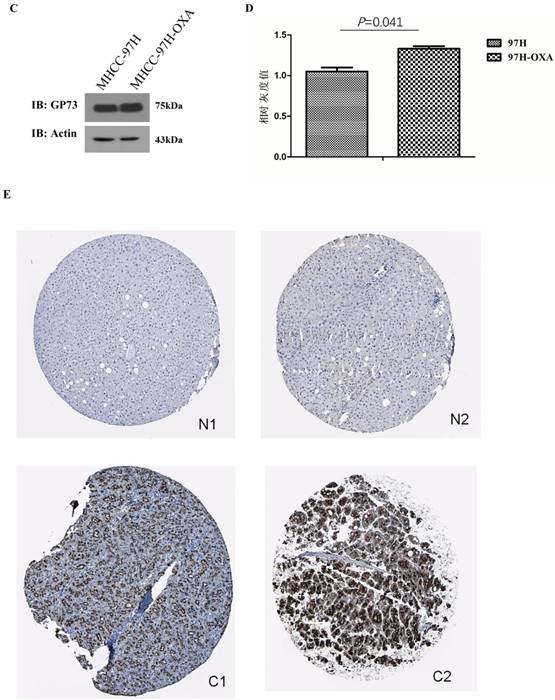
The expression of GP73 in 8 HCC cell lines and normal liver cell line L02 was shown by both western blot and immunofluorescence (Fig 1A and B), they consistently revealed that GP73 expressed highest in MHCC-97H cells and lowest in Hep3B cells. The expression of GP73 in normal liver cell line L02 is obviously lower than other liver cancer cell lines as shown by immunofluorescence. After resistance induction, the expression of GP73 in OXA-resistant MHCC-97H cells (MHCC-97H-OXA) was obviously higher than that in parental MHCC-97H cell lines (Fig 1C and D). To address the question about expression difference in normal and cancerous liver cells, we showed some representative figures from Human Protein Atlas (www.proteinatlas.org). It clearly demonstrated that GP73 express preferentially in liver cancer cells (Fig 1E).
The correlation between GP73 level and OXA resistance of HCC cells
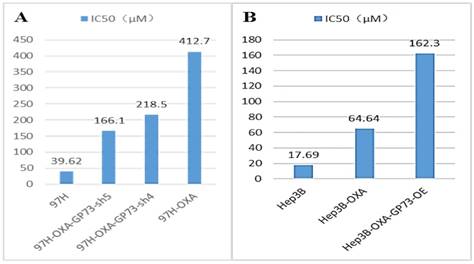
The IC50 of MHCC-97H cell line was 39.62 μM. After induction of drug resistance, the IC50 of MICC-97H-OXA cells was 412.7 μM, and drug-resistance multiple was 10.42. GP73 was further knocked down in MICC-97H-OXA cells, resulting to a decreased IC50 (218.5 μM of MHCC-97H-OXA-GP73-sh4 and 166.1 μM of MHCC-97H-OXA-GP73-sh5 respectively). Drug resistance reversal multiple of MHCC-97H-OXA-GP73-sh4 is 1.91 and MHCC-97H-OXA-GP73-sh5 is 2.48. The IC50 difference between MICC-97H-OXA and GP73 blocked MHCC-97H-OXA cell lines was statistically significant (Figure 2A).
The IC50 of Hep3B cell line was 17.69 μM. After induction of drug resistance, The IC50 of Hep3B-OXA was 64.64 μM, and the drug-resistance multiple was 3.65. After GP73 overexpression, the IC50 of Hep3B-OXA-GP73-OE was 162.3 μM and the drug-resistance reversal multiple was 0.398. There was significant difference in the IC50 among these three different Hep3B lines (Fig 2B).
GP73 and the proliferation of HCC cell
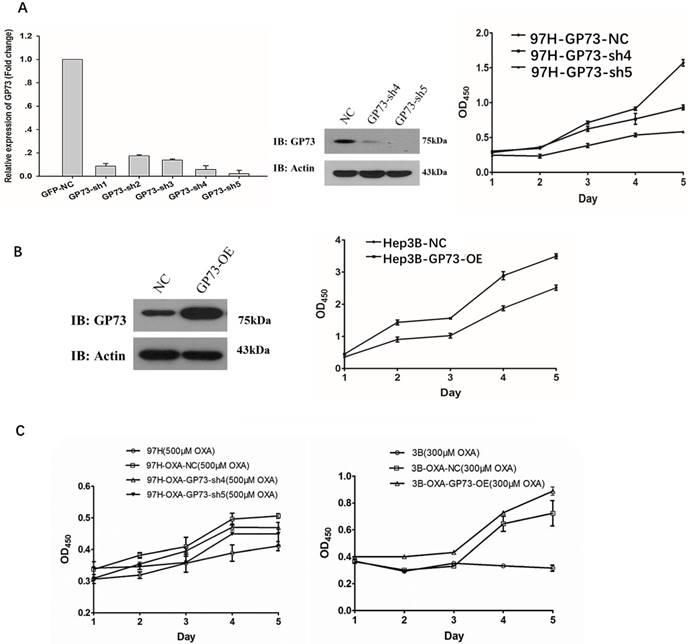
GP73 expression is successfully depleted in Sh4 and Sh5 groups (Fig 3A, left, middle). Importantly, the proliferation of MHCC-97H-GP73-sh4 and MHCC-97H-GP73-sh5 obviously decreased when compared with normal control (NC) cell line. On the fifth day, the differences of proliferation of each cell line was statistically significant (P<0.001, Fig. 3A, right).
The proliferation of GP73 overexpressed Hep3B cells (Hep3B-GP73-OE) was much higher than that of the parental cell lines (Hep3B). On the fifth day, the proliferation ability difference was statistically significant (p <0.01, Fig 3B).
OXA-resistant cells (MHCC-97H-OXA) were established by concentration-elevation method (OXA, 500μM, 48h). The proliferation of MHCC-97H-OXA cells was the highest. When GP73 was knocked down after the induction of drug resistance, the proliferation ability of the MHCC-97H-OXA-GP73-sh4 and MHCC-97H-OXA-GP73-sh5 were much lower than that of the MHCC-97H cells. On the fifth day, the proliferation ability of each cell line was statistically significant (P<0.001, Fig 3C, left).
The proliferation of OXA resistant Hep3B-OXA cells was shown to be significantly higher than that of Hep3B cells (P <0.05, Fig 3C, right). Overexpression of GP73 resulted in a higher proliferation ability in Hep3B-OXA-GP73-OE cells. On the fifth day, the proliferation ability of each cell line was statistically significant (p <0.01, Fig 3C, right).
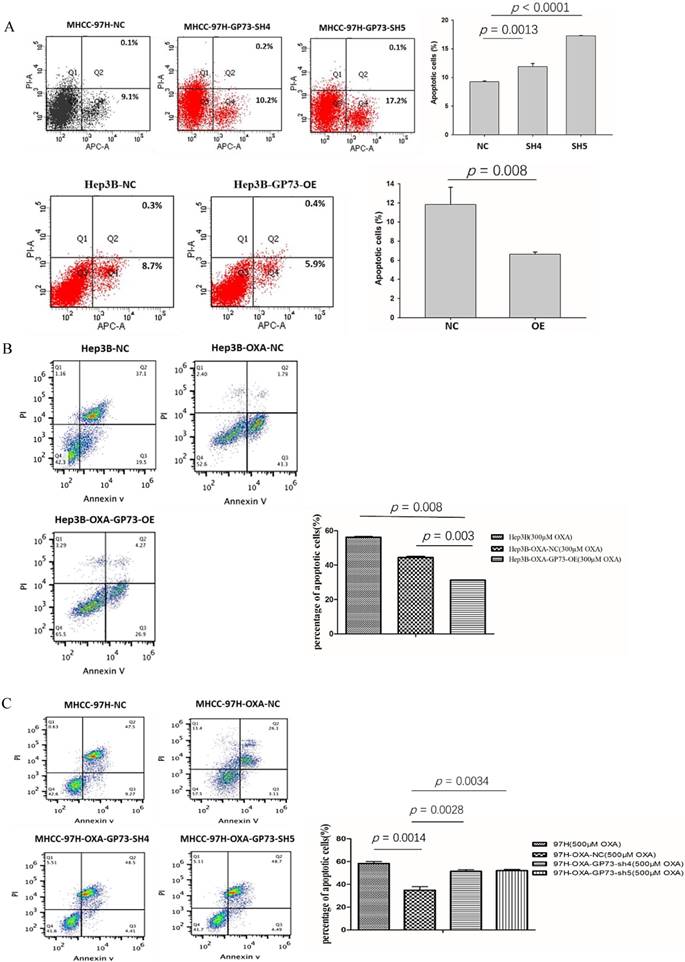
GP73 and apoptosis of HCC cell lines
As shown in Fig 4A, the knockdown of GP73 in MHCC-97H cells (MHCC-97H-GP73-sh4 and MHCC-97H-GP73-sh5 leads to increased apoptosis rates (p <0.001, Fig 4A, left). As shown in Fig 4A, the apoptosis rates of GP73 over-expressed cells(Hep3B-GP73-OE) was significantly lower than that of the parent Hep3B cells (p = 0.008, Fig 4A, right).
In order to study the effects of GP73 on the anti-apoptotic ability of drug-resistant HCC cell lines. After treatment with OXA (500 μM) for 72 h, the apoptosis of different types of 97H cell line was examined. The results showed that MHCC-97H-OXA possessed lowest apoptosis rates. MHCC-97-OXA-GP73-sh5 and MHCC-97-OXA-GP73-sh4 showed higher apoptosis rate than that of MHCC-97H-OXA cells (p <0.01, Figure 4B, left). The apoptosis rate of the Hep3B was the highest when compared with OXA resistant cell lines. After GP73 over expression, the apoptosis rate was further decreased when compared with OXA resistant Hep3B cells (p <0.01, Fig 4B, right).
The expression of GP73 in liver cancer cell lines and liver tissues. The expression of GP73 in 8 HCC cell lines and normal liver cell line L02, Scale bar = 20 μm (Figure 1A,1B); Expression of GP73 in HCC cell lines before and after OXA induction MHCC-97H-OXA was the cell line after induction of OXA resistance on MHCC-97H (Figure 1C,1D). Representative figures of GP73 expression by IHC from Human Protein Atlas (www.proteinatlas.org). N: normal tissue; C: cancer tissue (Fig 1E).
The comparison of the IC50 (μM) before and after the intervention of the HCC cell line. 97H-OXA was the cell line after induction of MHCC-97H resistance; 97H-OXA-GP73-sh4 and 97H-OXA-GP73-sh5 were the cell lines that knock-out of GP73 gene after OXA resistance induction of MHCC-97H cell; Hep3B-OXA was the Hep3B cells line induced by OXA resistance; Hep3B-OXA-GP73-OE was the cell line over-expressed GP73 after induction of OXA resistance of Hep3B cells.
GP73 and the proliferation activity. GP73 and the proliferation activity of MHCC-97H cells (Figure 3A). GFP-NC was the GP73 negative control MHCC-97H cells; GP73-sh1 to sh5 were the MHCC-97H cells that GP73 gene were knocked down, respectively. GP73 and The proliferation activity of Hep3B cells (Figure 3B, left) GP73-OE was the Hep3B cells that GP73 gene over-expressed; Hep3B-NC was the negative control Hep3B cells. GP73 and proliferation activity of OXA-resistance cell (Fig 3C, P<0.01) 97H-OXA was the cell line after induction of MHCC-97H resistance; 97H-OXA-nc is 97H-OXA cell as negative control; 97H-OXA-GP73-sh4 and 97H-OXA-GP73-sh5 were the cell lines with knocked down GP73 expression on the basis of 97H-OXA cells. Hep3B-OXA-nc was the negative control; Hep3B-OXA-GP73-OE was the cell line over-expressed GP73 after induction of OXA resistance of Hep3B cells.
The relationships between GP73 and sCLU
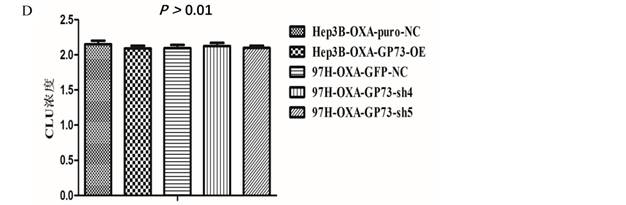
Secretory clusterin (sCLU) is thought to be an inhibitor of apoptosis, which can inhibit the apoptosis of HCC cells by interacting with GRP78 and caspase-9, and it is closely associated with HCC drug resistance to OXA and other chemotherapeutics. We detected sCLU level by Elisa as mentioned in Method section. We found that the manipulation of GP73 (overexpression or knockdown) have no effects on sCLU in both MHCC-97H and Hep3B cell lines. There was no significant difference in the concentration of sCLU secreted by different types of OXA resistant HCC cell lines (Fig 4D).
Discussion
GP73 is a transmembrane glycoprotein with a molecular weight of 73 kDa. GP73 is high expressed in a variety of tumors, such as in liver cancer, lung adenocarcinoma, prostate cancer and seminoma [12-15]. It is worth noting that in serum and liver tissue of patients with HCC, both virus infected and non-viral caused (alcoholic liver disease and autoimmune hepatitis), GP73 levels were significantly increased [6]. GP73 is considered as a valuable diagnostic marker of liver cancer in recent years. It has been shown that soluble GP73 in serum is derived from the Golgi apparatus. The amino terminus is a hydrophobic end, mainly located in the Golgi apparatus, constituting a transmembrane region and a signal peptide cleavage site. However, the carboxyl terminus is located outside the Golgi apparatus and consists of a continuous sequence of fourteen alkanoyl groups, five glycosylation sites, an acidic domain and a coiled-coil structure. These unique structures are the basis for GP73 to participate in the pathophysiology of intracellular signaling, phosphorylation and dephosphorylation of proteins, and to give full scenario to its biological function [16, 17]. At present, it is known that the carboxyl terminal of G73 is the main functional domain since the truncation of carboxyl terminus of GP73 shortened the survival time of the transgenetic mice and severely damaged the development of the kidney and liver system and the epithelial function [18]. GP73 participates in many physiological processes, such as immune regulation, cell adhesion and cell apoptosis [19]. Therefore, we speculate that the high expression of GP73 has important biological functions in the development and chemotherapeutic resistance of HCC.
GP73 and the apoptosis activity. GP73 and the apoptosis of MHCC-97H cell (Figure 4A, upper) and Hep3B cell (Figure 4A, lower). NC was the MHCC-97H cell as negative control; SH4 (97H-OXA-GP73-sh4) and SH5 (97H-OXA-GP73-sh5) were the cell lines with knocked down GP73 expression on the basis of MHCC-97H; NC (Hep3B-NC) was the Hep3B cell as negative control; OE (Hep3B-GP73-OE) was the cell line with over-expressed GP73. GP73 and the apoptosis of MHCC-97H OXA-resistance cell (Figure 4B) Hep3B OXA-resistance cell (Figure 4C) after treatment with OXA. MHCC-97H-NC was the MHCC-97H cell as negative control; MHCC-97H-OXA was OXA resistant; 97H-OXA-GP73-sh4 and 97H-OXA-GP73-sh5 were the cell lines with knocked down GP73 expression on the basis of MHCC-97H-OXA; Hep3B-NC was the Hep3B cell as negative control; Hep3B-OXA was the Hep3B cells line induced with OXA resistance; Hep3B-OXA-GP73-OE was the cell line with over-expressed GP73 after induction of OXA resistance of Hep3B cells. The expression of GP73 and the level of sCLU in OXA resistant HCC cell lines (Figure 4D). Hep3B-OXA was the Hep3B cells line induced by OXA resistance; Hep3B-OXA-puro-NC was the Hep3B-OXA cell as negative control; Hep3B-OXA-GP73-OE was the cell line with over expressed GP73 after induction of OXA resistance of Hep3B cells; 97H-OXA-GP73-NC was the MHCC-97H cell after induced OXA resistance; 97H-OXA-GP73-sh4 and 97H-OXA-GP73-sh5 were the cell lines with knocked down GP73 expressions after OXA resistance induction of MHCC-97H cell line.
In this study, we investigated the effects of GP73 on the proliferation, apoptosis, and resistance to OXA in hepatic carcinoma cells from vitro (cell culture). The results of the following two aspects revealed that the expression level of GP73 directly affects the proliferation, apoptosis and drug resistance of HCC cells: (1) The expression level of GP73 changed the proliferation and apoptosis of HCC cells; (2) The expression level of GP73 changed the IC50, proliferation activity and apoptosis of OXA resistant HCC cells, affecting the sensitivity of HCC cells to OXA.
Zhang et al reported that GP73 silence not only inhibited the proliferation of hepatocellular carcinoma cells, but also induced apoptosis [20]. Our study also showed that knock down of GP73 in MHCC-97H cells and over-expression of GP73 in Hep3B cells significantly changed the proliferation and apoptosis of HCC cells. OXA inhibits the proliferation and promote apoptosis of hepatic carcinoma cells [21, 22]. One of the main mechanisms of tumor cell resistance to chemotherapy is cancer cells can resist and escape chemotherapy-induced apoptosis [23]. We successfully constructed the OXA-resistant hepatic carcinoma cells in vitro and found that the expression level of GP73 in MHCC-97H resistant cell line was significantly higher than that in non-resistant cell line, suggesting that GP73 expression level correlated with drug resistance. Knock down of GP73 in MHCC-97H drug-resistance cells decreased its IC50 significantly. GP73 overexpression in Hep3B cells showed opposite phenomenon. In addition, the proliferation and apoptosis of HCC cell lines treated with different concentration of OXA and manipulation of GP73 were detected. The results showed that GP73 positively correlated with proliferative activity in the cell lines and negatively correlated with the apoptosis, indicating that GP73 directly affects the drug sensitivity of OXA. sCLU is considered to be an inhibitor of apoptosis by interacting with GRP78, caspase-9 and other factors [24, 25]. sCLU increase the resistance of HCC cells to chemotherapy by interacting with AKT and Bcl-2 / Bax pathway [25]. In pancreatic cancer, prostate cancer and other tumors, also observed increased sCLU expression, and increased resistance of tumor cells to chemotherapeutic drugs, while blocking the expression of sCLU protein increased the sensitivity of chemotherapy [26, 27]. To analyze the relationship between GP73 and sCLU, we detected the expression of sCLU in OXA resistant HCC cell lines with different GP73 expression levels. We failed to observe any significant change of sCLU levels between groups, suggesting that GP73 might regulate apoptosis in a sCLU independent pathway. However, this study only analyzed the expression level of sCLU protein, the interaction between sCLU and GP73 as well as the functional changes of sCLU needs further studies.
In conclusion, we demonstrated that GP73 is involved in the regulation of cellular proliferation, apoptosis and OXA resistance in HCC cells in vitro. GP73 can be a potential therapeutic target.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Grant No. 81660498 and Grant No. 81360347), Guangxi Natural Science Foundation (Grant No.2016GXNSFBA380090 and 2015GXNSFAA139128), China Postdoctoral Science Foundation, the 60th grant funding of general program for the post - doctoral funding program in the western region (Grant No. 2016M602919XB), Self-raised Scientific Research Funds of Health of Guangxi Zhuang Autonomous Region (Grant No. Z 2015605, Z 2015717 and Z 2016480) and Youth scientific funding of Guangxi medical university (Grant No.GXMUYSF201520).
Authors' contributions
Jia-Zhou Ye analyzed the data and wrote the manuscript; Rong Liang and Yan Lin designed the study and revised the manuscript; Chun-ling Yuan, Jin-yan Zhang, Zhi-Hui Liu and Yong-Qiang Li performed all the experiments and collected all the data; Shu-mei Yan, Hui-ni Wu and Xiao-Ling Luo analyzed the data.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mcglynn Katherine A. and London W. Thomas The Global Epidemiology of Hepatocellular Carcinoma, Present and Future[J]. Clinics in liver disease. 2011;15(2):223-x
2. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer. 2015;136(5E):359-86
3. Benzina S, Altmeyer A, Malek F. et al. High-LET radiation combined with OXA induce autophagy in U-87 glioblastoma cells[J]. Cancer Lett. 2008;264(1):63-70
4. Qin S, Cheng Y, Liang J. et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study[J]. Oncologist. 2014;19(11):1169-78
5. Kladney R. D, Bulla G. A, Guo L. et al. GP73, a novel Golgi-localized protein upregulated by viral infection[J]. Gene. 2000;249(1-2):53-65
6. Kladney R. D, Cui X, Bulla G. A. et al. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease[J]. Hepatology. 2002;35(6):1431-40
7. Marrero J. A, Romano P. R, Nikolaeva O. et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma[J]. J Hepatol. 2005;43(6):1007-12
8. Mao Y, Yang H, Xu H. et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma[J]. Gut. 2010;59(12):1687-93
9. Hann H. W, Wang M, Hafner J. et al. Analysis of GP73 in patients with HCC as a function of anti-cancer treatment[J]. Cancer Biomark. 2010;7(6):269-73
10. Sun Y, Yang H, Mao Y. et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival[J]. J Gastroenterol Hepatol. 2011;26(7):1207-12
11. Katada Takeyasu, Ishiguro Hideyuki, Kimura Masahiro. et al. GP73 contributes to the sensitivity of cisplatin in esophageal cancer[J]. Esophagus. 2009;6(3):173-176
12. Zhang Fangfang, Gu Yanli, Li Xiang. et al. Up-regulated Golgi phosphoprotein 2 (GOLPH2) expression in lung adenocarcinoma tissue[J]. Clinical biochemistry. 2010;43(12):983-991
13. Kristiansen G, Fritzsche F. R, Wassermann K. et al. GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: implications for tissue-based diagnostics[J]. Br J Cancer. 2008;99(6):939-48
14. Fritzsche F. R, Kristiansen G, Riener M. O. et al. GOLPH2 expression may serve as diagnostic marker in seminomas[J]. BMC Urol. 2010;10:4
15. Waidely E, Al-Yuobi A. R, Bashammakh A. S. et al. Serum protein biomarkers relevant to hepatocellular carcinoma and their detection[J]. Analyst. 2016;141(1):36-44
16. Kim Ha-Jeong, Lv Dandan, Zhang Yan. et al. Golgi phosphoprotein 2 in physiology and in diseases[J]. Cell & Bioscience. 2012;2:31-31
17. Puri Sapna, Bachert Collin, Fimmel Claus J. et al. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption[J]. Traffic. 2002;3(9):641-653
18. Wright Lorinda Marie, Yong Sheri, Picken Maria Mrozowicz. et al. Decreased Survival and Hepato-Renal Pathology in Mice with C-Terminally Truncated GP73 (GOLPH2)[J]. International Journal of Clinical and Experimental Pathology. 2009;2(1):34-47
19. Zhou Yan, Li Leike, Hu Longbo. et al. Golgi phosphoprotein 2 (GOLPH2/GP73/GOLM1) interacts with secretory clusterin[J]. Molecular Biology Reports. 2011;38(3):1457-1462
20. Zhang Yu-Long, Zhang You-Cheng, Han Wei. et al. Effect of GP73 silencing on proliferation and apoptosis in hepatocellular cancer[J]. World Journal of Gastroenterology: WJG. 2014;20(32):11287-11296
21. Qu Kai, Xu Xinsen, Liu Chang. et al. Negative regulation of transcription factor FoxM1 by p53 enhances OXA-induced senescence in hepatocellular carcinoma[J]. Cancer letters. 2013;331(1):105-114
22. Lim Sung-Chul, Choi Jeong Eun, Kang Ho Sung. et al. Ursodeoxycholic acid switches OXA-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma[J]. International Journal of Cancer. 2010;126(7):1582-1595
23. Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer[J]. Multi-drug resistance in cancer. 2010:47-76
24. Wang C, Jiang K, Gao D. et al. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78[J]. PLoS One. 2013;8(2E):55981
25. Xiu P, Dong X, Dong X. et al. Secretory clusterin contributes to OXA resistance by activating Akt pathway in hepatocellular carcinoma[J]. Cancer Sci. 2013;104(3):375-82
26. Jin J, Kim J. M, Hur Y. S. et al. Clinical significance of clusterin expression in pancreatic adenocarcinoma[J]. World J Surg Oncol. 2012;10:146
27. Mukherji Deborah, Eichholz Andrew, De Bono Johann S. Management of Metastatic Castration-Resistant Prostate Cancer[J]. Drugs. 2012;72(8):1011-1028
Author contact
![]() Corresponding authors: Rong Liang, Department of First Chemotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China; Email: article811com; Phone Number: +086-771-5335155; Fax number: +086-7715335155 and Yan Lin, Department of First Chemotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China; Email: linyanmgxcom; Phone Number: +086-771-5335155; Fax number: +086-7715335155
Corresponding authors: Rong Liang, Department of First Chemotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China; Email: article811com; Phone Number: +086-771-5335155; Fax number: +086-7715335155 and Yan Lin, Department of First Chemotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, P. R. China; Email: linyanmgxcom; Phone Number: +086-771-5335155; Fax number: +086-7715335155

 Global reach, higher impact
Global reach, higher impact