Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(3):479-487. doi:10.7150/jca.22157 This issue Cite
Research Paper
Clinicopathological Characteristics of the primary and metastatic Hepatic Neuroendocrine Tumors and the relevant Prognosis-Related Factors: A Retrospective Study of 81 Cases in a Single Chinese Center
1. Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China;
2. Department of Hepatic Surgery, Zhongshan Hospital, Fudan University, Shanghai, China;
3. Department of General Surgery, Nantong Second People's Hospital, Nantong, Jiangsu, China;
4. Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China.
* Equal contributors.
Received 2017-7-30; Accepted 2017-10-6; Published 2018-1-1
Abstract
Aims: We aim to describe the clinicopathological characteristics of hepatic neuroendocrine tumors (HNETs) and evaluate the relevant prognosis-related factors.
Methods: The clinical data of 81 consecutive patients with primary or metastatic HNETs from March 2000 to July 2014 were retrospectively analyzed.
Results: The mean (SD) age was 59.68 (11.64) years, 69.15% were men. The percentages of Grade G1, G2 and G3 tumors were 4.94%, 25.93% and 69.13%, respectively. Thirty-five cases were primary HNETs. Primary HNETs were more common in patients with larger tumors, lymph nodes invasions, tumor necrosis and portal vein tumor thrombus. The 1-, 3-, and 5-year overall survival rate were 88.89%, 32.10%, and 8.64%, separately. The relapse rate was 81.48% (66/81) and the mean (SD) relapse time was 18.79 (10.99) months. Reduced survival rate was associated with lymph node metastases (P=0.034), tumor necrosis (P=0.048), hard texture of tumor character (P=0.001), multifocality of tumor numbers (P=0.043), and the immunohistochemical expression of NSE (P=0.000) and Syn (P=0.037). Patients with metastatic HNETs were demonstrated with a more decreased period of Progression-free Survival (PFS) and Overall survival (OS) than their primary HNETs counterparts (P<0.05).
Conclusion: Primary HNETs cohort patients were more common with aggressive clinical presentation. The hard texture of tumor character, multifocality of tumor numbers, and the immunohistochemical expression of NSE and Syn were independent predictive factors. Patients who were pathologically diagnosed as the primary HNETs seemed to achieve a long-term survival.
Keywords: hepatic neuroendocrine tumors, clinicopathological characteristics, prognosis.
Introduction
Neuroendocrine tumors (NETs), once known as carcinoid tumors[1], signify a heterogeneous group of neoplasms that originate from various endocrine systems, including digestive system, respiratory system and urinary system. NETs arise from the digestive system account for the highest rates of the occurrence in NETs[2]. These tumors secret special peptides and present a significant challenge because of its peculiarity of occult malignancy. Of the NETs in digestive tumors, gastrointestinal tract and pancreas are the common primary locations[3,4], neoplasms in liver were often recognized as the metastatic lesion. It is reported that 80% of the hepatic NETs (HNETs) were metastatic with the simultaneous tumor masses in primary endocrine site[5]. However, the special oncological behaviors as well as the unclear molecular mechanisms of the NETs sometimes make the NETs patients appear hepatic tumor masses only, which often makes the clinical treatment process confused. Two possibilities of the concrete mechanisms can be explained about the hepatic masses: primary HNETs (PHNETs) or metastatic NETs occurred in the liver with no origin; and the final diagnosis were currently often based on the pathological examination of the specimen. Whichever the result is, a great issue is to summarize the clinicopathological characteristics about this rarely occurred disease to contribute to the diagnosis and treatment process.
The incidence rate of NETs has increased dramatically during the past decade according to the recent report of The Surveillance, Epidemiology, and End Results program and National Cancer Registry of Spain[1,3,6], which partly reflected the advanced endoscopic and radiological imaging techniques; and the enhanced clinical awareness and modified diagnostic techniques have resulted in the substantial improvement in the treatment and diagnosis on in the field of NETs. Besides, there is literature available on the clinical characteristics and related-factors predictive of outcome for NETs[7]. However, because of the rarity of HNETs, there are few data available on epidemiology and survival of HNETs in Chinese patients. In this study, we report our data on the clinicopathological features, survival, and prognosis-related factors of primary and secondary HNETs in a single Chinese center.
Methods and Materials
A total of 81 consecutive Chinese patients with Hepatic neuroendocrine tumors (HNETs) (including the primary HNETs cohort and the metastatic HNETs with no origin cohort) who were treated in Zhongshan hospital, Fudan University from March 2000 to July 2014 were included. In our study, the final diagnosis was based on pathological morphology and immunohistochemical assessment through surgical specimen, intraoperative biopsy and needle biopsy by experienced pathologists according to the site of origin and criteria of the World Health Organization (WHO) and graded according to the latest European Neuroendocrine Tumor Society (ENETS) proposal for grading and staging of NETs[8]. Subsequently, the following pathological features were recorded as being present or absent in the tumors: tumorous number, tumor necrosis and character, lymph node invasion, neural invasion and extra organ invasion (metastasis). Furthermore, the Ki67 index (regarded as the percentage of Ki-67-positive cells in 2000 tumor cells within areas of the highest immunostaining) and the mitotic count (based on counting 50 high-power fields and recorded as the number of mitoses per 10 high-power fields) were also documented from the immunohistochemical examination, which was performed on formalin-fixed, paraffin-embedded tissues (the tissue blocks were sectioned at 4μm). Slides were then stained using the Bond-Max Leica autostainer (Leica Biosystems, United Kingdom). Antibody detection was performed using the biotin-free Bond Polymer Refined Detection System (DS9800; Leica Microsystems, United Kingdom). The antibodies used were as follows: Ki67 index (MIB1 antibody, Dako, Denmark), CgA (Dako, Denmark), Syn (Dako, Denmark), NSE (Dako, Denmark), AFP (Dako, Denmark), SSTR2A and SSTR5 (Abcam, United Kingdom).
The enrolled patients were subjected to multiphasic CT and MRI that acquired less than 5 mm thin slices to evaluate the tumorous number and load. All the image diagnostic reports were all re-assessed by independent double-blinded reviewers. Selected patients underwent potentially curative resection of hepatic tumors with lymph node dissection. Other enrollees were subjected to a tumor biopsy. Selected ones underwent curative resection of masses in liver with lymph node dissection; other enrollees were subjected to a tumor biopsy. Comprehensive treatments were performed for patients with or without hepatic masses including liver-oriented strategies, such as hepatic segmental resection, hepatic Trans-catheter arterial chemoembolization (TACE), or radiofrequency ablation, and systemic therapy, which was octreotide long-acting release (LAR). In our study, different treatment modalities were divided as hepatic locoregional treatment which contains the resection, TACE and RFA; systematic treatment which included chemotherapy, targeted therapies and LAR and combined treatment modality.
The follow-up period was as follows: the first follow-up was performed within 2-3 months after the baseline and the subsequent follow-up cycle are usually range from 3 to 6 months or even shorter which depends on the clinical situations and (or) tumor relapse or metastasis was suspected. The data of overall survival (OS), and relapse/metastasis time were also documented. The duration of overall survival (OS) was calculated from the date of operation until tumor-specific death or the patient's last follow-up. The relapse time was computed from the date of remission to recurrence. The follow-up management was performed through the outpatient clinics and the telephone interview. All the patients were performed the endoscopic and (or) PET/CT examination during the follow-up period. Patients who were discovered the primary lesions after the diagnosis of HNETs were recognized as the metastatic cohort.
The statistical analyses were performed using the SPSS statistical package version 24.0 (SPSS Inc®, Chicago, Illinois, USA). Pearson χ2 test, Fisher exact test, Mann-Whitney U test, Pearson correlation test, and Spearman correlation test were used to evaluate the association between variables when appropriate. Survival was estimated according to the Kaplan-Meier product limit method and life tables method. Survival curves were compared by using the log-rank test. The analysis of risk factors was carried out by univariate and multivariate analyses by the Cox proportional hazards method. Multivariate analyses using the Cox proportional hazards model were performed to identify the factors independently associated with prognosis. Statistical significance was defined as P value less than 0.05.
Results
Common characteristics in 81 HNETs patients
During the study period from March 2000 to July 2014, 81 cases of liver NETs (including 35 cases of primary HNETs (PHNETs) and 46 metastatic HNETs patients with no primary location) were identified through the pathological examination and enrolled. Among these cases, 53 (65.4%) were man and 28 (34.6%) were women. The age range at diagnosis of all the HNETs was 29-85 (59.68±11.64), while that for PHNETs was 29-85 (60.69±13.60) and for metastatic HNETs with no primary site was 38-83 (58.91±9.98) (P=0.501). In our study, all the HNETs were nonfunctional. 53 (65.43%) of the 81 HNETs masses were detected through a regular physical examination, followed by the abdominal pain (22.22%), abdomen mass (4.95%), jaundice (3.70%), fever (2.47%) and weight loss (1.23%). On preoperative period, 67 (82.72%) patients were generally in good condition.
The median size of the hepatic tumor (in the case of multifocality, the largest lesion was recorded) was 6.5 cm (mean [SD] size, 6.84 [4.29] cm; range, 0.4-19 cm). Multifocality were detected in 40 (49.38%) patients. For the rest of the single focal HNETs, the majority of the lesion were located in the II (16.05%), III (14.81%) and IV (8.64%) segment in the liver. All the patients were detected the hepatic mass (or masses) only at the diagnosis. On the further study, our data from both pathological and radiographical examination showed that 71 patients (87.65%) had a localized disease, 10 patients (22.35%) presented with metachronous extra organ metastasis during the follow-up process; Regional lymph node metastases were detected in 14 patients (17.28%). The median number of lymph nodes detected in pathology reports was 3.8 (mean [SD] number, 5.4 [4.4]; range, 1.0-18.0), the median number of metastatic lymph nodes was 0 (mean [SD] number, 0.74 [1.15]; range, 0-6.0), and the positive rate was 24.8%. Regarding the World Health Organization (WHO) tumor grade, the percentages of G1, G2 and G3 tumors were 4.94%, 25.93%, and 69.13%, respectively. 42 (51.85%) tumor tissues were moderately-differentiated, 30 (37.04%) were poorly-differentiated and 9 (11.11%) were well-differentiated. Besides we found that the WHO histological grading was statistically linked with differentiation degree (P=0.032).
Comparison of clinicopathological characteristics between two HNETs groups
Table 1 shows the overall characteristics of the patients with HNETs during the study period. There was no statistically significant difference between PHNETs cohort and HNETs with unknown origin cohort in regard to sex and age. Among the two groups, there was a higher proportion of larger tumor load in the PHNETs patients, with the mean tumor load 211.1±30 cm3 and 76.66±35 cm3, respectively (P=0.036). Moreover, aggressive clinical manifestations, such as lymph node metastasis (P=0.020) and extra organ invasion (P=0.022) at diagnosis, were significantly more frequent in PHNETs group patients. Interestingly, the tumor of 3 patients (3.70%) (1 in PHNETs patient cohort and 2 in another group patients) appeared a soft texture. On the other hand, our data demonstrated that the percentage of Grade G3 NETs in primary group and secondary group were separately 74.29% and 65.23%. Then A strong correlation was observed between the two groups on the presentation of portal vein tumor thrombus, which means that Secondary NETs group were more likely to be with the portal vein tumor thrombus (23.91%), while we also found that 8.57% (3/35) of the PHNETs group appeared a portal vein thrombus (Pearson χ2 test, P =0.001). Finally, the mean ki-67 index of different group were 26.80±20.62 and 22.20±16.25, respectively (P=0.264); regarding to the mitotic count, 6.54±5.10 and 8.54±6.49 were detected in the separate groups (P=0.137).
The immunohistochemical staining were performed or re-performed for all the 81 cases (as is shown in Figure 1). The expression of Syn, NSE, CgA and AFP were recorded in all 81 cases and the positive rates of Syn, NSE, CgA and AFP were separately 64.20%, 26.63%, 46.91% and 9.88%. A comparison of immunohistochemical characteristics between patients with or metastatic HNETs is summarized in Table 2. The positive rates of Syn for PHNETs and metastatic HNETs were 74.29% (26/35) and 56.52% (26/46), respectively (P=0.048). Same differences did not exist between the two cohorts in the cellular expression of AFP, CgA and NSE (P > 0.05). Concurrently, no patients with HNETs had multiple endocrine neoplasia type 1 and von Hippel-Lindau disease.
The overall common characteristics of our enrolled 81 HNETs patients
| Characteristics | Primary HNETs | HNETs with unknown origin | P value |
|---|---|---|---|
| Age at onset, mean (SD) (median), y | 60.69 (13.60) (60) | 58.91 (9.98) (59) | 0.501 |
| Male, n (%) | 26 (74.29%) | 27 (58.7%) | 0.110 |
| Female, n (%) | 9 (25.71%) | 19 (41.3%) | |
| Hepatic tumor location (for single lesion), n (%) | 18 (51.43%) | 23 (50%) | 0.539 |
| III segment | 5 (14.29%) | 8 (17.39%) | |
| IV segment | 2 (5.71%) | 10 (21.74%) | |
| II segment | 4 (11.43%) | 3 (6.52%) | |
| Other segments | 7 (20%) | 2 (4.35%) | |
| Multifocal tumors, n (%) | 17 (48.57%) | 23 (50%) | 0.539 |
| Tumor diameter, mean (median) (SD), cm | 6.73 (6) (4.62) | 6.93 (6.5) (4.08) | 0.840 |
| Hepatic tumor load, mean (median) , cm3 | 211.1 (30) | 76.66 (35) | 0.036 |
| Detection of regional lymph node metastases, n (%) | 10 (28.57%) | 4 (8.70%) | 0.020 |
| Portal vein tumor thrombus, n (%) | 0.001 | ||
| Positive | 32 (91.43%) | 35 (76.09%) | |
| Negative | 3 (8.57%) | 11 (23.91%) | |
| Extra organ invasion at diagnosis, n (%) | 6 (17.14%) | 4 (8.70%) | 0.210 |
| Gall bladder | 1 | 2 | |
| Posterior peritoneum | 2 | 0 | |
| Lung | 2 | 1 | |
| Spleen | 1 | 0 | |
| Pancreas | 0 | 1 | |
| Necrosis, n (%) | 11 (31.43%) | 5 (10.87%) | 0.022 |
| Tumor texture, n (%) | 0.602 | ||
| Soft | 1 (2.94%) | 2 (4.35%) | |
| Hard | 34 (97.06%) | 44 (95.65%) | |
| WHO grade, n (%) | 0.564 | ||
| G1 | 2 (5.71%) | 2 (4.34%) | |
| G2 | 7 (20%) | 14 (30.43%) | |
| G3 | 26 (74.29%) | 30 (65.23%) | |
| Tumor differentiation, n (%) | 0.070 | ||
| Well-differentiated | 5 (14.29%) | 4 (8.70%) | |
| Moderately-differentiated | 21 (60%) | 21 (45.65%) | |
| Poorly-differentiated | 9 (25.71%) | 21 (45.65%) | |
| Ki-67 index, mean (SD) (median) | 26.80 (20.62) (21) | 22.20 (16.25) (20.50) | 0.264 |
| Mitotic count, mean (SD) (median) | 6.54 (5.10) (5) | 8.54 (6.49) (8) | 0.137 |
Treatment interventions
Of the 81 patients, 62 (76.54%) patients received a curative surgery of the primary tumor, of which 2 (2.47%) received the liver transplantation. 38 (46.91%) patients received a segmental hepatectomy and the other 22 (27.16%) patients received a hepalobectomy. On the other hand, a fine needle aspiration (FNA) biopsy was performed in 19 patients (23.46%).
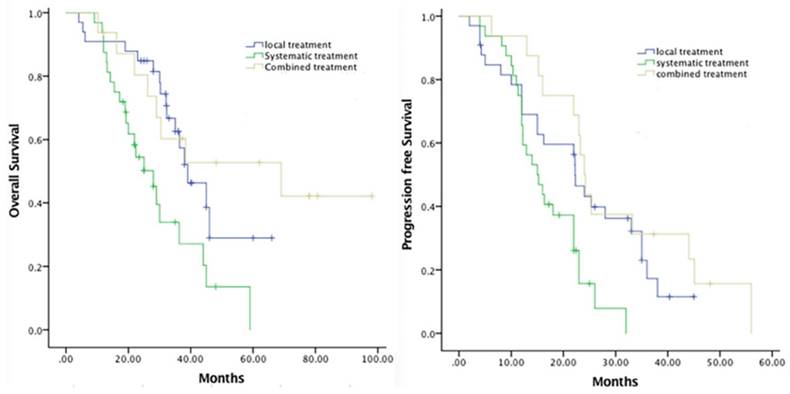
Comprehensive treatments were performed for selected patients with or without extra organ metastasis included liver-oriented strategies, such as TACE, or RFA, and systemic therapy, which was LAR and experienced chemotherapy. 13 (16.05%) patients received a TACE with or without exploratory laparotomy as a first treatment intervention. The following chemotherapeutic agents were selectively used for patients who had an arterial infusion chemotherapy: 5-fluorouracil, mitomycin C, doxorubicin, oxaliplatin, and gemcitabine. Radiofrequency ablation were performed in 24 (29.63%) patients. Nonliver-directed strategy referred to octreotide (Sandostatin LAR) injection (14.81%) and systematic chemotherapy (27.16%). The clinical correlation between the various treatment strategies and survival was also analyzed in this study, Figure 2 demonstrated the Kaplan-Meier survival curve for the duration of PFS and OS. In regard to the OS, the mean duration of local, systematic and combined treatment was separately 41.92, 29.57 and 60.48 months (p=0.03), in contrast, the mean duration of PFS was 23.26, 16.83 and 29.80 months (p=0.05), respectively.
The Positive expression of immunochemical examination in Primary and Secondary HNETs
| Primary HNETs | HNETs with unknown origin | P value | |
|---|---|---|---|
| Pathological staining | |||
| Syn | 0.048 | ||
| Negative | 10 | 18 | |
| Positive, n (+) (++) (+++) | 26 (12) (8) (4) | 26 (21) (6) (1) | |
| NSE | 0.837 | ||
| Negative | 25 | 32 | |
| Positive, (+) (++) (+++) | 10 (6) (3) (1) | 14 (13) (1) (0) | |
| CgA | 0.511 | ||
| Negative | 18 | 25 | |
| Positive, (+) (++) (+++) | 17 (6) (7) (4) | 21 (9) (10) (2) | |
| AFP | 0.085 | ||
| Negative | 29 | 44 | |
| Positive, (+) (++) (+++) | 6 (4) (1) (0) | 2 (2) (0) |
Survival analysis and related prognostic factors
The duration of follow-up was computed from the date of treatment intervention to the date of relapse, death, or last follow-up. On the last follow-up period, 45 (55.56%) patients died of tumor recurrence or distant metastasis. The mean follow-up time was 48.33 months. The median survival time for all 81 patients was 29.0 months (mean survival time, 32.23 months; Standard deviation, 18.19; Range: 4.2 to 98.2 months). The 1-, 2-, and 5-year accumulative OS rates were 88.89%, 32.10%, and 8.64%, respectively. Sixty-six (81.48%) patients had a tumor relapse or distant metastasis after the treatment interventions, of which the mean (SD) relapse time was 18.79 (10.99) months and the estimated median time of relapse was 16 months with the range from 2 to 56 months.
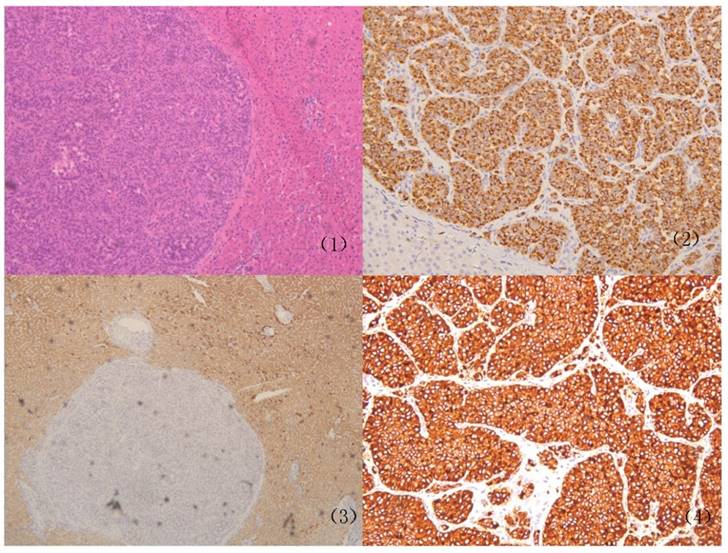
Representative case of Primary hepatic neuroendocrine tumors (PHNETs). Histopathological features of the PHNETs. (1) The hematoxylin-eosin staining of the PHNETs (×10); (2) the positive expression of chromogranin A in PHNETs (×20); (3) the positive expression of Hepa- in PHNETs (×20); (4) the positive expression of Syn in PHNETs (×20).
The comparison of PFS and OS between different treatment strategies.
Besides, the different 2010 WHO classification on NETs also indicate varied prognosis. Our data demonstrated that the median survival time for G1, G2 and G3 were separately 40.82 months, 51.87 months and 33.80 months and there was statistical significance on the OS between HNETs patients and HNEC patients (P=0.011); considering G1 and G2 patients, the difference in survival between the patients was not statistically significant (P>0.05). The 1-year survival rates for G1, G2 and G3 were 100%, 100% and 85.71%, respectively. Moreover, regarding to the varied differentiated degree, the mean OS for well-, moderately- and poorly-differentiated HNETs patients were 45.82 months, 43.39 months and 36.76 months (P=0.026).
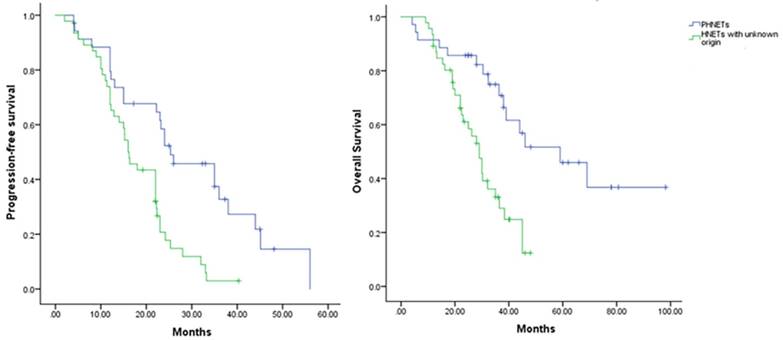
In the univariate analysis, survival rates in patients with regional lymph node metastases (P=0.034) were significantly lower than those in patients without; meanwhile, same differences were not found in sex (P=0.224), portal vein tumor thrombus (P=0.313). Moreover, a multivariate survival analysis was performed using the Cox proportional hazards model. It demonstrated that tumor character (P=0.001; 95% CI, 1.827-9.137), tumor numbers (P=0.043; 95% CI, 0.275-0.979), and the immunohistochemical expression of NSE (P=0.000; 95% CI, 1.691-5.763) and Syn (P=0.037; 95% CI, 0.375-0.985) were independent prognostic factors for survival. Finally, regarding to the prognosis in different group of HNETs patients, a survival curve was made to compared Progression-Free survival (PFS) and OS in two cohorts (Figure 3). From our study, Patients who were pathologically diagnosed as the PHNETs showed a more prolonged PFS (P=0.003, mean [SD] 24.92 months [13.94]) and OS (P=0.000, mean [SD] 40.39 months [22.59]) than their other counterparts patients (mean [SD] PFS: 17.31 months [8.04]; mean [SD] OS: 26.02 months [10.57]).
The comparison of PFS and OS between different HNETs groups from the initial diagnosis. HNETs, Hepatic neuroendocrine tumors; OS, overall survival; PFS, progression-free survival.
Univariate and Multivariate Analysis of the relevant Prognostic Factors associated with OS (n = 81) in Patients with HNETs
| Univariate analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P |
| OS | ||||
| Common characteristics | ||||
| Age(≥65 y/ < 65 y) | 0.995(0.966-1.025) | 0.746 | ||
| Sex (male/female) | 1.122(0.560-2.661) | 0.224 | ||
| Diameter (≥30 mm/ < 30 mm) | 1.046(0.964-1.136) | 0.278 | ||
| Tumor character (soft/hard) | 0.072(0.013-0.415) | 0.003 | 3.134(1.827-9.137) | 0.001 |
| Histologic grade (G3/G2,G1) | 0.449(0.144-1.402) | 0.168 | ||
| Lymphatic invasion (yes/no) | 0.100(0.015-0.698) | 0.034 | ||
| Multifocal tumor(yes/no) | 2.884(1.131-7.354) | 0.027 | 0.466(0.275-0.979) | 0.043 |
| Tumor differentiation (well/ median, poor) | 0.686(0.235-2.003) | 0.491 | ||
| Ki67 index (≥20 / <20) | 1.035(0.943-1.137) | 0.250 | ||
| Immunochemical expression | ||||
| Syn | 0.867(0.110-6.865) | 0.038 | 0.584(0.375-0.985) | 0.037 |
| NSE | 1.233(0.097-15.66) | 0.872 | 2.354(1.691-5.763) | 0.000 |
| CgA | 3.304(0.546-19.99) | 0.193 | ||
| AFP | 6.183(0.18-212.97) | 0.503 | ||
Discussion
NETs are a heterogeneous group of tumors with potentially malignant behavior. During the past decades, the concept of NETs has been substantially explored and some of the achievements have been applied for the clinical practice. Of the whole spectrum of the NETs, most of the current research has been performed on the clinical and basic science research of the Gastroenteropancreatic (GEP) NETs, while primary hepatic NETs (PHNETs) are occasionally reported to be considered to originate from neuroendocrine cells either in the intrahepatic biliary epithelium or in the intestinal metaplasia from the bile duct epithelium caused by chronic inflammation[9] and account for the very rare rates of the occurrence in NETs.
The literature point to the trend showing nonfunctional tumors presenting as a distinct majority among HNETs[10] and our study demonstrated in accordance with the previous reports with the nonfunctional tumors of the PHNETs counting to 100% (35/35). The high rate of nonfunctional rate of the PHNETs and indicated that the majority of the diagnosis were incidentally made, which was also in accordance with our demonstration that 22 of 35 PHNETs patients were diagnosed with no chief complaints (62.86%). Besides, Modlin[5] has reported that approximately 80% of the hepatic NETs were the metastatic lesion, in our study, 46 of the 81 patients were pathologically diagnosed as the metastatic HNETs (56.79%), which is not sustainable to the previous report. On clinicopathological characteristics of the 81 patients, the multifocality rates of the two cohorts were respectively 48.57% and 50%, which noted the significance of the intraoperative carefulness for the search of the multifocality through the thorough exposure or the intraoperative ultrasound. Notably, the tumor load was greater at the diagnosis in PHNETs cohorts compared with the those in the other group (P=0.036), meanwhile, for the longest tumor diameter in single lesion, there was no significance between the two groups (P=0.840), which demonstrated that the tumor size of sporadic HNETs were not an indicator showing the different tendency between primary and secondary groups; interestingly, on the occurrence rate of the aggressive clinical presentations, primary HNETs patients were more prone to have a regional lymph node invasion (P=0.020), portal vein tumor thrombus (P=0.001) and tumor necrosis (P=0.022) than metastatic HNETs group. The reason why primary HNETs were more aggressive may be that the expansibility of the invasion pattern and extensive distribution of bile duct epithelium make the PHNETs more apparent to appear the infiltration to circumferential tissue, whereas the metastatic HNETs were formed in a special molecular method with the particular growth pattern.
In our study, the majority of the 81 cases were pathologically proven to be the G3 patients (69.14%, 56/81) according to the WHO classification and no differences were found between the 2 groups (P=0.564), which indicated that the NETs of the liver, whatever its primary site is, are highly malignant as previously reported[11] and need a closer monitor and active management. By chance, we also found that WHO histological grading was statistically correlated with differentiation degree in HNETs patients (P=0.032). Admittedly, our survival analysis has showed that the varied differentiation grade was a prognosis-related factors for HNETs (P=0.026), while the different WHO grade cannot achieve that desired prognosis-predicting effects. There was no difference between the G1 and G2 HNETs patients (P>0.05). Our study also demonstrated in the univariate analysis that the regional lymph node metastases (P=0.034) remained the prognosis-related factors, which underscores the need for enhanced systemic therapies in those at advanced stage. On multivariate survival analysis, we found that tumor character (P=0.001), tumor numbers (P=0.043) were the independent factors for survival, which indicated that for the HNETs patients with multiplicity of the tumor numbers and the hard texture of the tumor character, a long-term and close follow-up is required. Besides, there was statistically difference for both the PFS and OS between the two groups (P<0.05). Thus the clinicopathological identification of PHNETs is in pressing need when the Hepatic NETs appeared with no other lesions in the body.
Currently, the diagnosis of PHNETs was made mainly from the pathological examination. When NETs masses were detected in the liver, we defined as PHNETs those additional tumors that met all of the following conditions: 1) located within the hepatic parenchyma and separated from the other organs; 2) a histological appearance in accordance to the characteristics of the NETs; 3) with no primary lesions found during a long-term follow-up through the examination of PET/CT and(or) endoscopy; and 4) unaccompanied by other lesions of hepatic tumor, such as HCC and cystoma. Backing up to the above viewpoints, our study suggests that the differences between primary and metastatic hepatic NETs could be identified through thorough pathological examinations from the result of immunohistochemistry and gene analysis. Both the primary and metastatic HNETs were in accordance with the histological features of the expression of the NETs and the expression of CgA, NSE, Syn were proven to be positive through the immunohistochemical staining in the HNETs[12,13]. Specially, recent studies have shown that the positive expression of CgA and Syn has proven to be more effective on the pathological diagnosis of the HNETs[9,14]. In our study, the positive rates of Syn, NSE, CgA and AFP were separately 64.19%, 29.63%, 46.91% and 9.88% and there was no difference between the groups. It is reported[15,16] that the majority of the PHNETs occurred in the right lobe of the liver and the metastatic HNETs were proven to show a clinical tendency of the presentation of multifocality. We found that the III segment of the liver was the mostly-occurred site for the HNETs and there was no difference for the rate of multifocality between the groups (P=0.539).
Efforts were continuously made to identified the HNETs, Ankur[16] has reported the imaging features from Ga-68 PET/CT in 68 metastatic HNETs patients and indicated that Ga-68 PET/CT is a promising imaging modality in clinical practice for identifying the primary site of the HNETs, while some other scholars have analyzed 38 cases of PHNET with the dynamic contrast-enhanced CT findings, indicating that of which 74% of the lesions in the arterial phase were enhanced, 52% were found with delayed enhancement and 48% showed hepatocellular carcinoma-like enhancement[17]. Besides, Xiaoqi[18] suggested that the CDX-2 and TTF-1 expression in the specimen is highly specific in identifying the origin of NETs.
On the other hand, reports have also demonstrated a favorable prognosis on PHNETs at 5 years in 74% of surgically treated cases with an 18% recurrence rate[13]. The prognosis of our cases was not that desirable with the 5-years OS rate counting to only 8.64%. However, it is still appropriate on the basis of our data to assume that a potentially radical resection correlates with a relatively long-term cure, with evidence that a 3-year OS of 24.68% was observed. The treatment role of surgical debulking in asymptomatic patients with the multifocality of the liver lesions and in patients where an R0/R1 resection cannot be achieved remain therapeutic dilemmas[19,20]. Besides, some nonliver-directed treatment strategies such as the octreotide injection and systematic chemotherapy have also achieved a certain effect in dealing with the invasion of NETs[21]. Wang[22] evaluated the effectiveness of the LAR for the treatment of advanced GEP-NETs disease and indicated that octreotide LAR was safe and effective in the treatment of patients with well-differentiated advanced GEP-NETs. In our study, the curative effect of LAR and chemotherapy in both PHNETs and metastatic HNETs patients were not specifically evaluated in 81 cases mainly owing to the delayed publication of the above treatment method which makes some of the enrolled patients no objective conditions for accepting LAR or other therapeutic methods, however, the clinical correlation between treatment modalities and survival were explored, we found that, in respect to OS, there was a statistical significance between local/combined treatment modalities and systematic treatment, which indicated that patients who underwent local treatment modality can achieve a better prognosis. Our findings were rightly consistent with the recent analysis[23].
HNETs, whatever the primary site is, are a rare form of hepatic tumors presenting with real hard clinical challenges. In this paper, we present the clinicopathological characteristics of 81 patients and evaluate the prognosis-related factors in a respective study. However, there are still some possible limitations in our study, the first and foremost limited factor is related to the deviation of the treatment method and the follow-up period in varied patients; the second factor is the absence of the molecular basis of this disease and the mechanisms involved in response and resistance to therapy, which will be practical tools to help us develop early diagnosis tools and newer more rationally designed treatment strategies that will potentially change the natural history of malignant HNETs. Thus more detailed research of the clinical characteristics of HNETs are still required in the future.
In summary, we conclude on the basis of our data that primary HNETs patients were more common with lymph node metastasis, portal vein tumor thrombus and larger tumor load and often achieved a long-term PFS and OS. Furthermore, the hard texture of tumor character, multifocality of tumor numbers, and the immunohistochemical expression of NSE and Syn were independent prognosis-related factors.
Acknowledgements
This study was funded by the Special Research Fund for Public Welfare from the Ministry of Health of China (201202007). We thanked all the doctors and nurses during the diagnosis, treatment and the detection process. The authors have neither financial nor non-financial competing interests to declare in relation to this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Delle Fave G, Kwekkeboom DJ, Van Cutsem E. et al. Enets consensus guidelines for the management of patients with gastroduodenal neoplasms [J]. Neuroendocrinology. 2012;95(2):74-87
2. Pavel M, Baudin E, Couvelard A. et al. Enets consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary [J]. Neuroendocrinology. 2012;95(2):157-176
3. Garcia-Carbonero R, Capdevila J, Crespo-Herrero G. et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (gep-nets): Results from the national cancer registry of spain (rgetne) [J]. Ann Oncol. 2010;21(9):1794-1803
4. Lepage C, Bouvier AM, Phelip JM. et al. Incidence and management of malignant digestive endocrine tumours in a well defined french population [J]. Gut. 2004;53(4):549-553
5. Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors [J]. Cancer. 1997;79(4):813-829
6. Modlin IM, Oberg K, Chung DC. et al. Gastroenteropancreatic neuroendocrine tumours [J]. Lancet Oncol. 2008;9(1):61-72
7. Ito T, Tanaka M, Sasano H. et al. Preliminary results of a japanese nationwide survey of neuroendocrine gastrointestinal tumors [J]. J Gastroenterol. 2007;42(6):497-500
8. Rindi G, Kloppel G, Couvelard A. et al. Tnm staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system [J]. Virchows Arch. 2007;451(4):757-762
9. Iimuro Y, Deguchi Y, Ueda Y. et al. Primary hepatic carcinoid tumor with metachronous lymph node metastasis after long-term follow up [J]. J Gastroenterol Hepatol. 2002;17(10):1119-1124
10. Pavel M, O'Toole D, Costa F. et al. Enets consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (nen) and nen of unknown primary site [J]. Neuroendocrinology. 2016;103(2):172-185
11. Alekseev D, Goralczyk A, Lorf T. et al. Ten years survival with excellent outcome after living donor liver transplantation from 70 years old donor for primary hepatic neuroendocrine carcinoma: Case report [J]. Int J Surg Case Rep. 2012;3(1):34-36
12. El Ali Z, Iwanik K, Sowinski J. et al. A jejunal stromal tumour in a patient with metastatic neuroendocrine cancer of unknown origin; a rare coexistence, diagnostic and therapeutic challenge [J]. Endokrynol Pol. 2009;60(3):216-220
13. Lin CW, Lai CH, Hsu CC. et al. Primary hepatic carcinoid tumor: A case report and review of the literature [J]. Cases J. 2009;2(1):90
14. Shetty PK, Baliga SV, Balaiah K. et al. Primary hepatic neuroendocrine tumor: An unusual cystic presentation [J]. Indian J Pathol Microbiol. 2010;53(4):760-762
15. Jia C, Zhang Y, Xu J. et al. Experience in primary hepatic neuroendocrine tumor [J]. Turk J Gastroenterol. 2012;23(5):546-551
16. Pruthi A, Pankaj P, Verma R. et al. Ga-68 dotanoc pet/ct imaging in detection of primary site in patients with metastatic neuroendocrine tumours of unknown origin and its impact on clinical decision making: Experience from a tertiary care centre in india [J]. J Gastrointest Oncol. 2016;7(3):449-461
17. Kim JE, Lee WJ, Kim SH. et al. Three-phase helical computed tomographic findings of hepatic neuroendocrine tumors: Pathologic correlation with revised who classification [J]. J Comput Assist Tomogr. 2011;35(6):697-702
18. Lin X, Saad RS, Luckasevic TM. et al. Diagnostic value of cdx-2 and ttf-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin [J]. Appl Immunohistochem Mol Morphol. 2007;15(4):407-414
19. Geramizadeh B, Kashkooe A, Malekhosseini SA. Liver metastasis of gastrointestinal neuroendocrine tumors: A single center experience [J]. Hepat Mon. 2016;16(5):e37293
20. Karavias DD, Tepetes K, Karatzas T. et al. Liver resection for metastatic non-colorectal non-neuroendocrine hepatic neoplasms [J]. Eur J Surg Oncol. 2002;28(2):135-139
21. Massironi S, Conte D, Sciola V. et al. Contrast-enhanced ultrasonography in evaluating hepatic metastases from neuroendocrine tumours [J]. Dig Liver Dis. 2010;42(9):635-641
22. Wang YH, Wang W, Jin KZ. et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in chinese patients with advanced gastroenteropancreatic neuroendocrine tumors [J]. Oncol Lett. 2017;13(3):1165-1174
23. Cavalcoli F, Rausa E, Conte D. et al. Is there still a role for the hepatic locoregional treatment of metastatic neuroendocrine tumors in the era of systemic targeted therapies? [J]. World J Gastroentero. 2017;23(15):2640-2650
Author contact
![]() Corresponding authors: Dr. Xuefeng Xu, Department of General Surgery, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, People's Republic of China. Tel: +86-13061626568; E-mail: xuefengxu87com and Dr. Wenhui Lou, Department of General Surgery, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, People's Republic of China. Tel: +86-18321110985; E-mail: lou.wenhuish.cn
Corresponding authors: Dr. Xuefeng Xu, Department of General Surgery, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, People's Republic of China. Tel: +86-13061626568; E-mail: xuefengxu87com and Dr. Wenhui Lou, Department of General Surgery, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, People's Republic of China. Tel: +86-18321110985; E-mail: lou.wenhuish.cn

 Global reach, higher impact
Global reach, higher impact