Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(3):494-501. doi:10.7150/jca.21562 This issue Cite
Research Paper
Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels in gastric and colorectal cancer patients
1. Department of Research, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region 530021, China;
2. Department of Gastrointestinal Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region 530021, China.
* These authors contributed equally to the work.
Received 2017-6-21; Accepted 2017-10-23; Published 2018-1-1
Abstract
Despite extensive progress in treatment for cancer in recent decades, the early diagnosis for gastric cancer (GC) and colorectal cancer (CRC) remains poor. In this study, we explore the diagnostic value of joint detection of thymidine kinase 1 (TK1), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9) and carbohydrate antigen 72-4 (CA 72-4) in the diagnosis of GC and CRC, and to evaluated the relationship between TK1 expression and clinical pathological characteristics in the patients. Serum TK1, CA 19-9, CA 72-4 and CEA levels were measured in 169 patients with GC, 344 patients with CRC and 75 healthy controls using electro-chemiluminescence. The TK1 concentration was significantly higher in patients with cancer than in healthy controls and patients with clinical stage Ⅲ+Ⅳ had higher TK1 levels than clinical stage Ⅰ+Ⅱ (P<0.05). The levels of TK1 is significantly associated with tumor stage, lymph node metastasis, distant metastasis, tumor differentiation and age (P<0.05). When the tumor markers (TK1, CA 19-9 and CA 72-4) were detected respectively, the area under receiver operating characteristics curve (AUC) of TK1 for three cancers was the highest (0.823-0.895). However, the combination of AUC was higher than that for each tumor marker detected respectively (0.934-0.953), and the Hosmer-Lemeshow test showed an adequate model of calibration (P>0.05). Moreover, the AUCs varied significantly between the combination tests and single biomarker tests (Z test, P<0.01). In conclusion, serum TK1 may be an independent tumor marker for GC and CRC patients, and the combination of TK1, CA 19-9 and CA 72-4 and CEA performed even better. This study suggests that combination detection of four tumor markers may prove to be useful for the diagnosis of GC and CRC.
Keywords: gastric cancer, colorectal cancer, TK1, CA 19-9, CA 72-4, CEA.
Introduction
Cancer is a major public health issue worldwide, and nowadays it represents a leading cause of death worldwide [1]. In order to deal with the increased incidence of cancer, the World Health Organization (WHO) has suggested focusing on early detection and prevention of tumors [2].
TK1 is an enzyme involved in the regulation of the mammalian cell cycle, and high levels have been reported in proliferating and malignant cells [3]. In proliferating cells, TK1 concentration and activity are enhanced at the late G1 stage of the cell cycle, and reaches peak levels at the late S-phase/early G2 stages, and the levels tend to degrade at the mitotic stage. However, TK1 is almost completely absent in quiescent cells [4]. Thus, TK1 activity in serum is a cell-proliferating biomarker that has been used for prognosis of tumor patients and for monitoring the outcome of cancer therapy [5, 6]. Measurement of TK1 levels in early grade CIN (cervical intraepithelial neoplasia) makes it possible to predict the risk for progression to malignancy in this cancer. TK1 was also found to be a prognostic factor for treatment outcome of cervical carcinomas, particularly in patients with advanced stages of the disease [7]. Previous research has indicated that TK1 is elevated markedly in serum from patients with stage Ⅰ and stage Ⅱ lung cancer [8].
For the early diagnosis of cancer, several tumor biomarkers have been widely used in order to improve sensitivity and specificity of diagnosis. Previous studies have demonstrated serum carcinoembryonic antigen (CEA) to be both an important prognostic factor as well as an indicator of the therapeutic effect and recurrence in patients with rectal cancer [9, 10]. CEA is produced in normal cells and at a higher level in the presence of certain cancers. Persistent elevation of CEA levels after surgery of colorectal cancer could indicate incomplete resection or occult metastatic disease, and it demonstrates a prognostic feature for relapse [11]. Some other serum tumor biomarkers, such as carbohydrate antigen 72-4 (CA 72-4), carbohydrate antigen 19-9 (CA 19-9) can also be elevated in tumors of the digestive system. CA 19-9 is a commonly researched tumor biomarker in gastric cancer. It has been investigated as a prognostic indicator or a predictive factor in patients with gastric cancer [12]. CA 72-4 was shown to be expressed in GC and CRC and it was considered to be more specific and sensitive than other bio-markers [13, 14]. However, a combined diagnosis based on the levels of CEA, CA 19-9 and CA 72-4 was able to considerably improve sensitivity without impairing specificity.
In this study, the serum expression levels of potential tumor biomarkers was investigated before the start of clinical treatment. The aim was to assess the diagnostic value of joint detection of TK1, CEA, CA19-9 and CA72-4 for the diagnosis for GC and CRC. We also evaluated the relationship between the serum TK1 levels and clinical pathological characteristics in the patients.
Materials and Methods
Patients and samples
Serum samples of 513 patients with carcinomas of the stomach (n=169), colon (n=177) and rectum (n=167) were collected from the Affiliated Tumor Hospital of Guangxi Medical University in 2015. All serum samples were collected from subjects before treatment. Clinical pathological features of the patients are presented in Table 1. Tumor staging was classified according to the AJCC (American Joint Committee on Cancer Staging), Tumor, Lymph nodes, Metastasis (TNM) staging classification. We also included 75 volunteers as the heathy controls (age range: 16-74 years). These volunteers were free from any vital infections or illnesses. This study was approved by the ethics committee of the Affiliated Tumor Hospital of Guangxi Medical University.
Patient Demographics and Clinical Pathological Features
| Number of patients | TK1≤2.0 (pmol/L) | TK1>2.0 (pmol/L) | P value | |
|---|---|---|---|---|
| Gender | 0.827 | |||
| Male | 328 | 138 | 190 | |
| Female | 185 | 76 | 109 | |
| Age | < 0.001 | |||
| ≤60 | 283 | 97 | 186 | |
| >60 | 230 | 117 | 113 | |
| T stage | < 0.001 | |||
| T1+T2 | 106 | 65 | 41 | |
| T3+T4 | 407 | 149 | 258 | |
| Lymoph node+ | < 0.001 | |||
| Negative | 194 | 111 | 83 | |
| Positive | 319 | 103 | 216 | |
| Metastasis | 0.002 | |||
| Absent | 368 | 169 | 199 | |
| Present | 145 | 45 | 100 | |
| Differentiation | < 0.001 | |||
| Moderate+ well | 327 | 159 | 168 | |
| Poor | 186 | 66 | 131 | |
| Stage | < 0.001 | |||
| Ⅰ+Ⅱ | 189 | 111 | 79 | |
| Ⅲ+Ⅳ | 324 | 104 | 220 |
Detection of serum tumor markers
Fresh blood samples were stored for a period of 30-60 minutes at room temperature (RT) in non-heparin tubes and allowed to clot, and then centrifuged at 3500 rpm for 10 min. The supernatant sera were collected for testing.
The concentrations of serum TK1 were measured by using a Thymidine Kinase 1 Cell Cycle Assay Kit (commercial kit; SSTK Ltd., Shenzhen, China). The procedure was implemented according to the manufacturer's protocol as previously described [27]. Serum samples (3 μL) were applied onto a nitrocellulose membrane and allowed to dry for 30-60 min at room temperature. The membranes were blocked with non-fat milk in tris-buffered saline and then incubated with primary anti-TK1 antibody. After three washes and they were incubated with secondary antibody, followed by the addition of ECL substrate. The antigen was then detected using a CIS-2 imaging system (SSTK Inc.). Normal reference values for TK1 was assumed to be 0-2.0 pmol/L. The concentrations of CA 19-9, CA 72-4 and CEA were quantitatively measured using electro-chemiluminescence immunoassay (ECLIA) assay kits, according to the manufacturer's instructions (Cobas, Roche Diagnostics, Germany). Normal reference values for CA 19-9, CA 72-4 and CEA were assumed to be 0-37 U/mL, 0-5.7 U/mL and 0-6.5ng/mL respectively.
Statistical analysis
Comparisons of tumor marker levels among the different groups were performed using one way ANOVA. The Х2 test was used to evaluate the correlation between clinical characteristics and the serum concentration of TK1 for the individual patient. The ROC (receiver operating characteristics) curve was displayed using SPSS 17.0. The areas under the ROC curve (AUC), 95% confidence interval (CI) and Youden's index (sensitivity + specificity - 1) were calculated for each tumor marker and the combination of all four markers. Sensitivity and specificity were produced according to Youden's index. Statistical analysis was conducted by logistic regression, analyzing diagnostic power of TK1, CA 19-9, CA 72-4, CEA and combined detection of the four tumor markers for the three cancers, and the Hosmer-Lemeshow goodness-of-fit test was used to assess the model. Correlation between the four different tumor markers were determined using Spearman's correlation. All of the above-mentioned statistical calculations were conducted using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The AUCs were compared through Z test using MedCalc V15.2 software. P < 0.05 was considered statistically significant.
Results
Associations between TK1 levels and clinical pathological characteristics
Of the clinical pathological characteristics included, a higher TK1 value was found in patients with T3+T4 stage compared to T1+T2 stage (P<0.05). The concentration of TK1 showed a positive correlation with presence of lymph node and distant metastases (P<0.05). In the group of patients with poor differentiation, a significantly higher concentration of TK1 was found compared to the group of patients with Moderate-well differentiation. There was a significant increase in the number of patients with TK1>2.0 pmol/L from stage Ⅰ+Ⅱ to stage Ⅲ+Ⅳ (P<0.05). A marked negative correlation was observed between increasing age and TK1 level (P<0.05). There was no significant difference in TK1 serum levels with respect to gender (P>0.05) (Table 1).
The mean serum levels of TK1, CEA, CA 19-9, and CA 72-4 in the healthy controls and patients prior to treatment
According to Table 2, the TK1 concentration was significantly higher in patients with cancer (gastric cancer and colorectal cancer) than in healthy controls (P<0.05). Among the three patient groups, there was no significant difference in TK1 value (P>0.05). There was significant difference in the mean serum CA 19-9/CA 72-4 level between the healthy control and patients with gastric and colon cancer (P<0.05). The average CEA levels in the colon cancer group was significantly higher than in healthy controls (P<0.05).
Positive rates of serum tumor markers in GC and CRC with different clinical stage of disease
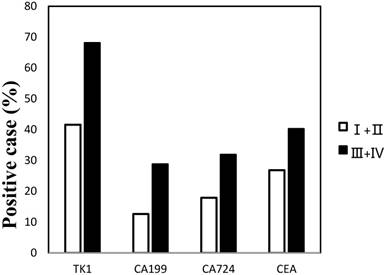
As shown in Figure 1, we separated all cancer groups into stage Ⅰ+Ⅱ and stage Ⅲ+Ⅳ, and then the positive rates of the four tumor markers were calculated in each group. Statistical analysis show that the positive rates of the four tumor markers increased with clinic stage, and statistically notable differences were found between stage Ⅰ+Ⅱ and stage Ⅲ+Ⅳ for TK1, CA 19-9, CA 72-4 and CEA (P<0.05).
Positive rate of serum tumor markers in gastric and colorectal cancer according to tumor stage.
Mean TK1 concentration levels in different groups
| Tumor type | N | TK1 (pmol/L) Mean ± SD | CA 19-9(U/mL) Mean ± SD | CA 72-4(U/mL) Mean ± SD | CEA(ng/mL) Mean ± SD |
|---|---|---|---|---|---|
| Healthy controls | 75 | 0.69±0.48 | 6.95±4.24 | 3.48±18.34 | 1.75±0.94 |
| Gastric cancer | 169 | 3.84±3.51* | 67.15±166.19* | 19.99±52.22* | 23.78±87.38 |
| Colon cancer | 177 | 3.50±3.64* | 95.04±214.59* | 21.12±54.35* | 50.36±161.48* |
| Rectal cancer | 167 | 3.28±4.10* | 42.56±114.62 | 15.35±49.64 | 24.85±62.38 |
Within the same columns, *represents differ significantly vs healthy controls (P<0.05).
The correlation analysis between four tumor markers in three cancer groups
Table 3 shows that there was no significant correlation between serum TK1 levels and CA 19-9, CA 72-4 and CEA in patients with gastric, colon and rectal cancer (all P>0.05). However, serum CEA levels was positively correlated with CA 19-9 and CA 72-4 in the three different cancer groups (all P<0.001).
The range of TK1 serum concentration levels in gastric and colorectal cancer
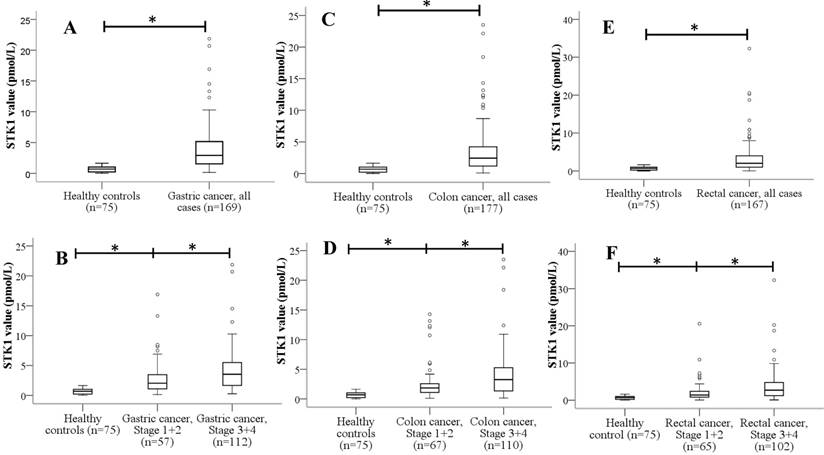
In GC, serum TK1 concentration levels ranged from 0.15 to 21.85 pmol/L (median = 2.91), and they showed significantly higher TK1 levels than healthy controls (Figure 2A). TK1 levels were also significantly higher in late-stage (Ⅲ+Ⅳ) compared with early-stage (Ⅰ+Ⅱ) (P<0.05) (Figure 2B). In patients with colon cancer, serum TK1 values ranged from 0.1 to 23.5 pmol/L (median = 2.44) and there was a statistically significant difference in TK1 concentration between colon cancer and control samples (P<0.05) (Figure 2C). There was also a significant difference between stage (Ⅰ+Ⅱ) colon cancer and stage (Ⅲ+Ⅳ) colon cancer (P<0.05) (Figure 2D). In rectal cancer serum, TK1 values ranged from 0.04 to 32.28 pmol/L (median = 2.03), the TK1 concentration levels were significantly different among the three groups including the controls group, the stage (Ⅰ+Ⅱ) and stage (Ⅲ+Ⅳ) (P<0.05) (Figures 2E-F).
Logistic regression and receiver operating characteristic (ROC) curve analysis in cancer groups versus healthy controls
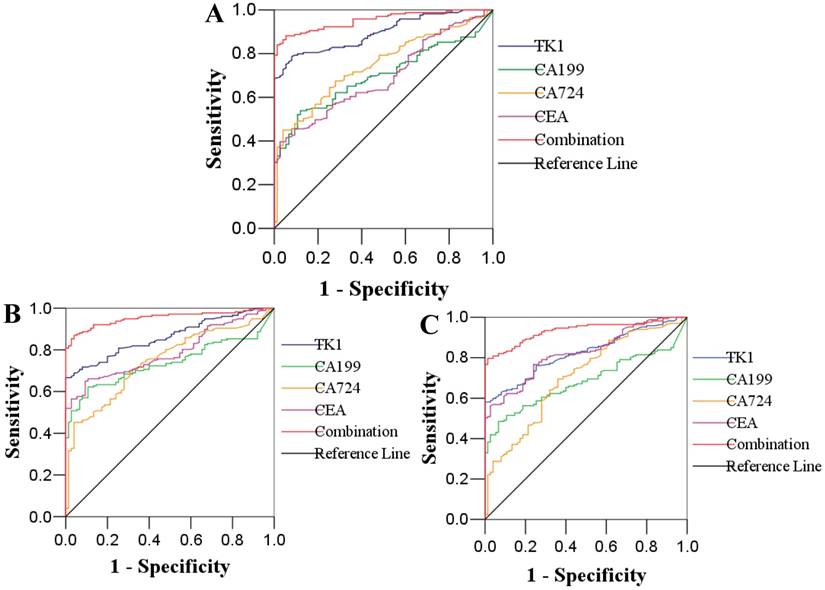
The ROC curve analysis of single TK1, CA19-9, CA72-4 and CEA and the combination in patients with gastric cancer, colon cancer, rectal cancer and healthy individuals are plotted in Figure 3. The AUC of the ROC analyses for the four tested tumor markers in gastric cancer were: TK1: 0.895; CA19-9: 0.697; CA72-4: 0.745; CEA: 0.693; the combination: 0.953. In patients with colon cancer, the AUC of the four tested tumor markers and the combination were: 0.863, 0.737, 0.746, 0.790 and 0.953, respectively. ROC curve analysis for rectal cancer patients revealed that the AUC of the four tested tumor markers and the combination were: 0.823, 0.682, 0.706, 0.826 and 0.934, respectively. Among the AUC of the three cancers for predicted probability connected with single tumor marker and logistic regression curves, when the tumor markers (TK1, CA19-9 and CA72-4) were detected respectively, the AUC of TK1 was the highest. Whereas, when the four tumor markers association with the logistic regression model are considered, the AUC of the combination was higher than that of each tumor marker detected respectively. The use of a single tumor marker (TK1) had good specificity (92.0-97.3%) but poor sensitivity (58.1-78.7%) for the diagnosis of GC and CRC. However, the sensitivity was improved to 80.8-88.2% without impairing specificity, when the four individual tumor marker measurements were used. The values for all parameters are shown in Table 4.
The Hosmer-Lemeshow test yielded Χ2 =3.347, P=0.851 in gastric cancer, Χ2 = 6.505, P=0.591 in colon cancer, Χ2 =14.492, P=0.07 in rectal cancer and this indicates the appropriateness of the logistic regression model to predict GC and CRC.
The AUCs of combination test and individual biomarkers were compared by Z test. Compared with the combination test in gastric cancer, the Z value of TK1, CA19-9, CA72-4 and CEA was respectively 4.137, 8.045, 6.307 and 7.888 (all P<0.01). In patients with colon cancer, the Z values between combination test and the four tested tumor markers were 4.812, 7.322, 6.365 and 6.344 (all P<0.01) respectively. In patients with rectal cancer, the Z values between combination test and the four tested tumor markers were 5.138, 7.490, 5.858 and 4.895 (all P<0.01) respectively. These results indicate the AUCs between the combination test and the individual biomarkers have significantly differences in GC and CRC patients (P<0.01).
Correlation between four different tumor markers in three cancer groups.
| Gastric cancer | Colon cancer | Rectal cancer | ||||
|---|---|---|---|---|---|---|
| rs* | P value | rs | P value | rs | P value | |
| TK1 vs CA 19-9 | -0.069 | 0.372 | 0.086 | 0.253 | 0.016 | 0.841 |
| TK1 vs CA 72-4 | -0.051 | 0.507 | 0.043 | 0.574 | 0.057 | 0.464 |
| TK1 vs CEA | -0.116 | 0.132 | 0.054 | 0.479 | 0.081 | 0.298 |
| CA 19-9 vs CA 72-4 | 0.117 | 0.129 | 0.207 | 0.006 | 0.168 | 0.030 |
| CA 19-9 vs CEA | 0.280 | <0.001 | 0.409 | <0.001 | 0.282 | <0.001 |
| CA 72-4 vs CEA | 0.333 | <0.001 | 0.320 | <0.001 | 0.270 | <0.001 |
*Spearman's correlation coefficient.
Box plot of distribution of TK1 in serum in three cancers (gastric, colon and rectal cancer) and healthy controls. The center line and box represent the median and interquartile ranges, respectively. The values of the maximum and minimum, eliminating outliers, are represented by the vertical lines. *P<0.05.
ROC curves of single TK1, CA 19-9, CA 72-4, CEA and the combination in predicting gastric cancer (A), colon cancer (B) and rectal cancer (C)
Area under the receiver operating curve (AUC) and the corresponding 95% confidence interval (CI) of the combination of TK1, CA 19-9, CA 72-4 and CEA (three different cancers versus healthy controls)
| Tumor markers | Sensitivity (%) | Specificity (%) | AUC | SE | 95% CI | P value |
|---|---|---|---|---|---|---|
| Gastric cancer | ||||||
| TK1 | 78.7 | 92.0 | 0.895 | 0.019 | 0.858-0.933 | <0.001 |
| CA 19-9 | 53.8 | 88.0 | 0.697 | 0.033 | 0.633-0.761 | <0.001 |
| CA 72-4 | 45.0 | 96.0 | 0.745 | 0.032 | 0.683-0.807 | <0.001 |
| CEA | 39.6 | 97.3 | 0.693 | 0.033 | 0.628-0.758 | <0.001 |
| Combination | 88.2 | 94.7 | 0.953 | 0.012 | 0.929-0.977 | <0.001 |
| Colon cancer | ||||||
| TK1 | 66.7 | 97.3 | 0.863 | 0.022 | 0.820-0.906 | <0.001 |
| CA 19-9 | 62.1 | 89.3 | 0.737 | 0.030 | 0.678-0.796 | <0.001 |
| CA 72-4 | 45.2 | 96.0 | 0.746 | 0.031 | 0.684-0.808 | <0.001 |
| CEA | 65.0 | 90.7 | 0.790 | 0.027 | 0.736-0.843 | <0.001 |
| Combination | 87.0 | 96.0 | 0.953 | 0.013 | 0.929-0.978 | <0.001 |
| Rectal cancer | ||||||
| TK1 | 58.1 | 97.3 | 0.823 | 0.026 | 0.773-0.874 | <0.001 |
| CA 19-9 | 48.5 | 93.3 | 0.682 | 0.033 | 0.618-0.747 | <0.001 |
| CA 72-4 | 69.5 | 64.0 | 0.706 | 0.035 | 0.637-0.775 | <0.001 |
| CEA | 56.3 | 97.3 | 0.826 | 0.026 | 0.775-0.876 | <0.001 |
| Combination | 80.8 | 96.0 | 0.934 | 0.015 | 0.904-0.964 | <0.001 |
SE, standard error
Discussion
In this study, we chose to study three of the most common digestive system cancers i.e., gastric, colon and rectal cancer. A previous study estimated that approximately 2,814,000 persons of Chinese origin would die from cancer in 2015, which is equivalent to over 7,500 cancer deaths daily [15]. The ranking for the 5 most common malignancies in China is lung, esophagus, stomach, colorectal and liver. Digestive system cancers, especially gastric and colorectal cancers, form the bulk of overall malignant conditions. According to the statistics of National Central Cancer Registry of China (NCCR), with an estimate of about 679,100 cases of GC and 376,300 new CRC cases, the mortality from GC and CRC accounted for approximately 498,000 and 191,000 deaths, respectively, in China by 2015 [15]. Despite extensive progress in treatment for cancer in recent decades, the early diagnosis for GC and CRC remains poor. Diagnostic and prognostic markers play an essential part in classifying tumors and determining the best therapeutic treatment for a patient.
TK1 is a pyrimidine salvage pathway enzyme important for DNA precursor synthesis and repair. Serum TK1 activity detection has been used as a tumor proliferation biomarker for the diagnosis and prognosis of various solid-tumor diseases. Early studies suggested that TK1 levels in the serum of patients with malignancies are markedly increased when compared to healthy persons. For instance, Jagarlamudi et al [16] observed that mean serum TK1 activities and TK1 protein levels from prostate and breast cancer patients were significantly higher than the healthy control group. Bolayirli et al [17] reported that serum TK1 activity is markedly elevated in patients with colorectal cancer, as compared with healthy individuals. In this study, the TK1 concentration was significantly higher in patients with cancers (rectal, gastric and colon cancer) than in healthy controls (P<0.05). Our results are consistent with these findings.
The high levels of TK1 in patients with malignancies also indicate that TK1 may be a tumor growth-related marker for the assessment of its progression. Here we studied 513 patients with different types of carcinomas to determine possible relationships between TK1 values and clinical stages and to confirm whether TK1 can be used for early diagnosis and assessment of tumor progression in cancer patients.
In the present study, the levels of TK1 was associated with clinical stages and tumor differentiation but not with gender. Previous clinical investigations suggested that elevated TK1 levels indicated active tumor growth. Mao et al. [18] found a significantly higher TK1 expression in stage Ⅱ patients with non-small cell lung adenocarcinoma compared to stage Ⅰ. Liu et al. [19] suggested that there was a significant increase in the mean TK1 level from stage Ⅰ+Ⅱ to stage Ⅲ+Ⅳ in patients with gastric cancer. Chen et al. [20] have proven that in patients with lung, gastric or thyroid cancers, high TK1 levels with the same tumor size indicate a later clinical stage. Xu et al. [21] previously observed higher TK1 expression in patients with lung adenocarcinoma which correlates to pathological stages.
The mechanisms behind the differences in the TK1 values between cancer patients in earlier and later clinical stages are not fully understood. However, it is likely that TK1 is closely associated with growth and that the level of TK1 may also reflect the growth rate of tumors in patients. In this study, a marked negative correlation was observed between increasing age and TK1 serum levels. Previous research has shown a decrease in mean TK1 activity with increasing age of patients [5]. Others have found the TK1 values are not correlated with age [19]. However, some researchers have measured the TK1 serum levels in 11880 persons for screening of apparently healthy persons for malignancies; the results show that the average age of the TK1-positive group was markedly higher than that of the TK1-negative group [22]. Thus, more investigations are needed to confirm this intriguing finding.
In order to evaluate the diagnostic capacity of TK1 and other biomarkers, ROC curves were constructed and the areas under the ROC curves (AUCs) were calculated. The AUC for TK1 in gastric, colon and rectal cancer were 0.895, 0.863 and 0.823, respectively. AUCs in the range of 0.97 and over are widely considered to have excellent accuracies, the range between 0.93 and 0.96 are considered very good, the range between 0.75 and 0.92 are considered good and AUCs of less than 0.75 are considered to be deficient in accuracy and are close to random [23]. According to this standard, the accuracy of TK1 in the diagnosis for GC and CRC in our study was not considered to be good enough. Therefore, we introduced other potential biomarkers, such as CEA, CA 19-9 and CA 72-4. Although these biomarkers have been widely used for diagnosis of various types of cancer, when they are used individually for the diagnosis of cancer, inconsistent results have been obtained.
Previous research showed that the expression of CEA is high in most gastrointestinal tumors [24]. However, Liang et al. concluded that CEA had little value for the diagnosis of gastric cancer [25]. CEA reflect different properties of tumor cells rather than directly surveying the growth rate of tumors [2] and this is indicated in this study by the lack of accuracy for cancer diagnosis. CA 19-9 is a ganglioside lipoprotein and its serum level is associated with gastrointestinal cancers. Serum CA 19-9 is elevated markedly in tumors of the digestive system and gallbladder. However, CA 19-9 has the highest sensitivity with relatively low specificity, so it cannot be used alone as an effective biomarker to diagnose gallbladder cancer [26]. Srivastava et al. suggested CA 19-9 should be combined with imaging tests to identify gallbladder cancer [27]. In a previous study, CA 72-4 has been considered as the most sensitive and specific biomarker for GC. However, the detection of only serum CA 72-4 is limited in sensitivity and accuracy. Therefore, a single test for CA 72-4 could not satisfy the demands of clinical practice [12].
In this study, the AUCs for CA 19-9 and CA 72-4 in gastric, colon and rectal cancer varied from 0.682 to 0.826, which indicates that CA 19-9 and CA 72-4 had limited value for the diagnosis of GC and CRC. To obtain a better accuracy for GC and CRC detection, the four tumor markers (TK1, CEA, CA 19-9 and CA 72-4) combined with the logistic regression model was applied in this study. Our results show that serum TK1 may be an independent tumor marker based on the associated AUC score being above 0.820 which reflects a good specificity. However, the four tumor markers combined in the logistic regression model had a better diagnostic performance for GC and CRC, which is consistent with the results of previous research [23, 25, 26].
In conclusion, the levels of TK1 is significantly associated with advanced tumor stages, poor differentiation and elderly patients with gastric or colorectal cancer. Moreover, the serum TK1 level may be an independent tumor marker for GC and CRC patients, and the combination of TK1, CA 19-9, CA 72-4 and CEA performed even better as a diagnostic tool. The combined detection of four tumor markers may prove to be useful for the diagnosis of GC and CRC.
Abbreviations
GC: gastric cancer; CRC: colorectal cancer; TK1: thymidine kinase 1; WHO: World Health Organization; CEA: carcinoembryonic antigen; CA 19-9: carbohydrate antigen 199; CA 72-4: carbohydrate antigen 724; TNM: Tumor, Lymph nodes, Metastasis; CIN: cervical intraepithelial neoplasia; ECLIA: electro-chemiluminescence immunoassay; ROC: receiver operating characteristics; AUC: area under the ROC curve; CI: confidence interval.
Acknowledgements
The authors would like to thank Dr. Dev Sooranna, Imperial College London, for editing the manuscript. This work was supported by the Guangxi Scientific Research and Technical Development Project (No. 1298003-2-8) and the Scientific Research & Technical Development Project of Qingxiu District, Nanning city (No. 2015S03).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Stewart BW WC. World cancer report 2014. Lyon: International Agency for Research on Cancer. 2014
2. Wang Y, Jiang X, Dong S, Shen J, Yu H, Zhou J. et al. Serum TK1 is a more reliable marker than CEA and AFP for cancer screening in a study of 56,286 people. Cancer Biomark. 2016;16:529-36
3. Gasparri F, Wang N, Skog S, Galvani A, Eriksson S. Thymidine kinase 1 expression defines an activated G1 state of the cell cycle as revealed with site-specific antibodies and ArrayScan assays. European journal of cell biology. 2009;88:779-85
4. Chen YL, Eriksson S, Chang ZF. Regulation and functional contribution of thymidine kinase 1 in repair of DNA damage. The Journal of biological chemistry. 2010;285:27327-35
5. Nisman B, Nechushtan H, Biran H, Gantz-Sorotsky H, Peled N, Gronowitz S. et al. Serum thymidine kinase 1 activity in the prognosis and monitoring of chemotherapy in lung cancer patients: a brief report. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:1568-72
6. Aufderklamm S, Todenhofer T, Gakis G, Kruck S, Hennenlotter J, Stenzl A. et al. Thymidine kinase and cancer monitoring. Cancer letters. 2012;316:6-10
7. Chen G, He C, Li L, Lin A, Zheng X, He E. et al. Nuclear TK1 expression is an independent prognostic factor for survival in pre-malignant and malignant lesions of the cervix. BMC cancer. 2013;13:249
8. Alegre MM, Weyant MJ, Bennett DT, Yu JA, Ramsden MK, Elnaggar A. et al. Serum detection of thymidine kinase 1 as a means of early detection of lung cancer. Anticancer research. 2014;34:2145-51
9. Song S, Hong JC, McDonnell SE, Koong AC, Minsky BD, Chang DT. et al. Combined modality therapy for rectal cancer: the relative value of posttreatment versus pretreatment CEA as a prognostic marker for disease recurrence. Annals of surgical oncology. 2012;19:2471-6
10. Tarantino I, Warschkow R, Schmied BM, Guller U, Mieth M, Cerny T. et al. Predictive Value of CEA for Survival in Stage I Rectal Cancer: a Population-Based Propensity Score-Matched Analysis. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2016;20:1213-22
11. Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT. et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. International journal of colorectal disease. 2011;26:1135-41
12. Chen XZ, Zhang WK, Yang K, Wang LL, Liu J, Wang L. et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Molecular biology reports. 2012;39:9031-9
13. Chen XZ, Zhang WH, Chen HN, Liu JP, He D, Liu Y. et al. Associations between serum CA724 and HER2 overexpression among stage II-III resectable gastric cancer patients: an observational study. Oncotarget. 2016;7:23647-57
14. Wang W, Li Y, Zhang X, Jing J, Zhao X, Wang Y. et al. Evaluating the significance of expression of CEA mRNA and levels of CEA and its related proteins in colorectal cancer patients. Journal of surgical oncology. 2014;109:440-4
15. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115-32
16. Jagarlamudi KK, Hansson LO, Eriksson S. Breast and prostate cancer patients differ significantly in their serum Thymidine kinase 1 (TK1) specific activities compared with those hematological malignancies and blood donors: implications of using serum TK1 as a biomarker. BMC cancer. 2015;15:66
17. Bolayirli M, Papila C, Korkmaz GG, Papila B, Aydogan F, Karatas A. et al. Serum thymidine kinase 1 activity in solid tumor (breast and colorectal cancer) patients treated with adjuvant chemotherapy. Journal of clinical laboratory analysis. 2013;27:220-6
18. Mao Y, Wu J, Skog S, Eriksson S, Zhao Y, Zhou J. et al. Expression of cell proliferating genes in patients with non-small cell lung cancer by immunohistochemistry and cDNA profiling. Oncology reports. 2005;13:837-46
19. Liu Y, Ling Y, Qi Q, Tang Y, Xu J, Tong Z. et al. Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer. Experimental and therapeutic medicine. 2011;2:1177-81
20. Chen Y, Ying M, Chen Y, Hu M, Lin Y, Chen D. et al. Serum thymidine kinase 1 correlates to clinical stages and clinical reactions and monitors the outcome of therapy of 1,247 cancer patients in routine clinical settings. International journal of clinical oncology. 2010;15:359-68
21. Xu Y, Shi QL, Ma H, Zhou H, Lu Z, Yu B. et al. High thymidine kinase 1 (TK1) expression is a predictor of poor survival in patients with pT1 of lung adenocarcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33:475-83
22. Chen Z, Zhou H, Li S, He E, Hu J, Zhou J. et al. Serological thymidine kinase 1 (STK1) indicates an elevated risk for the development of malignant tumours. Anticancer research. 2008;28:3897-907
23. Li H, Zhang B, Hu X, Dong Y, Fan Q, Guo F. et al. Serum Helicobacter pylori FliD antibody and the risk of gastric cancer. Oncotarget. 2016;7:22397-408
24. Tsouma A, Aggeli C, Lembessis P, Zografos GN, Korkolis DP, Pectasides D. et al. Multiplex RT-PCR-based detections of CEA, CK20 and EGFR in colorectal cancer patients. World journal of gastroenterology. 2010;16:5965-74
25. Liang Y, Wang W, Fang C, Raj SS, Hu WM, Li QW. et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7:49565-73
26. Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z. et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World journal of gastroenterology. 2014;20:4085-92
27. Srivastava K, Srivastava A, Mittal B. Potential biomarkers in gallbladder cancer: present status and future directions. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2013;18:1-9
Author contact
![]() Corresponding author: Litu Zhang, Professor, Department of Research, Affiliated Tumor Hospital of Guangxi Medical University, 71 He Di Rd, Nanning 530021, Guangxi Zhuang Autonomous Region, China. E-mail: zhanglitucom; Tel: +86-771 5310593.
Corresponding author: Litu Zhang, Professor, Department of Research, Affiliated Tumor Hospital of Guangxi Medical University, 71 He Di Rd, Nanning 530021, Guangxi Zhuang Autonomous Region, China. E-mail: zhanglitucom; Tel: +86-771 5310593.

 Global reach, higher impact
Global reach, higher impact