Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(6):1121-1126. doi:10.7150/jca.24397 This issue Cite
Research Paper
Inhaled Immunotherapy Administration for Lung Cancer; Efficient? Certainly Possible
1. 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
2. Pulmonary Department-Oncology Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece
3. Department of Respiratory and Critical Care Medicine, Changhai Hospital, Second Military Medical University, Shanghai, China
4. Department of Respiratory Diseases, The Affiliated Jiangning hospital of Nanjing Medical University, Nanjing, China
5. Radiation-Oncology Department, “Theageneio” Cancer Hospital, Thessaloniki, Greece
6. Ear, Nose and Throat Department, “Saint Luke“ Private Hospital, Panorama, Thessaloniki, Greece
7. 1 st University Surgery Department, University General Hospital of Alexandroupolis, Democritus University of Alexandroupolis, Alexandroupolis, Greece
8. Anatomy Department, Democritus University of Alexandroupolis, Alexandroupolis, Greece
9. Surgery Department, Interbalkan European Medical Center, Thessaloniki, Greece
10. Department of Pharmacology & Clinical Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
11. Medical Clinic I, "Fuerth" Hospital, University of Erlangen, Fuerth, Germany
12. Department of Pathology, Hackensack Meridian Health-Hackensack University Medical Center, NJ, USA
13. Anesthesiology Department, “AHEPA” University General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
14. Sana Clinic Group Franken, Department of Cardiology / Pulmonology / Intensive Care / Nephrology, ''Hof'' Clinics, University of Erlangen, Hof, Germany
* contributed equally to this work.
Received 2017-12-17; Accepted 2018-1-29; Published 2018-3-2
Abstract
Lung cancer is still diagnosed at a late stage in most lung cancer patients. Regarding Non-small Cell lung cancer there are novel therapies such as; tyrosine kinase inhibitors and immunotherapy. Currently we have two immunotherapies that can be used either as first-line treatment or second line treatment; pembrolizumab and nivolumab. A third one is being investigated as a combination of immunotherapy; ipilimumab. Aerosol treatment has been investigated for many diseases not only for the lung, but also for systematic diseases. The design of cups was found the most significant factor in producing significant effects. The comparison of cups reveals the design J as the most capable of reducing the droplets at a minimum size of mass median aerodynamic diameter (MMAD) MMAD=1.99. Drug effect comes second in sequence (F=62.04) showing that nivolumab is the most drastic preparation at low particle sizes (1.89), two drugs share an intermediate particle diameter (pembrolizumab and ipilimumab). In total drugs demonstrate a decreasing droplet size: Ipilimumab>Pembrolizumab> Nivolumab.
Keywords: lung cancer, ipilimumab, pembrolizumab, nivolumab, nsclc
Introduction
Although we have new equipment for lung cancer diagnosis such as the radial and convex endobronchial ultrasound, lung cancer is still diagnosed at a late stage due to lack of early symptoms. [1-5] Non-specific chemotherapy agents used to be the tip of the arrow for treatment of this diseases, however; during the last ten years huge steps have been made based on the genome of the tumor. The following gene expressions are connnected with targeted treatment. The epidermal growth factor receptor (EGFR) expression, anaplastic lymphoma kinase (ALK) expression, proto-oncogene B-Raf (BRAF), proto-oncogene tyrosine-protein kinase ROS (ROS-1). In the case where we have positive expression of these genes then tyrosine kinase inhibitors (TKIs) specific for each gene are being administared.[6] Regarding immunotherapy we have two drugs; pembrolizumab and nivolumab. Firstly we investigate the expression of programmed death-ligand 1 (PD-L1), in the case where it is >50% then pembrolizumab can be used as first line treatment, in the case where PD-L1 <50% then it can be used as second line treatment. Indifferent of the PD-L1 expression, nivolumab can be used as second line treatment for advanced nsclc. Inhaled therapies have been used for many years not and not only for lung diseases. In the case of pulmonary hypertension, diabetes and cystic fibrosis inhaled therapies have been used for many years. [7-10] The lung is actually huge bed sheet of vessels and the administration of an inhaled agent is usually absorbed immediately. There are several factors that affect the absorption and distribution of a drug such as (most important); drug production system, underlying lung disease, airway humidity, temperature, solution salts, viscosity, electric load and sustain release systems.[11, 12] Both aerosol droplets and aerosol powders are affected mostly from the humidity within the airways (90%) as their size can increase up to 50%. Moreover; the speed of the aerosol can also affect the deposition especially powder formulations if the powder has <1.5μm mass median aerodynamic diameter (MMAD).[11] In current experimental project we have evaluated the best combination of aerosol production system and residual cup.
Materials and Methods
Aerosol Production Systems
Jet-Nebulizers and residual cups
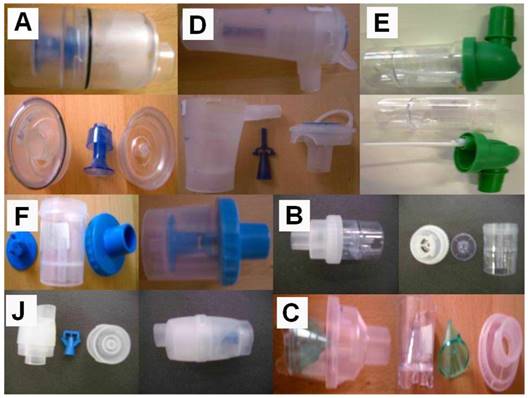
Three nebulizers were chosen from our department for the experiment: Maxineb® (6 liters/minute and 35 psi), Sunmist® (5-7 liters/minute and 35 psi) and Invacare® (4-8 liters-minute and 36 psi). (Figure 1) In total 7 residual cups were chosen for evaluation, four with a capacity of no more than 6 mls and two with a capacity no more than 10 mls. The designs for the large residual cups will be mentioned as A, D and E. The residual cups for the small residual cups will be mentioned as C, F, B and J. (Figure 2) The large residual cups were not used with a capacity of more than 8 mls as explained in the discussion section.
Jet-nebulizers: Maxineb, Sunmist and Invacare

Ultrasound Nebulizers
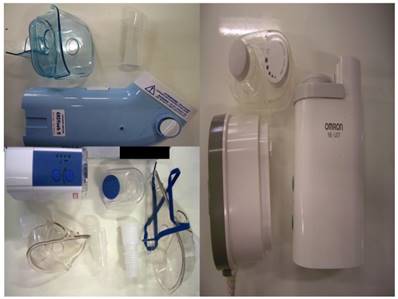
Three new ultrasound nebulizers were chosen from the market based on their cost-effectiveness. The first was Omron® NE-U07, Tokyo, Japan. Compact and weight less than 350gm, includes 10ml medication cup. Generates uniform micromillimetre-sized vapor particles. The second was a portable EASYneb® II, FLAEMNUOVA, Martino, Italy. with the following operating specifications; drug max capacity: 8ml`s, Frequency: 2.4 MHz, Nebulization capacity (adjustable) 0-0.7ml/min approximately (tests performed with saline 0.9%), Particle size: 2.13 μm (ΜΜΑD), sound level at 10cm: 50 db (A), Operating temperature: min. 10oC, max. 40oC and air humidity: min. 10%, max. 95%RH. The third was a portable GIMA, Gessate, Italy (Choice Smart Health Care Company Limited, Wan Chai, Hong Kong, No. G2061259328002) with the following operating specifications; Particle size: 3-5μm, Frequency: 2.5 MHz, Medication Cup Capacity: 1-6 ml`s, sound level at 10cm: <50 db, Operating temperature: min. 10oC, max. 40oC and air humidity: min. 10%, max. 95% RH. (Figure 3)
Drugs
Opdivo® (nivolumab), Bristol-Myers Squibb, 10mg/l, Yervoy® (ipilimumab), Bristol-Myers Squibb and Keytruda® (pembrolizumab), Merck. (Figure 4)
Droplet Measurement
The size distribution of the droplets and their mean diameter (d32) were calculated using a Malvern Mastersizer 2000 apparatus (Malvern Instruments Ltd, Malvern, Worcestershire, UK) equipped with a Scirocco module (Malvern Instruments Ltd, Malvern, Worcestershire, UK). The device has been modified as for the used to be able to spray the generated droplets directly perpendicular to the laser beam (i.e. [4, 8, 13, 14]). A refractive index of 1.33 was been used for the sprayed droplets. The measurements were made under ambient temperature. (Figure 5)
Z Potential
Zeta potential is a scientific term for electrokinetic potential in colloidal dispersions. The zeta potential is the electric potential in the interfacial double layer (DL) at the location of the slipping plane relative to a point in the bulk fluid away from the interface. Zeta potential is the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle.[15]
The zeta potential is caused by the net electrical charge contained within the region bounded by the slipping plane, and also depends on the location of that plane. Thus it is widely used for quantification of the magnitude of the charge. However, zeta potential is not equal to the Stern potential or electric surface potential in the double layer, because these are defined at different locations. Such assumptions of equality should be applied with caution. Nevertheless, zeta potential is often the only available path for characterization of double-layer properties.
Designs for the large residual cups will be mentioned as A, D and E. The residual cups for the small residual cups will be mentioned as C, F, B and J.
The zeta potential is a key indicator of the stability of colloidal dispersions. The magnitude of the zeta potential indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in a dispersion. For molecules and particles that are small enough, a high zeta potential will confer stability, i.e., the solution or dispersion will resist aggregation. When the potential is small, attractive forces may exceed this repulsion and the dispersion may break and flocculate. So, colloids with high zeta potential (negative or positive) are electrically stabilized while colloids with low zeta potentials tend to coagulate or flocculate as outlined in the table.[16] (Figure 6)
The z potential was measured for all drugs (five times each) and the results were as follows: a) Nivolumab mean: -1.20, b) Ipilimumab mean: -3.85, c) mean: -14.01.
Results
Inhalation process at three load levels (2, 4, 6 ml)
The design of cups was found the most significant factor in producing significant effects. The comparison of cups reveals the design J as the most capable of reducing the droplets at a minimum size of mass median aerodynamic diameter (MMAD) MMAD=1.99. Design C is the less efficient (4, 16) and a group of five cups is arrayed in a size range of 3, 16 to 3,01.
Drug effect comes second in sequence (F=62.04) showing that nivolumab is the most drastic preparation at low particle sizes (1.89), two drugs share an intermediate particle diameter (pembrolizumab and ipilimumab). In total drugs demonstrate a decreasing droplet size: Ipilimumab>Pembrolizumab>Nivolumab.
MAXINEB nebulizer produces the smallest droplet size (F=15, 01, MMAD=1.99) differing only significantly from INVACARE whose confidence intervals do not overlap.
Low but significant effect (F= 4,03) is produced by load level particularly at 6ml concentration (1.99) as compared to that of 2ml (2.30).
Drug and residual cup interact significantly (F=7, 41) providing a unique and clear indormation: Nivolumab combined with design cup B drops MMAD down to 1,40mm. This finding is very important because the analysis reveals hidden otherwise effects since no Nivolumab and cup B contributed to any best solution.
The interaction effect between residual cups and nebulizers (F=3, 63) brings in another effective combination, MAXINEB x cup J, reducing even lower the droplet size (MMAD=1.66) than acting themselves individually.
Ultrasound nebulizers: EASYneb® II (upper left), GIMA (lower left), and Omron® NE-U07 (right)
Drugs
Matersizer 2000 (In the Laboratory of Department of Food Technology, School of Food Technology and Nutrition, Alexander Technological Educational Institute, Thessaloniki, Greece)

Zeta potential equipment (In the Laboratory of Department of Food Technology, School of Food Technology and Nutrition, Alexander Technological Educational Institute, Thessaloniki, Greece)

Inhalation process at dose level 8ml
At the highest dose level the pattern of drugs, nebulizers and residual cups changes dramatically. Now, none of the drugs prevails in effect although they perform comparatively more strongly (F=75.53), followed by the cup designs (F=13.62) then by the nebulizers (F=6.69) and finally by the interactive effect between drug and cup design (F=11.60). Six drugs share equally the lowest droplet size, ranging between 1.9 and 3.12 while Ipilimumab was the least drastic preparation.
Residual cup D and SUNMIST nebulizer is the best combination for large residual cups reducing the droplets to MMAD=2.12 and 2.17 respectively.
The drugs Nivolumab and Pembrolizumab in combination with cup D and drug Ipilimumab in joint with cup A provide good droplet size reductions. These effects are not unique as compared to others' effects but they show clear action without overlapping confidence intervals with the two other residual cups.
Nebulizers
The results of the ultrasound procedure reveal that factor main effects follow a different pattern of significance effect order as compared to the aforesaid results: nebulizer (F=43,70), drug (F=29.81) and loading (F=12.03). The mask or inlet did not exert any particular effect (F=0.61, p=0.422). MAXINEB is by far the best nebulizer performer, MMAD=2.01), Nivolumab between the drugs, MMAD=1.59) and 4ml dose reacts better than that of 2ml attaining nevertheless moderate levels of droplet size (2.57 against 3.11). However, when the 4ml dose is combined with MAXINEB nebulizer (F=8.64), it results in an amazing size reduction of 1.69mm. Significant interactive effects were observed between drug and nebulizer (F=6.04) and by the second order interaction drug x nebulizer x loading (F=9.61). Both interactions did not come up with any clear or unique effect at low level droplet sizes and therefore their results are not depicted.
Discussion
Several chemotherapy drugs that have been previously used for lung cancer were investigated as aerosol forms. Drugs such as; cisplatin, carboplatin, taxane derivatives, bevacizumab and gemcitibine have been administered as aerosol.[17-19] Different aerosol production systems were used in these studies, however; extensive concern regarding the most efficient system was not given. In specific, most studies stopped experimenting with the aerosol production system when the droplets produced had a mass median aerodynamic diameter of ≤5μm. In several of these drugs further experimentation with additional aerosol production systems (APSs) could reveal that another APS could produce smaller droplets and therefore deeper penetration into the lung parenchyma. Moreover; it was previously observed that not only the APS, but also the inlet that several devices use can produce even smaller droplet size in an aerosol.[20, 21] Inhaled drugs that are already on the market can be possibly improved if an inlet is added to the production device or a different inlet design.[20, 21] Regarding the efficiency of a systematic administered drug as aerosol, there have been many in vitro and in vivo studies.[22] Furthermore; there have been numerous clinical trials.[22] There are several parameters that affect the efficiency and safety of the aerosol administration and the most important are underlying pulmonary disease and time of administration. Regarding the underlying pulmonary disease a patient might have asthma, chronic obstructive pulmonary disease (COPD), or a very large tumor mass (≥3cm) and this patient might have disease exacerbation. Regarding time of administration we have to include the time where the patient inhales the aerosol drug formulation and the time where the drug remains in the alveoli until it is absorbed through the vessels of the alveoli. The issue of safety for aerosol chemotherapy has been partially investigated, not all studies addressed this issue, most of them focused just on the efficiency.[19, 22] Solubility of the drug formulation is very important because it has been connected with the interaction of the alveoli membrane were toxicity might be observed. Toxicity has been observed either with the production of cytokines locally, or with the appearance of pneumonitis.[17] Certainly, it has been observed at least for the drug cisplatin that chemotherapy drugs through the alveoli are distributed to the lymph nodes.[17] This issue is also very important in the case of advanced lung cancer disease where a patient usually has metastatic disease in the lymph nodes. Immunotherapy has been previously established for lung cancer and we are expecting in the near future new data on combinations of immunotherapy drugs or immunotherapy plus chemotherapy.[23] In the present study we presented data where the three immunotherapeutic drugs nivolumab, ipilimumab and pembrolizumab can be produced as aerosol from their current form using water as a drug solvent 1/10. Further experimentation in animal models will focus on the safety and efficiency issue.
Acknowlegments
We would like to thank our patients for their kind donation of several pharmaceutical products in order to perform this study (drugs and equipment).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, Linsmeier B, Tsavlis D, Kioumis I. et al. Drug Eluting Stents for Malignant Airway Obstruction: A Critical Review of the Literature. Journal of Cancer. 2016;7:377-90 doi:10.7150/jca.13611
2. Haidong H, Yunye N, Wei Z, Zarogoulidis P, Hohenforst-Schmidt W, Man YG. et al. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: a single-center study. Journal of Cancer. 2017;8:3514-21 doi:10.7150/jca.20035
3. Oezkan F, Khan A, Zarogoulidis P, Hohenforst-Schmidt W, Theegarten D, Yasufuku K. et al. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. OncoTargets and therapy. 2014;7:2061-5 doi:10.2147/OTT.S72974
4. Zarogoulidis P, Darwiche K, Tsakiridis K, Teschler H, Yarmus L, Zarogoulidis K. et al. Learning from the Cardiologists and Developing Eluting Stents Targeting the Mtor Pathway for Pulmonary Application; A Future Concept for Tracheal Stenosis. Journal of molecular and genetic medicine: an international journal of biomedical research. 2013;7:65. doi:10.4172/1747-0862.1000065
5. Zarogoulidis P, Huang H, Bai C, Kosmidis C, Trakada G, Veletza L. et al. Endobronchial ultrasound convex probe for lymphoma, sarcoidosis, lung cancer and other thoracic entities. A case series. Respiratory medicine case reports. 2017;22:187-96 doi:10.1016/j.rmcr.2017.08.016
6. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR. et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2017;15:504-35
7. Pitsiou G, Zarogoulidis P, Petridis D, Kioumis I, Lampaki S, Organtzis J. et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug design, development and therapy. 2014;8:1753-63 doi:10.2147/DDDT.S70277
8. Zarogoulidis P, Kioumis I, Ritzoulis C, Petridis D, Darwiche K, Porpodis K. et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam. International journal of pharmaceutics. 2013;455:182-8 doi:10.1016/j.ijpharm.2013.07.040
9. Zarogoulidis P, Kioumis I, Porpodis K, Spyratos D, Tsakiridis K, Huang H. et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug design, development and therapy. 2013;7:1115-34 doi:10.2147/DDDT.S51303
10. Zarogoulidis P, Papanas N, Kouliatsis G, Spyratos D, Zarogoulidis K, Maltezos E. Inhaled insulin: too soon to be forgotten? Journal of aerosol medicine and pulmonary drug delivery. 2011;24:213-23 doi:10.1089/jamp.2011.0876
11. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. British journal of clinical pharmacology. 2003;56:588-99
12. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. British journal of clinical pharmacology. 2003;56:600-12
13. Zarogoulidis P, Petridis D, Ritzoulis C, Darwiche K, Spyratos D, Huang H. et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: back to drawing the residual cup. Int J Pharm. 2013;453:480-7 doi:10.1016/j.ijpharm.2013.06.011 S0378-5173(13)00520-6 [pii]
14. Zarogoulidis P, Petridis D, Ritzoulis C, Darwiche K, Kioumis I, Porpodis K. et al. Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics. International journal of pharmaceutics. 2013;456:325-31 doi:10.1016/j.ijpharm.2013.09.004
15. de Kruijf W, Ehrhardt C. Inhalation delivery of complex drugs-the next steps. Current opinion in pharmacology. 2017;36:52-7 doi:10.1016/j.coph.2017.07.015
16. Backstrom E, Boger E, Lundqvist A, Hammarlund-Udenaes M, Friden M. Lung Retention by Lysosomal Trapping of Inhaled Drugs Can Be Predicted In Vitro With Lung Slices. Journal of pharmaceutical sciences. 2016;105:3432-9 doi:10.1016/j.xphs.2016.08.014
17. Zarogoulidis P, Darwiche K, Krauss L, Huang H, Zachariadis GA, Katsavou A. et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future oncology. 2013;9:1307-13 doi:10.2217/fon.13.111
18. Zarogoulidis P, Eleftheriadou E, Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T. et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investigational new drugs. 2012;30:1628-40 doi:10.1007/s10637-011-9714-5
19. Zarogoulidis P, Giraleli C, Karamanos NK. Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered. Therapeutic delivery. 2012;3:1021-3
20. Zarogoulidis P, Porpodis K, Kioumis I, Petridis D, Lampaki S, Spyratos D. et al. Experimentation with inhaled bronchodilators and corticosteroids. International journal of pharmaceutics. 2014;461:411-8 doi:10.1016/j.ijpharm.2013.12.010
21. Zarogoulidis P, Petridis D, Ritzoulis C, Li Q, Huang H, Ning Y. et al. Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays' production systems. International journal of pharmaceutics. 2013;458:39-47 doi:10.1016/j.ijpharm.2013.10.019
22. Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP. et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. International journal of nanomedicine. 2012;7:1551-72 doi:10.2147/IJN.S29997
23. Mayor M, Yang N, Sterman D, Jones DR, Adusumilli PS. Immunotherapy for non-small cell lung cancer: current concepts and clinical trials. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2016;49:1324-33 doi:10.1093/ejcts/ezv371
Author contact
![]() Corresponding author: Konstantinos Sapalidis, 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece telephone: 6944706828. Paul Zarogoulidis, M.D, Pulmonary Oncology-Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom
Corresponding author: Konstantinos Sapalidis, 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece telephone: 6944706828. Paul Zarogoulidis, M.D, Pulmonary Oncology-Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom

 Global reach, higher impact
Global reach, higher impact