Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(7):1152-1164. doi:10.7150/jca.23344 This issue Cite
Research Paper
Nomograms to Predict Individual Prognosis of Patients with Primary Small Cell Carcinoma of the Bladder
1. Department of Urology, Fudan Institute of Urology, Huashan Hospital, Fudan University, Shanghai, China
2. Department of Urology, Ruijin Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
3. Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
Received 2017-10-16; Accepted 2018-1-28; Published 2018-3-8
Abstract
Objectives: To develop reliable nomograms to estimate individualized overall survival (OS) and cancer specific survival (CSS) for patients with primary small cell carcinoma of the bladder (SCCB) and compare the predictive value with the AJCC stages.
Patients and Methods: 582 eligible SCCB patients identified in the Surveillance, Epidemiology, and End Results (SEER) dataset were randomly divided into training (n=482) and validation (n=100) cohorts. Akaike information criterion was used to select the clinically important variables in multivariate Cox models when establishing nomograms. The performance of nomograms was bootstrapped validated internally and externally using the concordance index (C-index) with 95% confidence interval (95% CI) and calibration curves and was compared with that of the AJCC stages using C-index, Kaplan-Meier curves and decision curve analysis (DCA).
Results: Two nomograms shared common indicators including age, tumor size, T stage, lymph node ratio, metastases, chemotherapy, radiation and radical cystectomy, while marriage and gender were only incorporated in the OS nomogram. The C-indices of nomograms for OS and CSS were 0.736 (95%CI 0.711-0.761) and 0.731(95%CI 0.704-0.758), respectively, indicating considerable predictive accuracy. Calibration curves showed consistency between the nomograms and the actual observation. The results remained reproducible when nomograms were applied to the validation cohort. Additionally, comparisons between C-indices, Kaplan-Meier curves and DCA proved that the nomograms obtained obvious superiority over the AJCC stages with wide practical threshold probabilities.
Conclusions: We proposed the first two nomograms for individualized prediction of OS and CSS in SCCB patients with satisfactory predictive accuracy, good robustness and wide applicability.
Keywords: small cell carcinoma of the bladder, nomogram, overall survival, cancer specific survival, decision curve analysis
Introduction
Small cell carcinoma of the bladder (SCCB) is a rare, poorly differentiated and aggressive neuroendocrine malignancy [1]. In spite of the proof that SCCB might have the same clonal origin as urothelial bladder cancer [2], clinicopathological characteristics and treatment choices are significantly different between these two diseases. SCCB accounts for estimated less than 1% of bladder malignancies [3, 4], leading to an unclear understanding of this disease. Besides, the survival outcomes of patients with SCCB reported in the previous studies were very poor, with a median survival between 11 months and 23 months if treatments were given [5-7]. If patients didn't receive any treatment, the survival could be no more than half a year [6]. What's more, management of SCCB, although involving a multimodal approach including chemotherapy, radiation and surgery, still lacks a standard guideline [8]. Up to now, the characterization and clinical management of the disease are based mostly on retrospective reviews and case reports [6, 9-15] with few prospective researches available.
Owing to the rarity of this cancer, so far there has been a lack of agreement about how to predict patient's survival outcomes. Currently, the AJCC staging system for urothelial bladder cancer based on tumor, node and metastasis are also used to predict survival in SCCB patients [16]. However, previous studies didn't found good predictive value of AJCC staging system for SCCB patients [17] and obvious overlapping of the survival curves was observed among I-IV AJCC stages [5, 10]. Apart from such unfavorable results, we should notice that the AJCC classification only accounts for three tumor-related indicators which reflected the tumor morphology and pathology, without taking patients' clinical features and diverse therapeutic regimens in to consideration. In addition, the current AJCC classification merely classified patients into various groups, but fails to estimate the individualized survival outcomes.
Prognostic nomograms are graphic calculating scales for predictive models to maximize the predictive accuracy of individual prognosis [18, 19], and they have been established for several cancers [20-22]. As for urothelial bladder cancer (UBC), various nomograms were also developed and has been proved to be useful in management of bladder cancer [23]. Obvious advantages have been observed in nomograms such as better predictive accuracy, strong robustness as well as user-friendliness, which enhances their potentials in clinical practice [19, 22]. Therefore, nomograms have been suggested as alternative methods or even as new standard to guide the management of cancer patients [24-26]. However, in terms of SCCB patients, so far no nomogram that predict overall survival (OS) or cancer specific survival (CSS) has been established, which might be attributed to the limited number of SCCB cases in each single institution.
In this study, SCCB patients recorded in the Surveillance, Epidemiology, and End Results (SEER) dataset, which is a U.S. population-based cancer dataset that collects information of cancer patients in 18 registries of the U.S. and covers approximately 30% of total U.S. population, were identified. We aimed to develop validated prognostic nomograms, which included their demographic variables (age, gender, marital status), clinicopathological information (tumor size, T stage, node status, metastasis) as well as treatment methods (surgery, radiation, chemotherapy) for predicting OS and CSS of SCCB patients. And we deeply compared the performance of the nomograms with the currently used AJCC staging system for bladder cancer.
Patients and Methods
Patient eligibility and variables
Patients diagnosed with small cell carcinoma of the bladder from 2004 to 2014 were identified from Surveillance, Epidemiology, and End Results (SEER) database. Only patients who met the following criteria were included: 1) age > 18 years; 2) microscopically diagnosed with primary SCCB as the first malignancy; 3) histological type limited to small cell carcinoma or combined small cell carcinoma (ICD-O-3 codes: 8002, 8041-8045); 4) active follow-up with complete date and known survival months and known cause of death; 5) adequate/consistent information on the TNM stages and other variables including age, gender, number of regional lymph node removed, number of regional lymph node positive, surgery of the primary tumor, radiation and chemotherapy. Patients were excluded if controversial information was recorded (e.g. patients at M0 stage with visceral metastases recorded). Covariates of interest extracted for each case are age, gender, marriage, pathology, TNM stage, surgery of the primary tumor, radiation, chemotherapy, sites of metastases and metastasectomy. Cancer stages reported using the 6th AJCC stages were converted based on the 7th edition [16]. Besides, for patients received pelvic lymphadenectomy, the number of regional lymph nodes dissected and the number of positive lymph nodes were retrieved and lymph node ratio (LNR) was calculated by dividing the positive node number by the examined nodes number. For the purpose of properly assessing the prognostic value of lymph node ratio in SCCB patients, positive LNR was stratified into two categories (cut-off point 0.46) by X-tile program, a practical tool for cut-point optimization, according to the minimal p-value approach [20]. Hence, the variable LNR was finally divided into four categories: patients didn't receive lymphadenectomy; LNR=0; 0<LNR≤0.46 and LNR>0.46. Using the similar approach, we also identified 8.3 cm as the cut-off point for patients with definite size of tumor. The follow-up information including survival status, survival months and cause of death were all extracted from the dataset. The primary endpoints of the study were overall survival (OS) and cancer specific survival (CSS). Survival time was calculated from the date of diagnosis to the date of 1) death from any cause (OS); 2) death from SCCB (CSS); or 3) the last follow-up.
After patient identification, 582 eligible patients were enrolled and made up the primary cohort of SCCB. The primary cohort was randomized into a training cohort (n=482) and a validation cohort (n=100) in order to develop and validate the nomograms.
Statistical analysis
Continuous variable (age) was presented as median with range, while categorical variables were shown as the number of patient with respective percentages. Univariate and multivariate Cox proportional hazards regression analyses were employed to evaluate the prognostic factors. Nomograms for 1- and 3- year OS and 1- and 3-year CSS were formulated based on the results of the multivariate Cox regression analyses. The backward step-down process based on Akaike information criterion (AIC) was used to finally recruit independent prognostic factors into the constructions of the nomograms [27]. Based on the training cohort and validation cohort, both internal and external validations of the nomograms were completed. The performances of the nomograms as well as the AJCC staging system were assessed by Harrell's concordance-index (C-index), which is similar to the area under curve (AUC) of the receiver operating characteristic (ROC) curve, but proved to be more suitable for censored data [27]. Comparisons between nomogram models and the AJCC staging system were performed with the rcorrp.cens function in the Hmisc package in R [28]. Calibration curves of the nomograms were applied to evaluate the consistency between predicted survival and observed survival and bootstraps with 1000 resamples were used for the validation [24]. In the external validation of the nomograms, the total scores of each case in the validation cohort were calculated according to the established nomograms, and the scores were then used as factors in the Cox regression model, from which the validation C-index and calibration curves were derived [24].
To further compare the nomograms with the present AJCC stating system, the primary cohort was divided in to four quartiles (four nomogroups) (for OS: nomogroup I: 0-20; nomogroup II: 20-24; nomogroup III:24-29; and nomogroup IV: >29; for CSS: nomogroup 1:0-18; nomogroup 2:18-23; nomogroup 3:23-28; nomogroup 4:>28) using quartile.exc function of Microsoft Excel. After grouping, the prognostic discrimination of the nomograms as well as the AJCC stages were assessed by Kaplan-Meier analysis. Due to the very limited number of patients (only 3 patients) with stage 0is (Tis patients), they were not included in the Kaplan-Meier curves. Furthermore, decision curve analysis (DCA) was employed using the rmda package of R to outline the ranges of threshold probabilities within which the nomograms were clinically applicable [29].
The primary cohort was randomly allocated using the SPSS version 22.0 software (IBM SPSS Statistics, Chicago, IL, US). The other analyses were processed with the R program (v 3.4.0) using rms and the above-mentioned packages and Kaplan-Meier curves were drawn using the SPSS 22.0. Two-sided P values of less than 0.05 were considered statistically significant. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Patient baseline characteristics
The baseline characteristics of the primary cohort (582 patients), training cohort (482 patients) and validation cohort (100 patients) were listed in Table 1. 400 (68.7%) patients had died by the end of the follow-up, among which 326 (56.0%) died from SCCB and 74 (12.7%) died from other causes. The median OS were 13 months for the training and validation cohorts. For both training and validation cohorts, the majority of patients were with T2, N0 and M0 stages. Besides of chemotherapy, which remained to be the main treatment for all patient cohorts, 171 (29.4%) and 145 (25.4%) of enrolled patients received radiotherapy and radical cystectomy (RC), while less than 2% patients had metastasectomy. In the primary cohort, 429 (73.7%) patients didn't receive lymphadenectomy. Results of pelvic lymphadenectomy showed that 98 were found lymph nodes negative (LNR=0), while 40 and 15 patients were in “LNR≤0.46” and “LNR>0.46” groups, respectively, based on the cut-off point determined by the X-tile plots.
Univariate and multivariate Cox regression of the training cohort
We performed the univariate analyses to identify the clinical parameters that were significantly associated with OS and CSS. As shown in Table 2, age, marital status, T stage, metastases, tumor size, radical cystectomy, chemotherapy and LNR were significantly associated with OS. For CSS, marital status lost its significance while radiation became a significant parameter. In the next-step multivariate Cox regression, at first all the original factors entered into the Cox regression model [21]. For the purpose of identifying the independent predictors which were strikingly contributed to patients' prognosis and could be admitted into the nomograms, least value of AIC was used to do variable selection in accordance with the previous studies [30]. As shown in Table 3, ten key indicators for predicting OS were identified including age, gender, marital status, T stage, metastases, radical cystectomy, radiation, chemotherapy, tumor size and LNR. As for CSS, gender and marital status were also excluded from the selection (Table 4).
Patient demographics and clinical characteristics
| Variables | Primary cohort (n=582) | Training cohort (n=482) | Validation cohort (n=100) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age (median, range) | 71 | 37-99 | 71 | 37-99 | 70 | 47-94 |
| Gender | ||||||
| Male | 442 | 75.9 | 374 | 77.5 | 68 | 68.0 |
| Female | 140 | 24.1 | 108 | 22.4 | 32 | 32.0 |
| Marital status | ||||||
| Married | 329 | 56.5 | 275 | 57.1 | 54 | 54.0 |
| Unmarried | 231 | 39.7 | 188 | 39.0 | 43 | 43.0 |
| Unknown | 22 | 3.8 | 19 | 3.9 | 3 | 3.0 |
| Pathology | ||||||
| Small cell carcinoma | 538 | 92.4 | 450 | 93.4 | 88 | 88.0 |
| Combined small cell carcinoma | 44 | 7.6 | 32 | 6.6 | 12 | 12.0 |
| T stage | ||||||
| Tis | 3 | 0.5 | 3 | 0.6 | 0 | 0.0 |
| T1 | 91 | 15.6 | 71 | 14.7 | 20 | 20.0 |
| T2 | 324 | 55.7 | 273 | 56.6 | 51 | 51.0 |
| T3 | 92 | 15.8 | 77 | 15.9 | 15 | 15.0 |
| T4 | 72 | 12.4 | 58 | 12.0 | 14 | 14.0 |
| N stage | ||||||
| N0 | 465 | 79.9 | 381 | 79.0 | 84 | 84.0 |
| N1 | 53 | 9.1 | 49 | 10.2 | 4 | 4.0 |
| N2 | 54 | 9.3 | 46 | 9.5 | 8 | 8.0 |
| N3 | 10 | 1.7 | 6 | 1.2 | 4 | 4.0 |
| Metastases | ||||||
| M0 | 471 | 80.9 | 391 | 81.1 | 80 | 80.0 |
| Distant lymph nodes | 11 | 1.9 | 8 | 1.7 | 3 | 3.0 |
| Other organs | 81 | 13.9 | 66 | 13.7 | 15 | 15.0 |
| Distant nodes & Organs | 19 | 3.3 | 17 | 3.5 | 2 | 2.0 |
| Radical cystectomy | ||||||
| No | 434 | 74.6 | 362 | 75.1 | 72 | 72.0 |
| Yes | 148 | 25.4 | 120 | 24.9 | 28 | 28.0 |
| Radiation | ||||||
| No evidence of radiation | 411 | 70.6 | 340 | 70.5 | 71 | 71.0 |
| Yes | 171 | 29.4 | 142 | 29.5 | 29 | 29.0 |
| Chemotherapy | ||||||
| Yes | 372 | 63.9 | 303 | 62.9 | 69 | 69.0 |
| No evidence of chemotherapy | 210 | 36.1 | 179 | 37.1 | 31 | 31.0 |
| Metastasectomy | ||||||
| No | 572 | 98.3 | 476 | 98.8 | 96 | 96.0 |
| Yes | 10 | 1.7 | 6 | 1.2 | 4 | 4.0 |
| Tumor size | ||||||
| ≤8.3 cm | 352 | 60.5 | 277 | 57.5 | 75 | 75.0 |
| >8.3 cm | 30 | 5.2 | 26 | 5.4 | 4 | 4.0 |
| Unknown | 200 | 34.4 | 179 | 37.1 | 21 | 21.0 |
| Lymph node ratio | ||||||
| No lymphadenectomy | 429 | 73.7 | 357 | 74.1 | 72 | 72.0 |
| LNR=0 | 98 | 16.8 | 79 | 16.4 | 19 | 19.0 |
| 0<LNR≤0.46 | 40 | 6.9 | 33 | 6.8 | 7 | 7.0 |
| LNR>0.46 | 15 | 2.6 | 13 | 2.7 | 2 | 2.0 |
LNR, lymph node ratio
Univariate Cox regression analysis for OS and CSS in SCCB patients from the training cohort
| Characteristics | Overall survival | Bladder cancer specific survival | |||
|---|---|---|---|---|---|
| Hazard ratio (95%CI) | p value | Hazard ratio (95%CI) | p value | ||
| Age at diagnosis | 1.028(1.018-1.038) | <0.001 | 1.025(1.014-1.036) | <0.001 | |
| Gender | |||||
| Male | Reference | Reference | |||
| Female | 0.886(0.682-1.151) | 0.364 | 1.051(0.797-1.386) | 0.724 | |
| Marital status | |||||
| Married | Reference | Reference | |||
| Unmarried | 1.317(1.059-1.644) | 0.015 | 1.245(0.975-1.591) | 0.079 | |
| Unknown | 1.584(0.901-2.784) | 0.110 | 1.568(0.850-2.895) | 0.150 | |
| T stage | |||||
| Tis | 0.590(0.081-4.286) | 0.602 | 0.841(0.115-6.169) | 0.864 | |
| T1 | Reference | Reference | |||
| T2 | 1.166(0.842-1.614) | 0.355 | 1.440(0.974-2.127) | 0.067 | |
| T3 | 1.304(0.881-1.930) | 0.184 | 1.621(1.026-2.560) | 0.038 | |
| T4 | 2.048(1.358-3.087) | 0.001 | 2.558(1.591-4.112) | <0.001 | |
| Metastases | |||||
| M0 | 0.238(0.139-0.405) | <0.001 | 0.267(0.144-0.496) | <0.001 | |
| Distant LNs only | 0.323(0.151-0.917) | 0.032 | 0.360(0.125-1.042) | 0.059 | |
| Organs other than LNs | 0.666(0.377-1.175) | 0.161 | 0.832(0.435-1.594) | 0.580 | |
| LNs & Organs | References | References | |||
| Radical cystectomy | |||||
| No | Reference | Reference | |||
| Yes | 0.536(0.409-0.704) | <0.001 | 0.564(0.419-0.760) | <0.001 | |
| Radiation | |||||
| No evidence of radiation | Reference | Reference | |||
| Yes | 0.790(0.624-1.001) | 0.051 | 0.727(0.557-0.948) | 0.018 | |
| Chemotherapy | |||||
| No evidence of chemotherapy | Reference | Reference | |||
| Yes | 0.499(0.403-0.619) | <0.001 | 0.495(0.390-0.629) | <0.001 | |
| Tumor size (cm) | |||||
| ≤8.3 | Reference | Reference | |||
| >8.3 | 1.728(1.099-2.716) | 0.018 | 1.805(1.120-2.909) | 0.015 | |
| Unknown | 1.251(1.001-1.562) | 0.049 | 1.139(0.888-1.463) | 0.305 | |
| LNR | |||||
| No lymphadenectomy | 0.666(0.364-1.220) | 0.188 | 0.683(0.350-1.334) | 0.264 | |
| LNR=0 | 0.293(0.148-0.578) | <0.001 | 0.308(0.145-0.653) | 0.002 | |
| 0<LNR≤0.46 | 0.524(0.256-1.073) | 0.077 | 0.584(0.267-1.277) | 0.178 | |
| LNR>0.46 | Reference | Reference | |||
| Pathology | |||||
| Small cell | Reference | Reference | |||
| Combined small cell | 0.858(0.546-1.349) | 0.507 | 0.870(0.525-1.441) | 0.588 | |
| Metastasectomy | |||||
| No | Reference | Reference | |||
| Yes | 0.808(0.301-2.173) | 0.673 | 1.100(0.409-2.954) | 0.851 | |
| N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 1.109(0.782-1.571) | 0.562 | 1.260(0.873-1.820) | 0.217 | |
| N2 | 1.409(0.997-1.992) | 0.052 | 1.505(1.035-2.187) | 0.032 | |
| N3 | 2.407(0.991-5.849) | 0.052 | 2.346(0.870-6.328) | 0.092 | |
CI, confidence interval; LNs, lymph nodes; LNR, lymph node ratio
Prognostic nomograms for OS and CSS
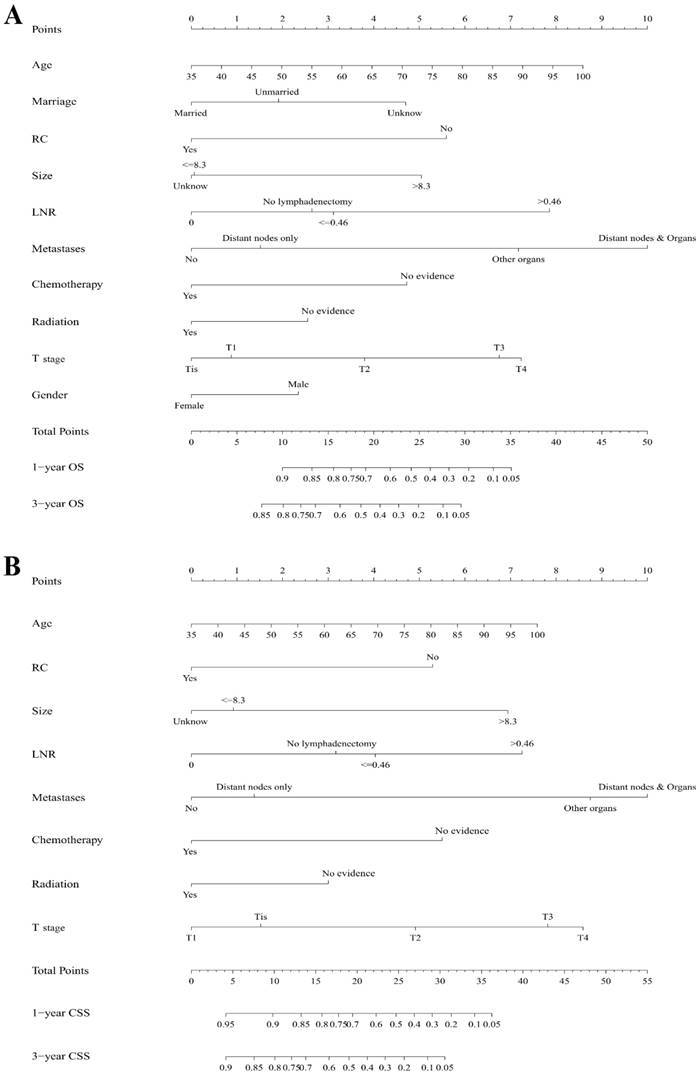
As selected by the AIC, the above-mentioned covariates were employed in the nomograms for 1- and 3-year OS (Figure 1A) and CSS (Figure 1B). A certain score was assigned to each subtype of the variables. By adding up the total score from all the variables and locating it to the total point scale, we could determine the probabilities of the outcomes by drawing a vertical line to the total score. Detailed scores of each nomogram predictor were listed in Table 5.
Multivariate Cox regression analysis for OS in SCCB patients from the training cohort
| Characteristics | Full | AIC-based | |||
|---|---|---|---|---|---|
| Hazard ratio (95%CI) | p value | Hazard ratio (95%CI) | p value | ||
| Age at diagnosis | 1.017(1.007-1.028) | 0.001 | 1.017(1.007-1.028) | 0.001 | |
| Gender | |||||
| Male | Reference | Reference | |||
| Female | 0.732(0.550-0.9794) | 0.032 | 0.738(0.556-0.979) | 0.035 | |
| Marital status | |||||
| Married | Reference | Reference | |||
| Unmarried | 1.289(1.005-1.653) | 0.045 | 1.304(1.020-1.668) | 0.049 | |
| Unknown | 1.654(0.880-3.111) | 0.118 | 1.725(0.942-3.158) | 0.044 | |
| T stage | |||||
| Tis | 0.867(0.118-6.391) | 0.889 | 0.884(0.120-6.509) | 0.903 | |
| T1 | Reference | Reference | |||
| T2 | 1.424(1.013-2.002) | 0.042 | 1.452(1.036-2.035) | 0.030 | |
| T3 | 2.098(1.336-3.293) | 0.001 | 2.134(1.362-3.343) | 0.001 | |
| T4 | 2.211(1.412-3.463) | 0.001 | 2.254(1.445-3.517) | <0.001 | |
| Metastases | |||||
| M0 | 0.296(0.165-0.530) | <0.001 | 0.288(0.163-0.511) | <0.001 | |
| Distant LNs only | 0.325(0.123-0.853) | 0.018 | 0.358(0.141-0.909) | 0.031 | |
| Organs other than LNs | 0.717(0.396-1.299) | 0.273 | 0.719(0.397-1.305) | 0.278 | |
| LNs & Organs | References | References | |||
| Radical cystectomy | |||||
| No | Reference | Reference | |||
| Yes | 0.492(0.285-0.851) | 0.011 | 0.482(0.278-0.835) | 0.009 | |
| Radiation | |||||
| No evidence of radiation | Reference | Reference | |||
| Yes | 0.714(0.550-0.929) | 0.012 | 0.716(0.551-0.930) | 0.012 | |
| Chemotherapy | |||||
| No evidence of chemotherapy | Reference | Reference | |||
| Yes | 0.534(0.418-0.683) | <0.001 | 0.547 (0.430-0.696) | <0.001 | |
| Tumor size | |||||
| ≤8.3 | Reference | Reference | |||
| >8.3 | 1.813(1.111-2.958) | 0.017 | 1.857(1.149-3.000) | 0.012 | |
| Unknown | 0.991(0.783-1.253) | 0.947 | 0.990(0.784-1.251) | 0.936 | |
| LNR | |||||
| No lymphadenectomy | 0.555(0.220-1.401) | 0.213 | 0.500(0.211-1.180) | 0.114 | |
| LNR=0 | 0.397(0.175-0.898) | 0.027 | 0.354(0.171-0.731) | 0.005 | |
| 0<LNR≤0.46 | 0.544(0.249-1.190) | 0.127 | 0.538(0.248-1.163) | 0.115 | |
| LNR>0.46 | Reference | Reference | |||
| Pathology | Not selected | ||||
| Small cell | Reference | — | — | ||
| Combined small cell | 0.836(0.528-1.322) | 0.443 | — | — | |
| Metastasectomy | Not selected | ||||
| No | Reference | — | — | ||
| Yes | 0.811(0.289-2.280) | 0.692 | — | — | |
| N stage | Not selected | ||||
| N0 | Reference | — | — | ||
| N1 | 1.112(0.720-1.712) | 0.632 | — | — | |
| N2 | 1.095(0.676-1.776) | 0.712 | — | — | |
| N3 | 1.562(0.562-4.343) | 0.393 | — | — | |
CI, confidence interval; LNs, lymph nodes; LNR, lymph node ratio
Multivariate Cox regression analysis for CSS in SCCB patients from the training cohort
| Characteristics | Full | AIC-based | |||
|---|---|---|---|---|---|
| Hazard ratio (95%CI) | p value | Hazard ratio (95%CI) | p value | ||
| Age at diagnosis | 1.014(1.002-1.025) | 0.020 | 1.014(1.003-1.026) | 0.014 | |
| Gender | Not selected | ||||
| Male | Reference | — | — | ||
| Female | 0.931(0.685-1.266) | 0.651 | — | — | |
| Marital status | Not selected | ||||
| Married | Reference | — | — | ||
| Unmarried | 1.142(0.866-1.506) | 0.348 | — | — | |
| Unknown | 1.564(0.769-3.182) | 0.217 | — | — | |
| T stage | |||||
| Tis | 1.230(0.164-9.202) | 0.840 | 1.198(0.160-8.949) | 0.860 | |
| T1 | Reference | Reference | |||
| T2 | 1.808(1.204-2.715) | 0.004 | 1.831(1.227-2.735) | 0.003 | |
| T3 | 2.591(1.542-4.352) | <0.001 | 2.609(1.562-4.360) | <0.001 | |
| T4 | 2.828(1.695-4.720) | <0.001 | 2.884(1.733-4.799) | <0.001 | |
| Metastases | |||||
| M0 | 0.337(0.172-0.659) | 0.001 | 0.303(0.157-0.586) | <0.001 | |
| Distant LNs only | 0.339(0.110-1.041) | 0.059 | 0.364(0.123-1.078) | 0.068 | |
| Organs other than LNs | 0.920(0.467-1.813) | 0.809 | 0.883(0.449-1.738) | 0.719 | |
| LNs & Organs | References | References | |||
| Radical cystectomy | |||||
| No | Reference | Reference | |||
| Yes | 0.502(0.271-0.930) | 0.028 | 0.518(0.280-0.959) | 0.036 | |
| Radiation | |||||
| No evidence of radiation | Reference | Reference | |||
| Yes | 0.672(0.501-0.900) | 0.008 | 0.688(0.515-0.918) | 0.011 | |
| Chemotherapy | |||||
| No evidence of chemotherapy | Reference | Reference | |||
| Yes | 0.496(0.379-0.651) | <0.001 | 0.508 (0.391-0.661) | <0.001 | |
| Tumor size | |||||
| ≤8.3 | Reference | Reference | |||
| >8.3 | 1.980(1.177-3.332) | 0.010 | 2.051(1.238-3.400) | 0.005 | |
| Unknown | 0.892(0.686-1.159) | 0.392 | 0.894(0.688-1.161) | 0.400 | |
| LNR | |||||
| No lymphadenectomy | 0.718(0.258-1.996) | 0.525 | 0.595(0.229-1.549) | 0.288 | |
| LNR=0 | 0.493(0.202-1.200) | 0.119 | 0.402(0.181-0.895) | 0.026 | |
| 0<LNR≤0.46 | 0.629(0.268-1.475) | 0.286 | 0.671(0.290-1.556) | 0.353 | |
| LNR>0.46 | Reference | Reference | |||
| Pathology | Not selected | ||||
| Small cell | Reference | — | — | ||
| Combined small cell | 0.842(0.505-1.403) | 0.508 | — | — | |
| Metastasectomy | Not selected | ||||
| No | Reference | — | — | ||
| Yes | 1.071(0.384-2.985) | 0.896 | — | — | |
| N stage | Not selected | ||||
| N0 | Reference | — | — | ||
| N1 | 1.342(0.848-2.125) | 0.209 | — | — | |
| N2 | 1.237(0.740-2.067) | 0.417 | — | — | |
| N3 | 1.300(0.403-4.190) | 0.661 | — | — | |
CI, confidence interval; LNs, lymph nodes; LNR, lymph node ratio
Internal and external validation of the nomograms
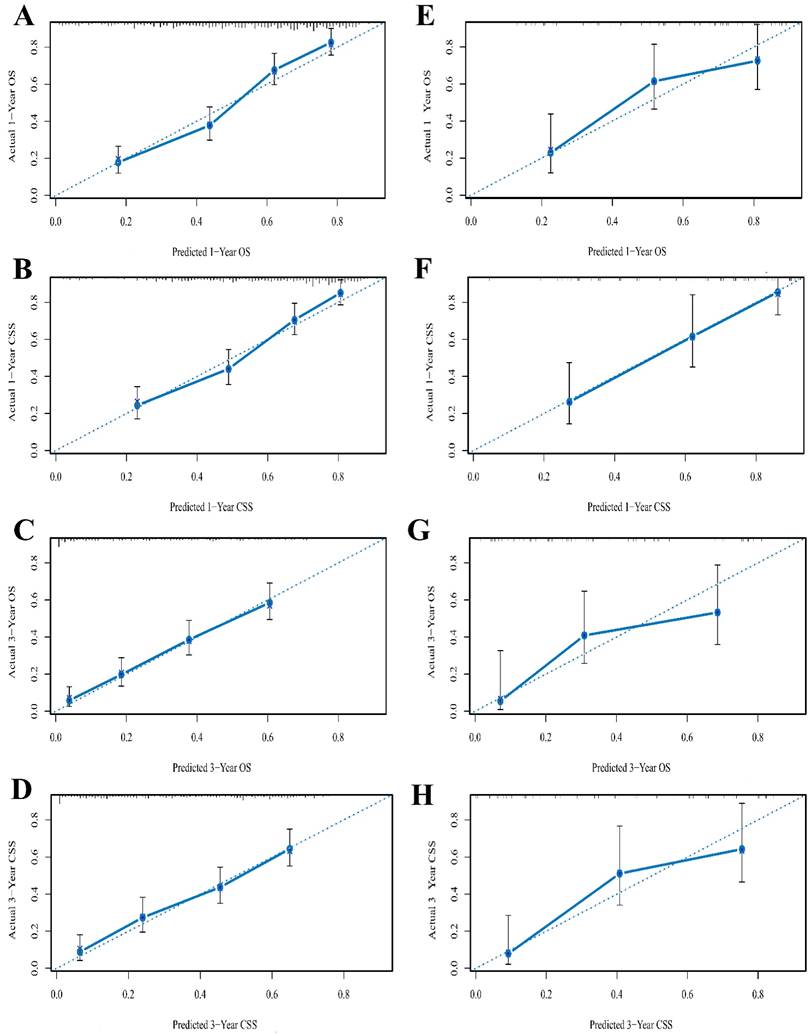
The C-index of the nomograms for predicting OS and CSS were 0.736 (95%CI 0.711-0.761) and 0.731 (95%CI 0.704-0.758) respectively in the training cohort (Table 6). When the validation cohort was applied to the nomograms for OS and CSS, the C-index were 0.713 (95%CI 0.650-0.775) and 0.766 (95%CI 0.708-0.823), respectively, which were all greater than 0.7 [31], indicating the suitability of the established nomograms for patients with SCCB. The C-indices, AIC and log-likelihood values of the nomograms for OS and CSS were all listed in Table 6. Internal and external calibration plots for 1- and 3-year OS and CSS also showed fair agreement between nomograms' prediction and observed outcomes in the training cohort (Figure 2A-D) and validation cohort (Figure 2E-H), suggesting appreciable reliability of the nomograms.
Detailed scores of all predictors in the nomograms
| Variables | Nomogram Points | |
|---|---|---|
| OS | CSS | |
| Gender | ||
| Female | 0 | Not selected |
| Male | 2 | |
| T stage | ||
| Tis | 0 | 2 |
| T1 | 1 | 0 |
| T2 | 4 | 5 |
| T3 | 7 | 8 |
| T4 | 7 | 9 |
| Radiation | ||
| Yes | 0 | 0 |
| No evidence of radiation | 3 | 3 |
| Chemotherapy | ||
| Yes | 0 | 0 |
| No evidence of chemotherapy | 5 | 5 |
| Metastases | ||
| No | 0 | 0 |
| Distant nodes only | 2 | 1 |
| Other organs | 7 | 9 |
| Distant nodes & Organs | 10 | 10 |
| LNR | ||
| No lymphadenectomy | 3 | 3 |
| LNR = 0 | 0 | 0 |
| LNR ≤ 0.46 | 3 | 4 |
| LNR > 0.46 | 8 | 7 |
| Tumor size | ||
| ≤ 8.3 cm | 0 | 1 |
| > 8.3 cm | 5 | 7 |
| Unknown | 0 | 0 |
| Radical cystectomy | ||
| No | 6 | 5 |
| Yes | 0 | 0 |
| Marriage | ||
| Married | 0 | Not selected |
| Unmarried | 2 | |
| Unknown | 5 | |
| Age (continuous variables) | ||
| 35 | 0 | 0 |
| 45 | 1 | 1 |
| 55 | 3 | 2 |
| 65 | 4 | 4 |
| 75 | 5 | 5 |
| 85 | 7 | 6 |
| 95 | 8 | 7 |
LNR, lymph node ratio
Comparison of nomograms with AJCC staging system
We do a comprehensive comparison between SCCB nomograms for OS/CSS and the widely used 7th AJCC staging system. First, the nomograms yielded larger log-likehoods and C-indices along with smaller AIC values for both OS and CSS in all cohorts compared with the AJCC stages (Table 6), with all between-group p values <0.001. These results implied that our nomograms were more robust than the existing AJCC stages in predicting 1- and 3-year survival outcomes.
Comparison of nomograms with 7th AJCC staging system
| Nomogram | AJCC classification | P | |
|---|---|---|---|
| Training cohort, OS | |||
| AIC | 3606 | 3696 | — |
| Log-likelihood | -1784 | -1840 | <0.001 |
| C-index(95% CI) | 0.736(0.711-0.761) | 0.620(0.589-0.651) | <0.001 |
| Training cohort, CSS | |||
| AIC | 2983 | 3045 | — |
| Log-likelihood | -1472 | -1514 | <0.001 |
| C-index(95% CI) | 0.731(0.704-0.758) | 0.633(0.599-0.667) | <0.001 |
| Validation cohort, OS | |||
| AIC | 437 | 464 | — |
| Log-likelihood | -218 | -225 | <0.001 |
| C-index(95% CI) | 0.713(0.650-0.775) | 0.604(0.528-0.680) | <0.001 |
| Validation cohort, CSS | |||
| AIC | 349 | 387 | — |
| Log-likelihood | -174 | -187 | <0.001 |
| C-index(95% CI) | 0.766(0.708-0.823) | 0.607(0.525-0.689) | <0.001 |
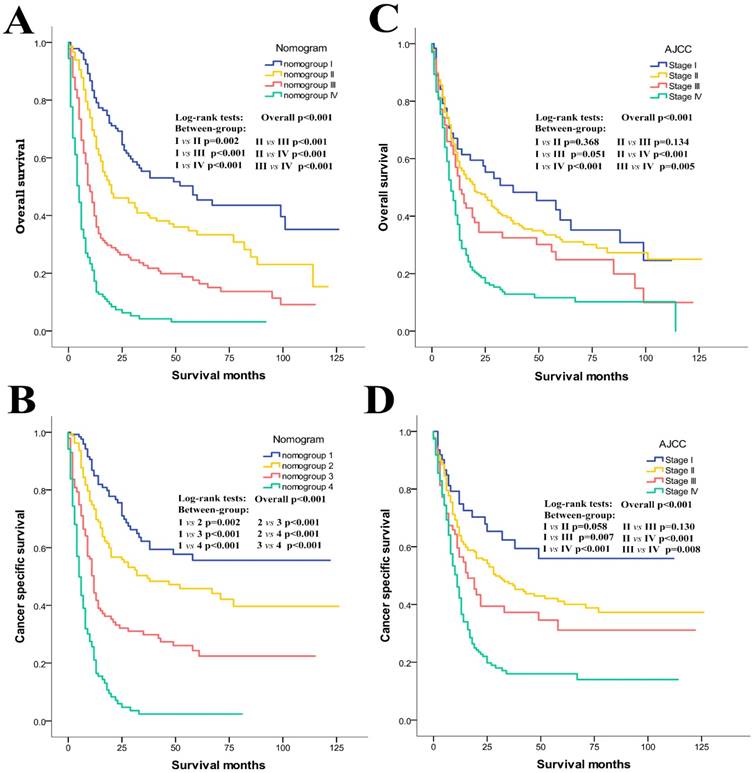
Moreover, as shown in the Kaplan-Meier curves of the primary cohort (Figure 3), although both the AJCC stages and the nomogroups showed good prognostic stratification (p<0.001 for all cases), the nomogroups still showed much better prognostic discrimination than the AJCC stages. For example, the overall survival of AJCC stage I could not be differentiated from those of the stage II (p=0.368) and III (p=0.051), and no difference could be observed between stage II and III (p=0.134). Besides, some overlapping of the survival curves for the AJCC stages was observed (Figure 3C). As for cancer specific survival, the difference of AJCC stage I vs II (p=0.058) and stage II vs III (p=0.130) was still not significant (Figure 3D). However, the nomogroups performed consistently much better for both OS (Figure 3A) and CSS (Figure 3B). They could accurately stratify patients into the 4 risk groups with significant differences in the 1- and 3-year OS and CSS rates (1-/3-year OS rate: 80.8/55.4% in nomogroup I, 67.5/40.9% in nomogroup II, 39.9/22.7% in nomogroup III, 17.2/4.2% in nomogroup IV; 1-/3-year CSS rate: 84.9/66.2% in nomogroup 1, 73.4/49.3% in nomogroup 2, 44.3/29.8% in nomogroup 3, 21.1/2.4% in nomogroup 4).
Nomograms estimating 1- and 3-year (A) overall survival and (B) cancer specific survival of primary SCCB patients. Instruction of the nomograms: Firstly, each covariate of an individual patient is located on the corresponding axis, by drawing a vertical line from that variable to the points scale, we could obtain its point. Second, we need to add up the points of each characteristic to obtain a total point, then by drawing a vertical line from the Total points scale to the 1- and 3-year OS or CSS scale, we can get the estimated probabilities of survival. RC, radical cystectomy; LNR, lymph node ratio; OS, overall survival; CSS, cancer specific survival
The calibration curves of 1- and 3-year OS (A, C) and CSS (B, D) for training cohort and OS (E, G) and CSS (F, H) for validation cohort. Nomogram-predicted probability of survival is plotted on the x-axis, and the actual survival is plotted on the y-axis. Dashed lines through the point of origin represent the perfect calibration models where the predicted probabilities are identical to the actual probabilities. OS, overall survival; CSS, cancer specific survival
Kaplan-Meier curves of risk group stratification for OS and CSS in the primary cohort. A, nomogram for OS; B, nomogram for CSS; C, AJCC staging system for OS; D, AJCC staging system for CSS.
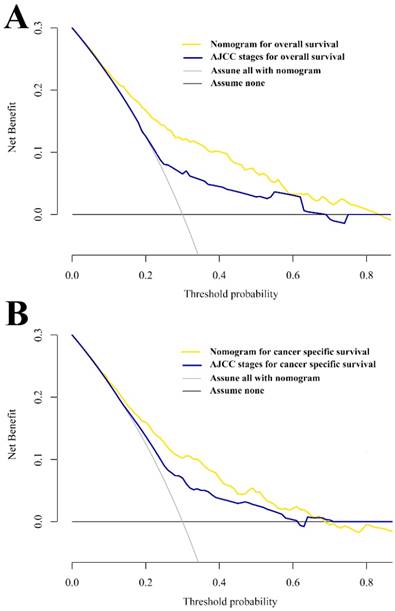
Additionally, decision curve analysis (DCA) was applied to render the clinical validity to the nomograms using the primary cohort. The results strengthened good clinical applicability of the nomograms in predicting OS and CSS of patients with SCCB due to the wide and practical ranges of threshold probabilities (Figure 4). When further comparing to the conventional AJCC stages, the nomograms still showed superiority over the AJCC system based on the fact that the net benefits for patients was enhanced in a rather wide range of threshold probabilities when using the nomograms compared with using the AJCC stages (Figure 4A and 4B).
Discussion
In the present study, using a large patient cohort of SCCB from the SEER dataset, we establish two novel, comprehensive and convenient nomograms for estimating individual overall survival and cancer specific survival outcomes for patients with a very rare cancer—primary SCCB. The nomograms showed satisfactory accuracy and robustness when applied to both training and validation cohorts, which indicated good clinical applicability of the nomograms for this rare genitourinary tumor.
Decision curve analysis of nomograms and AJCC staging system for predicting OS (A) and CSS (B).
Distinct from urothelial bladder cancer, several unique features of primary SCCB should be mentioned. First, the extremely low incidence led to blurry understanding of SCCB [9]. Besides, the majority of SCCB patients presented with either locally advanced or metastatic disease [5], which was associated with very poor prognosis [5, 14]. Naito et al reviewed 31 patients with primary SCCB between 2001 and 2014, and showed a median survival time of only 12.7 months, which was in line with the results of our study. As for treatment, management of primary SCCB involves multimodal methods [32]. Up till now there are no standard guidelines of SCCB and no multicenter randomized controlled trials are available to guide treatment decisions. Hence, patients' survival might be significantly influenced by their choices of therapies. Owing to the particularity of this malignancy, so far there was no staging system or predicting model that was specially designed for primary SCCB or was widely accepted. Currently, the 7th AJCC staging system based on T, N and M information for urothelial cancers was also applied to SCCB. However, Mackey et al did not observe any correlation between AJCC staging and patient's survival when 106 SCCB patients were analyzed retrospectively [17]. Koay et al also revealed obvious overlapping of the survival curves of the I-IV AJCC stages [5], suggesting that SCCB should be differently staged in comparison with other bladder malignancies. Additionally, the staging systems of AJCC that depend solely on pathological characteristics retain limited prognostic impact on SCCB because of ignoring the effect of diverse treatments on patients' survival.
Prognostic nomograms are the visualization of complicated statistical model that were used to predicting individual survival outcomes, and numerous advantages were observed in prognostic nomograms including good accuracy, user-friendliness and comprehensibility, allowing for wide application in clinical practice [18, 19, 22]. As for bladder malignancies, several nomograms had been established for patients with urothelial bladder cancer [33-35]. Using population-based data, our study for the first time applied the nomograms to evaluating the prognosis of patients with primary small cell bladder cancer, which might be considered an update and extension of the previously published researches that also used population-based data but failed to establish a robust prognostic prediction model for SCCB patients [5, 36]. Via the establishment of two nomograms, we validated some clinicopathological factors as important prognostic predictors for OS and CSS. Some of these predictors are worth noting here. Age at diagnosis showed strong impact on both OS and CSS. This finding was also revealed in Koay et al's study, in which age at diagnosis was identified as an independent prognostic factor for OS [5]. Based on the AIC values, gender was included in the OS nomogram but not in the CSS nomogram, and male patients was found to have a significantly greater risk of overall mortality compared with female. The role of gender in predicting prognosis of SCCB patients seemed to be totally different from that in patients with urothelial bladder cancer. Numerous studies proved that female patients were associated with higher cancer specific mortality in urothelial bladder cancer [37-39]. Such disparity might be a reflect of differences between SCCB and UBC in genetic, hormonal, societal, and environmental factors, which all had significant impact on gender-related cancer prognosis [40]. Additionally, this is the first time that marital status was introduced to prognostic nomogram for SCCB. In the present study, marriage was proved to make a prognostic difference and deserved more attention since it might include complicated mechanisms for the enhancement of overall survival.
It's reasonable to deduce that some tumor-related factors like infiltration depth, metastatic status, lymph nodes status and tumor size were to some extent associated to overall survival and cancer specific survival. They are typical features of tumor development and are closely related to patient death at various but statistically significant levels. The AJCC staging system is based on three elements: T stage which reflects infiltration depth, N stage which reflects nodes status and M stage which reflects metastatic status. Our nomograms also include T stage of SCCB and the roles it played in nomograms were similar to those in the AJCC classification (T1 represented the best prognosis while T4 represented the worst). As for the metastatic status, previous study had already pointed out that in SCCB patients, distant lymph nodes were much more common metastatic foci compared with other organs [10]. Our study innovatively subdivided the M1 stage into three different levels: 1) distant nodes only; 2) other organs and 3) distant node & organs, which were consequently proved to have different prognostic value and were all embedded in the two nomograms together with M0 stage. Lymph node status was one of the most controversial among these tumor-related factors in our study. Out of our expectation, the present N classification was not an independent prognostic indicator for OS and CSS and was not included in our nomograms via the AIC-based selections of factors. Lymph node ratio (LNR) has been proposed as a quality indicator in urothelial bladder cancer and might has superiority over the N staging system [41-43]. In view of LNR's potential value in predicting prognosis, we paid a special attention to the roles it played in our study. According to the results of multivariate analysis, patients with LNR=0 had a significantly better OS and CSS than patients with LNR>0.46, indicating its applicability in SCCB. Simultaneously, the AIC-based selection incorporated LNR into the established nomograms, which became the representative of lymph node status and made up for the absence of N stages.
The proposed nomograms included three treatment factors: radical cystectomy, chemotherapy and radiation, which performed well in predicting the survival outcomes of SCCB patients. Among all therapies, chemotherapy as adjuvant or neoadjuvant therapy still appeared to be the major treatment modality for SCCB patients [8]. The common regimen for SCCB is platinum based chemotherapy which was associated with significantly improved survival [17]. Moreover, radiation was also an option for some patients. Several previous studies showed the benefits of the combination of radiation with chemotherapy [12, 44]. A literature review in 2015 concluded that radiation seemed to work only when given in a sequential manner following chemotherapy [32]. We gave this issue a supplementary explanation that according to the results of our multivariate analyses, radiation seemed to be a totally independent prognostic factor for both OS and CSS. Hence, further studies are needed to fully reveal the therapeutic roles of radiation in SCCB and to discover its synergistic effect with chemotherapy as well as surgeries. In 2004, Cheng et al asserted that radical cystectomy alone had no curative function in the majority of cases [9]. However, a Mayo Clinic study found that if metastatic disease is absent, RC should be performed [10]. Our study also confirmed the significant prognostic value of RC. It is now believed that neo adjuvant chemotherapy followed by surgery appears to be the optimal treatment, since it could completely resect all tumor burden, downstage the cancer and improve patients' survival [7, 10, 45].
The C-index, log-likehood value and calibration curves were regular methods to do the validation of a nomogram [20, 30, 31]. Our study also produced some novel results using various methods. Above all, based on the nomograms of OS and CSS, four nomogroups were established and could efficiently classified patients into different risk groups with a single predictive score. Such risk classification was even superior to the current AJCC classification and might be quite useful for clinicians to identify patients with high risks in order to give intensified follow-up. The nomogroups also helped to figure out the degree of survival heterogeneity in AJCC stages, which frequently brought confusion and uncertainty to patient consulting. We need to realize that the optimal thresholds for risk classification of the nomograms might be individualized, so further validations and optimization of our nomograms by us as well as other urologists all over the world are definitely needed to maximize nomograms' clinical potential. Besides, we should note that high predictive accuracy does not necessarily mean better usefulness in clinical practice. In fact, well performed models might also have limited applicability when the threshold probabilities of the net benefits are impractical [18, 29]. Therefore, we introduced DCA which was recommended by previous studies and was used in evaluating nomograms [35, 46-48]. The results proved the clinical validity of our nomograms.
Generally speaking, the new nomograms in our study are innovative in the following aspects. First and most importantly, no prognostic nomogram has been designed for SCCB patients before. We established the first two nomograms for these patients and made the individualized prediction of prognosis become possible. Second, we revealed that the current AJCC stages for bladder cancer was actually not quite suitable for small cell carcinoma. Hence, we established a novel nomogroup system based on the scores of the nomogram and further proved that the new-established nomogroup could easily divided patients into different risk stratifications. Third, enlightened by one of our previous study [49], we for the first time revealed that in SCCB patients, distant lymph node metastasis represented better survival outcomes compared with other organ metastases. Hence, in the “metastasis” item of the nomograms, we creatively divided it into four categories (M0; distant node only; other organs; distant node & other organs), which increased the predictive accuracy. Fourth, we for the first time proved that lymph node ratio was more suitable as a node-related index for SCCB patients in terms of prognosis prediction, and was imbedded into our nomograms. Fifth, as we mentioned above, it was the first time that marital status was shown to have prognostic value for SCCB patients and was included into the nomograms for OS prediction. Last but not least, decision curve analysis, a relatively new approach for analyzing net benefit, was applied to our nomograms and revealed that the new nomograms had wider clinical applicability than the current AJCC stages.
Although to the best of our knowledge, these are the first two prognostic nomograms for OS and CSS in primary SCCB patients using population-based data, some potential limitations should still be considered. The major limitation came from the SEER dataset itself. For example, the recently updated SEER dataset used “No/Unknown” as one inseparable group in terms of chemotherapy and radiation, which referred to patients with no evidence of chemotherapy or radiation in the medical records. This led to the limitations of the completeness of the variables and might cause other relevant bias. To show our acknowledgement and understanding of the limitations of the SEER radiation and chemotherapy data, we specially included the description about this issue in our study and used group named “no evidence” rather than “no” as the counterpart of “yes” for chemotherapy and radiation. As for the establishment of nomograms, improved model accuracy sometimes comes at the cost of increased complexity. It's not easy to balance the tradeoffs between comprehensiveness and comprehensibility, which is a common issue in developing new nomograms. Considering this, we only chose variables that were clinically essential and practical with high reproducibility and low time-varying effects. Moreover, because of the limited number of patients in the validation cohort, the calibration curves of 3-year CSS showed slightly reduced level of agreement. Frankly speaking, it's a recognized problem that the validation cohort of nomograms for rare diseases sometimes contained too few people [24]. For the purpose of minimizing the bias caused by limited validation cases, the validation cohort was set with 100 SCCB patients which was a recommended standard by previous study [50]. Additionally, the study was conducted retrospectively and selection bias might exist.
Conclusion
In conclusion, we established and validated the first two nomograms predicting individual overall and cancer specific survival for patients with primary SCCB. The proposed nomograms in our study showed consistently reliability and clinically practicality with wide threshold probabilities. Besides, the nomograms outperformed the current AJCC classification and offered a useful tool for patient counseling and clinical assessments. Further external valuations by other independent patient groups are still required.
Abbreviations
OS: overall survival; CSS: cancer specific survival; SCCB: small cell carcinoma of the bladder; AJCC: American Joint Committee on Cancer; SEER: Surveillance, Epidemiology, and End Results; C-index: concordance index; CI: confidence interval; DCA: decision curve analysis; LNR: lymph node ratio; AIC: akaike information criterion; AUC: area under curve; ROC: receiver operating characteristic; RC: radical cystectomy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81472379 & No.81702507), the Leading Talent Project of Shanghai and Zhejiang Provincial Natural Science Foundation of China (Grant No. LY16H160016). We thank Dr. Josephine Lu (Education faculty, Monash University) for helping to edit English language.
Data access
We obtained permission to access SEER dataset with the reference number 11587-Nov2016. Extraction of data from the SEER database does not require informed consent.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. Histopathology. 2005;46:57-63
2. Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT. et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol. 2005;166:1533-9
3. Blomjous CE, Vos W, De Voogt HJ, Van der Valk P, Meijer CJ. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989;64:1347-57
4. Lopez JI, Angulo JC, Flores N, Toledo JD. Small cell carcinoma of the urinary bladder. A clinicopathological study of six cases. Br J Urol. 1994;73:43-9
5. Koay EJ, Teh BS, Paulino AC, Butler EB. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer. 2011;117:5325-33
6. Bex A, Nieuwenhuijzen JA, Kerst M, Pos F, van Boven H, Meinhardt W. et al. Small cell carcinoma of bladder: a single-center prospective study of 25 cases treated in analogy to small cell lung cancer. Urology. 2005;65:295-9
7. Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL. et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481-4
8. Thota S, Kistangari G, Daw H, Spiro T. A clinical review of small-cell carcinoma of the urinary bladder. Clin Genitourin Cancer. 2013;11:73-7
9. Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H. et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer. 2004;101:957-62
10. Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 2005;103:1172-8
11. Agheli A, Arora A, Kodali S, Kalavar M. Pure small cell carcinoma of the urinary bladder. Clin Adv Hematol Oncol. 2008;6:380-4 discussion 5-6
12. Bastus R, Caballero JM, Gonzalez G, Borrat P, Casalots J, Gomez de Segura G. et al. Small cell carcinoma of the urinary bladder treated with chemotherapy and radiotherapy: results in five cases. Eur Urol. 1999;35:323-6
13. Grignon DJ, Ro JY, Ayala AG, Shum DT, Ordonez NG, Logothetis CJ. et al. Small cell carcinoma of the urinary bladder. A clinicopathologic analysis of 22 cases. Cancer. 1992;69:527-36
14. Pasquier D, Barney B, Sundar S, Poortmans P, Villa S, Nasrallah H. et al. Small Cell Carcinoma of the Urinary Bladder: A Retrospective, Multicenter Rare Cancer Network Study of 107 Patients. Int J Radiat Oncol Biol Phys. 2015;92:904-10
15. Chen Z, Liu Q, Chen R, Liu Z, Li M, Ling Q. et al. Clinical analysis of small cell carcinoma of the bladder in Chinese: nine case reports and literature reviews. World J Surg Oncol. 2017;15:33
16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FI, Trotti A. Urinary bladder. AJCC Cancer Staging Manual, 7th ed. NewYork: Springer. 2010:494-505
17. Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998;159:1624-9
18. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-80
19. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-70
20. Shi X, Hu WP, Ji QH. Development of comprehensive nomograms for evaluating overall and cancer-specific survival of laryngeal squamous cell carcinoma patients treated with neck dissection. Oncotarget. 2017;8:29722-40
21. Pan H, Shi X, Xiao D, He J, Zhang Y, Liang W. et al. Nomogram prediction for the survival of the patients with small cell lung cancer. J Thorac Dis. 2017;9:507-18
22. Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14:4400-7
23. Nguyen CT, Stephenson AJ, Kattan MW. Are nomograms needed in the management of bladder cancer? Urol Oncol. 2010;28:102-7
24. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z. et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-95
25. Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24:3819-20
26. Mariani L, Miceli R, Kattan MW, Brennan MF, Colecchia M, Fiore M. et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402-8
27. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-87
28. Frank E, Harrell Jr. Harrell Miscellaneous. R Package version 3.9-2. https://CRAN.R-project.org/package=Hmisc. Accessed 10 Feb. 2012
29. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-74
30. Zhang ZY, Luo QF, Yin XW, Dai ZL, Basnet S, Ge HY. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer. 2016;16:658
31. Wan G, Gao F, Chen J, Li Y, Geng M, Sun L. et al. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer. 2017;17:91
32. Shatagopam K, Kaimakliotis HZ, Cheng L, Koch MO. Genitourinary small cell malignancies: prostate and bladder. Future Oncol. 2015;11:479-88
33. Al-Daghmin A, English S, Kauffman EC, Din R, Khan A, Syed JR. et al. External validation of preoperative and postoperative nomograms for prediction of cancer-specific survival, overall survival and recurrence after robot-assisted radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2014;114:253-60
34. Brooks M, Godoy G, Sun M, Shariat SF, Amiel GE, Lerner SP. External Validation of Bladder Cancer Predictive Nomograms for Recurrence, Cancer-Free Survival and Overall Survival following Radical Cystectomy. J Urol. 2016;195:283-9
35. Ishioka J, Saito K, Sakura M, Yokoyama M, Matsuoka Y, Numao N. et al. Development of a nomogram incorporating serum C-reactive protein level to predict overall survival of patients with advanced urothelial carcinoma and its evaluation by decision curve analysis. Br J Cancer. 2012;107:1031-6
36. Geynisman DM, Handorf E, Wong YN, Doyle J, Plimack ER, Horwitz EM. et al. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med. 2016;5:192-9
37. Tracey E, Roder D, Luke C, Bishop J. Bladder cancer survivals in New South Wales, Australia: why do women have poorer survival than men? BJU Int. 2009;104:498-504
38. Jeldres C, Isbarn H, Capitanio U, Zini L, Bhojani N, Shariat SF. et al. Development and external validation of a highly accurate nomogram for the prediction of perioperative mortality after transurethral resection of the prostate for benign prostatic hyperplasia. J Urol. 2009;182:626-32
39. Tilki D, Reich O, Svatek RS, Karakiewicz PI, Kassouf W, Novara G. et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. J Urol. 2010;183:1757-63
40. Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI. et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29:457-63
41. Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943-5
42. Osawa T, Abe T, Shinohara N, Harabayashi T, Sazawa A, Kubota K. et al. Role of lymph node density in predicting survival of patients with lymph node metastases after radical cystectomy: a multi-institutional study. Int J Urol. 2009;16:274-8 discussion 8
43. Wiesner C, Salzer A, Thomas C, Gellermann-Schultes C, Gillitzer R, Hampel C. et al. Cancer-specific survival after radical cystectomy and standardized extended lymphadenectomy for node-positive bladder cancer: prediction by lymph node positivity and density. BJU Int. 2009;104:331-5
44. Bex A, de Vries R, Pos F, Kerst M, Horenblas S. Long-term survival after sequential chemoradiation for limited disease small cell carcinoma of the bladder. World J Urol. 2009;27:101-6
45. Siefker-Radtke AO, Kamat AM, Grossman HB, Williams DL, Qiao W, Thall PF. et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592-7
46. Zastrow S, Brookman-May S, Cong TA, Jurk S, von Bar I, Novotny V. et al. Decision curve analysis and external validation of the postoperative Karakiewicz nomogram for renal cell carcinoma based on a large single-center study cohort. World J Urol. 2015;33:381-8
47. Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW. et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302-11
48. Roupret M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR. et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189:1662-9
49. Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang X. et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res. 2017;9:611-26
50. Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58:475-83
Author contact
![]() Corresponding author: Zhoujun Shen, Department of Urology, Huashan Hospital, Fudan University, Shanghai, China, No.12 Middle Urumqi Road, Shanghai, 200040, China. Tel: +86-21-52888228; Fax: +86-21-62485237; Email address: 11307120113edu.cn
Corresponding author: Zhoujun Shen, Department of Urology, Huashan Hospital, Fudan University, Shanghai, China, No.12 Middle Urumqi Road, Shanghai, 200040, China. Tel: +86-21-52888228; Fax: +86-21-62485237; Email address: 11307120113edu.cn

 Global reach, higher impact
Global reach, higher impact