Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(8):1476-1485. doi:10.7150/jca.23290 This issue Cite
Research Paper
EGFR Target Therapy Combined with Gemox for Advanced Biliary Tract Cancers: a Meta-analysis based on RCTs
1. Department of Medical Oncology, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
2. Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
3. Cancer Institute, the Affiliated People's Hospital of Jiangsu University, Jiangsu, Zhenjiang, China
Received 2017-10-12; Accepted 2018-1-19; Published 2018-4-6
Abstract
Background: Controversy exists regarding whether EGFR-targeted therapy combined with GEMOX (gemcitabine and oxaliplatin) provides additional benefits over GEMOX alone for biliary tract cancer patients. Therefore, this meta-analysis of randomized controlled trials (RCTs) was performed to assess the efficacy and safety of the GEMOX + EGFR-targeted regimen, and subgroup analysis was conducted to identify groups that might benefit from targeted therapy.
Methods: The PubMed, Cochrane Library, and ClinicalTrials.gov registries were searched for published studies. Hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS) were pooled using a fix-effect model. Risk-ratios (RRs) were used to analyse the objective response rate (ORR) and adverse events.
Results: Four RCTs were assessed. GEMOX + EGFR-targeted therapy significantly improved PFS (HR = 0.80, 95% CI 0.66-0.94, P = 0.03) and was associated with a better ORR (RR = 1.52, 95% CI 1.13-2.04, P = <0.01), whereas the TKI group achieved a better ORR in subgroup analysis. Patients with cholangiocarcinoma responded well to the GEMOX + EGFR-targeted regimen, leading to a better ORR (RR = 1.78, 95% CI 1.21-2.61, P = <0.01). Unfortunately, PFS benefits were not translated into OS benefits (HR = 0.92, 95% CI 0.75-1.08, P = 0.39).
Conclusion: GEMOX + EGFR-targeted therapy is a considerable and tolerable treatment option for patients with advanced BTCs, improving both PFS and ORR but not prolonging patient survival. Patients with cholangiocarcinoma would benefit the most from EGFR-targeted therapy.
Keywords: Biliary Tract Cancer, Meta-analysis, Gemcitabine, Oxaliplatin, Target therapy
Introduction
Biliary tract cancers (BTCs) belong to a group of malignancies arising in different parts of the biliary tree and can be divided into intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC), gallbladder cancer (GBCA) and ampullary cancer (ampulla of Vater) according to anatomical location. BTC incidence is much lower than that of any other gastrointestinal neoplasia. The number of Americans who received a BTC diagnosis in 2015 was 10,910, and there were 3,700 deaths. Additionally, BTCs have a higher incidence in Asia, particularly in Thailand, Korea, India, and Japan[1]. Although the incidence and mortality from gallbladder cancer has been decreasing[2, 3], the incidence of cholangiocarcinoma has significantly increased in recent years[4]. The five-year survival rate is estimated to be 5% to 30% when considering all patients[5, 6], and the estimated five-year survival rate varies with stage: 50% for American Joint Committee on Cancer (AJCC) stage I, 30% for stage II, 10% for stage III, and 0% for stage IV[5, 7]. Most patients are unable to receive a complete resection, which is considered the only potentially curative treatment[7, 8]. Therefore, systematic treatment is essential for patients with advanced BTC. G.Q. Zhu performed a network meta-analysis showing that chemotherapy with gemcitabine is the optimal adjuvant treatment, as it has a balanced benefit-toxicity ratio for resected BTC, whereas chemoradiation is more likely to cause toxic effects[9].
Chemotherapy options for BTC patients with unresectable or metastatic disease include gemcitabine-based and fluoropyrimidine-based regimens[1]. The BT22 and ABC 02 studies suggested the potential of gemcitabine and platinum-based regimens as first-line treatments for advanced BTCs[10]. The results of two randomized clinical trials exploring adjuvant chemotherapy, PRODIGE-12/ACCORD-18, assessed the benefits of adjuvant chemotherapy (gemcitabine and oxaliplatin) and found no significant difference in relapse-free survival compared with observation alone (HR = 0.83, 95% CI 0.58-1.19, P = 0.31). The BilCap clinical trial exploring capecitabine compared with observation alone after surgery showed a benefit from capecitabine in terms of overall survival (OS) (HR = 0.71, 95% CI 0.55-0.92, P < 0.01). Based on these results, capecitabine can be considered for standard chemotherapy following surgery for BTC[11, 12].
KRAS mutations and epidermal growth factor receptor (EGFR) overexpression has been observed in many BTC studies[13-15]. EGFR-TKIs and EGFR-mAbs are two main types of EGFR-targeted drugs. Recently, the combination of EGFR-targeted drugs and GEMOX (gemcitabine and oxaliplatin) regimens has demonstrated various clinical trial outcomes in clinical trials[16-18]. It is likely that although BTCs are histologically similar, they have different aetiologies, and recent genomic footprint results have called into question their biological similarity. According to recent reports, KRAS mutations are observed in intra- and extra-hepatic cholangiocarcinoma more frequently than in gallbladder cancer[19-22], which was observed 4-13% in GBCA, 8.3-42% in ICC and 8.6-24.2% in ECC[23]. Additionally, clinical trials might not have generated convincing, conclusive results because of sample limitations or differences between patient groups[24-26].
Therefore, we conducted a meta-analysis based on randomized controlled trials (RCTs) to investigate the effectiveness and safety of the GEMOX + EGFR-targeted regimen in patients with advanced BTC as well as to identify groups that might benefit from targeted therapy.
Materials and methods
Search strategy and study collection
A wide search of the main computerized databases, including the PubMed, Cochrane Library, and ClinicalTrials.gov registries, was performed with both mesh and free text words. The overall search strategy included the following search terms: “Gemcitabine”, “Oxaliplatin”, “Gemox”, “EGFR-TKIs”, “EGFR-mAbs”, “EGFR”, “Target Therapy”, “Biliary Tract Cancers/Carcinomas”, “Bile Duct Cancers/Carcinomas”, and “clinical trial”, with no language or date restrictions. The studies were limited to human subjects and RCTs.
Two of the researchers (Y.Y. and W.G.) evaluated the titles and abstracts of all of the retrieved studies. Both reviewers independently selected trials for inclusion according to prior agreement regarding the study population and intervention. If one of the reviewers determined that an abstract was eligible, then the full text of the article was retrieved and reviewed in detail by all of the reviewers. If disagreements about the inclusion of an article occurred, a third reviewer (H.H.) reviewed the entire article and contributed to a consensus decision. In addition, the reference lists of identified articles were manually checked to include other potentially eligible trials (Fig. 1).
Selection criteria
Well-designed RCTs comparing GEMOX and EGFR-targeted therapy with GEMOX alone were included. The studies were required to clearly report the inclusion and exclusion criteria for patients, the chemotherapy regimens, and the definition and evaluation of prognostic outcomes. The study characteristics and methodological quality of the included trials were then summarized in tables. The risk of bias in the included studies was assessed according to The Cochrane Handbook.
Statistical extraction and analysis
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
We considered sufficient quantitative data as including the necessary descriptive and statistical information to perform a meta-analysis. The study population characteristics, inclusion and exclusion criteria, chemotherapy regimens, adverse event reports and study designs, such as concealment of the sequence of randomization, blinding, application of intention-to-treat analysis, loss of follow-up description, and whether the trial was multi-centric were separately extracted by two reviewers (Y.Y. and W.G.). The data on the PFS, OS and number of patients who had objective response and disease control according to RECIST 1.0 (Response Evaluation Criteria in Solid Tumors 1.0) were collected by two reviewers (Y.Y. and W.G.) from the full publications and supplementary data downloaded from online.
Four RCTs were identified that included 629 patients, of which 623 were evaluated for PFS, OS and adverse events. We calculated OS from survival curves and from data provided in publications that directly reported HRs for OS, as described by Jayne F Tierney[27]. The number of patients used to analyse the ORR was 612. We further divided patients into cholangiocarcinoma and non-cholangiocarcinoma subgroups. Cholangiocarcinoma was described by anatomical location, including intrahepatic (iCCA), perihilar (pCCA) and distal CCA (dCCA) diagnosed throughout the biliary tree, and was typically classified as intrahepatic and extrahepatic cholangiocarcinoma in RCTs. The non-cholangiocarcinoma group included gallbladder carcinomas and ampulla of Vater carcinomas[1]. Only three publications provided sufficient data to analyse ORR by subgroup.
RRs were used to analyse dichotomous variables, including the ORR and adverse events. HRs for PFS and OS, P-values and 95% confidence intervals (95% CIs) were calculated for survival outcomes. The meta-analysis was performed using STATA version 12.0 software (Stata Corporation, College Station, Texas, USA). The studies were considered with a homogeneous and a fixed-effect model, and Mantel-Haenszel's (M-H) method was used to combine and weigh the individual studies. Heterogeneity was evaluated with the Chi2 and I2 values: I2 < 25% reflects a small level of inconsistency, whereas I2 > 50% implies significant inconsistency. For all analyses, P < 0.05 was considered statistically significant[28, 29].
Trails flow diagram.
Characteristics of Included Randomized Controlled Trials
| Study | Phase | NCT No. | Participant | Male | Median age (G+EGFR vs G) | Disease site (G+EGFR vs G) | Outcome | Intervention | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS(HR) | OS(HR) | ORR | Gemox+EGFR | Gemox | |||||||
| Chen.2015 | II | NCT01267344 | 1.Advanced BTC 2.Systemic therapy-naive 3.Adequate organ functions 4.ECOG PS 0 or 1 | 58(47.5%) | 61 vs 59 | I(44 vs 45); E(9 vs 10); G(9 vs 5) | Y | N | Y | Cetuximab | Gemox |
| Lee.2012 | III | NCT01149122 | 1.Advanced BTC 2.No adjuvant treatment <6 mo 3.Adequate organ functions 4.ECOG PS 0 -2 | 170(63.4%) | 59 vs 61 | C(96 vs 84); G(35 vs 47); A(4 vs 2) | Y | Y | Y | Erlotinib | Gemox |
| Leone.2015 | II | NCT01389414 | 1.Unresectable or metastatic biliary tract adenocarcinoma 2.Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2 3.Adequate organ functions 4.Therapy-naive | 32(36.0%) | 63.9 vs 64.2 | I(21 vs 21); E(21 vs 7); G(12 vs 16) | Y | Y | Y | Panitumumab | Gemox |
| Malka.2014 | II | NCT00552149 | 1.Advanced BTC 2.Therapy-naive 3.Adequate organ functions 4.WHO performance status (WHO PS) of 0 or 1 5.Expected life expectancy of more than 3 months | 85(56.7%) | 61 vs 62 | C(62 vs 61); G(11 vs 11); A(1 vs 0) | Y | N | Y | Cetuximab | Gemox |
PFS: Progression-free survival; ORR: Object response rate; I/E/G/C/A=intrahepatic/extraheptatic/gallbladder/cholangiocarcinoma/ampulla of vater; G: Gemox regimen; EGFR: EGFR Targeted therapy;
Results
Description of studies
Four RCTs were available for this analysis, and 623 patients were assessed for PFS, OS and adverse events; four patients in the Vecti-BIL trial and two patients in J. S. Chen's study were not included. Sufficient data for analysing ORR were available for 612 patients, as a BINGO trial with six patients in the chemotherapy plus cetuximab group and 11 patients in the chemotherapy alone group were not assessed for disease response. Two publications reported OS directly, whereas another two publications reported only survival curves. Therefore, we calculated OS from the survival curves and data provided in these publications[27]. One study did not report disease response data for original patient sites; therefore, 536 patients, including 411 cholangiocarcinoma patients and 125 non-cholangiocarcinoma patients, were assessed to compare ORR in the cholangiocarcinoma and non-cholangiocarcinoma groups. The study characteristics are reported in Table 1. Enrolled patient descriptions from the four RCTs were well balanced; this information is summarized in Table 2. Table 3 presents the methodological quality of the included trials. All four studies reported acceptable randomization methods. One study did not describe the allocation of concealment details, and one study did not describe the blinding of patients or outcome assessors. All of the analyses were performed with an intention-to-treat basis. The number of patients lost to follow-up was acceptable (< 10%) in all of the studies.
Efficacy
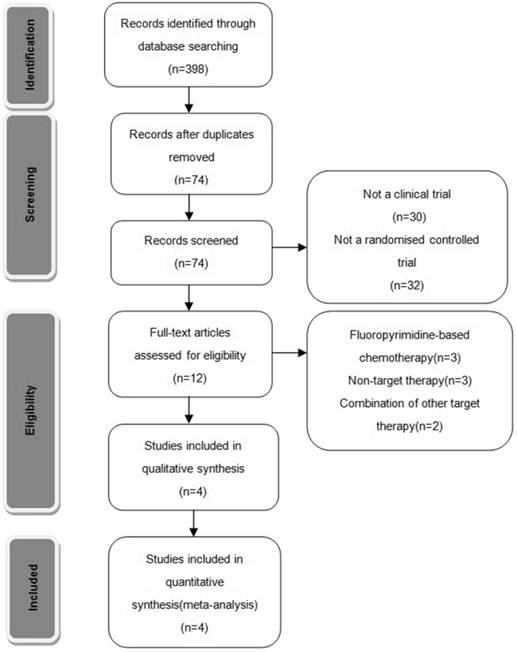
Compared with GEMOX alone, the combination of GEMOX and EGFR-targeted therapy was associated with improved PFS (HR = 0.80, 95% CI 0.66-0.94, P = 0.03), though this did not translate into a larger OS (HR = 0.92, 95% CI 0.75-1.08, P = 0.39). Next, we divided the EGFR-targeting drugs into EGFR-TKI and EGFR-mAb groups and found that heterogeneity between the two groups was not significantly different (P = 0.34 and P = 0.87, respectively) (Fig. 2 and Fig. 3). GEMOX and EGFR-targeted chemotherapy showed a better ORR (RR = 1.52, 95% CI 1.13-2.04, P = 0.006), whereas the EGFR-TKI group achieved a better ORR (Fig. 4).
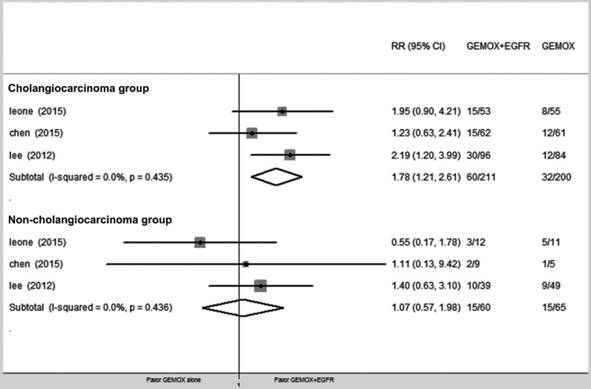
We divided patients into cholangiocarcinoma and non-cholangiocarcinoma groups and evaluated the ORR for each group (RR = 1.78, 95% CI 1.21-2.61, P = 0.003 and RR = 1.07, 95% CI 0.59-1.98, P = 0.840, respectively) (Fig. 5).
Characteristics of Enrolling Patients
| GEMOX +EGFR-targeted | GEMOX | |
|---|---|---|
| Sex | ||
| Female/Male | 139/179(77.65%) | 145/166(87.34%) |
| Primary site | ||
| Non-cholangiocarcinoma/Cholangiocarcinoma | 74/254(29.64%) | 73/228(32.02%) |
| ECOG performance status | ||
| Score 2/Score 0-1 | 10/308(3.25%) | 19/292(6.51%) |
| Disease stage | ||
| Locally advance/ Metastatic | 79/239(33.05%) | 61/250(24.4%) |
Assessment of Methodological Quality of Included Studies
| Reference | Country | Allocation generation | Allocation concealment | Blinding of patients and assessors | Data analysis | Loss to follow-up (%) | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|---|
| Chen et al. | Taiwan | Computer generated | unclear | Assessor blinded | ITT | 3.28 | Low risk | / |
| Lee et al. | Korea | Computer generated | Adequate | Not blinded | ITT | 0.75 | Low risk | Changed the primary endpoint because >90% deaths were caused by disease |
| Leone et al. | Italy | Computer generated | Adequate | unclear | ITT | 5.62 | Low risk | / |
Malka et al. | France Germany | Computer generated | Adequate | Not blinded | ITT | 2.00 | Low risk | / |
Risk of bias was assessed according to the method recommended by the Cochrane
Forest plot of comparison between Gemox-targeted and GEMOX alone; the outcome was hazard ratio (HR) of progression free survival (PFS).
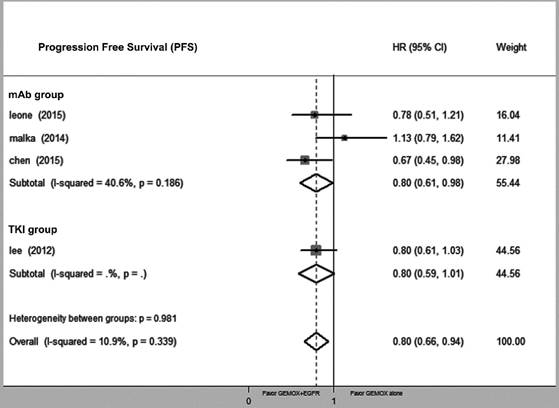
Forest plot of comparison between Gemox-targeted and GEMOX alone; the outcome was hazard ratio (HR) of overall survival (OS).
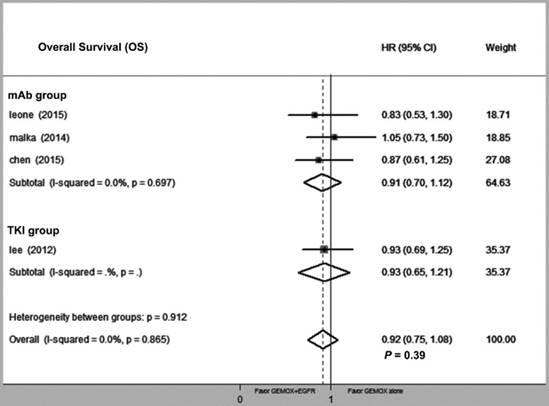
Forest plot of comparison between GEMOX-targeted and GEMOX alone; the outcome was risk ratio (RR) of objective response rate (ORR).
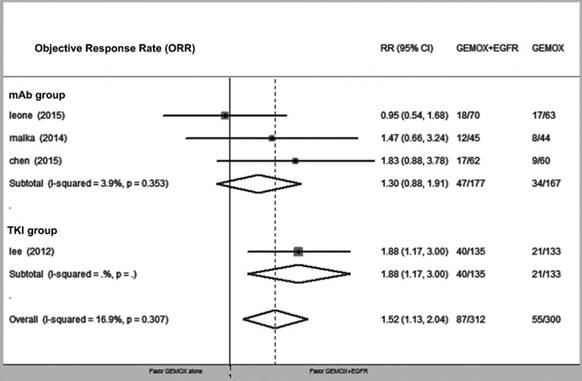
Forest plot of comparison between GEMOX-targeted and Gemox alone in subgroup of cholangiocarcinomas and non-cholangiocarcinomas, risk ratio (RR) of objective response rate (ORR).
Toxicity
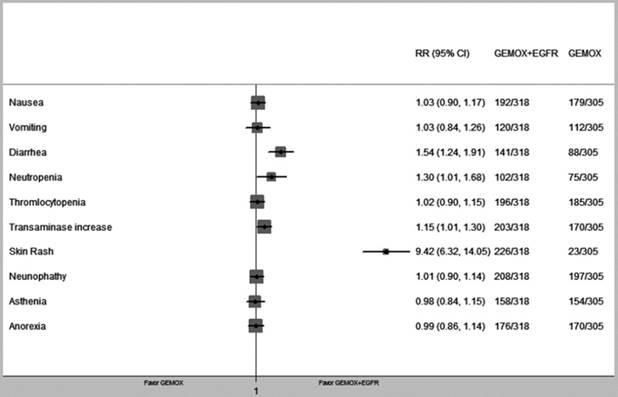
We pooled nine types of commonly occurring adverse events from the four RCTs. Comparing patients that received GEMOX chemotherapy, those treated with GEMOX and EGFR-targeted chemotherapy showed higher risks of diarrhoea (RR = 1.54, 95% CI 1.24-1.91, P < 0.01), neutropaenia (RR = 1.30, 95% CI1.01-1.68, P = 0.04) and transaminase increase (RR = 1.15, 95% CI 1.01-1.30, P = 0.04). A skin rash was frequently observed in the GEMOX+EGFR-TKIs group, which was significant compared with the GEMOX alone group (RR = 9.42, 95% CI 6.32-14.05 P < 0.01) (Fig. 6). In addition, we compared the grade 3-4 adverse events that occurred in the GEMOX combined EGFR-target therapy group and the GEMOX alone group and observed no significant difference. All of the above is summarized in Table 4.
Discussion
BTCs are a heterogeneous group of relatively rare tumours that often have extremely poor prognoses. The low incidence of BTCs limits the potential for prospective studies. Furthermore, studies often combine patients with GBCA and cholangiocarcinoma, which is problematic because the biology of these tumours is different. Adequately powered clinical studies are needed to generate convincing results.
Summary of Adverse Events
| Adverse event | All grades of adverse events 3-4 grades | |
|---|---|---|
| RR(95%CI) | P value RR(95%CI) P value | |
| Nausea | 1.03 (0.90-1.17) | 0.67 0.73 (0.28-1.91) 0.51 |
| Vomiting | 1.03 (0.84-1.26) | 0.79 0.53 (0.16-1.77) 0.31 |
| Diarrhea | 1.54 (1.24-1.91) | 0.00 1.32 (0.62-2.78) 0.47 |
| Neutropenia | 1.30 (1.01-1.68) | 0.04 1.25 (0.79-1.99) 0.35 |
| Thromlocytopenia | 1.02 (0.90-1.15) | 0.80 1.11 (0.60-2.05) 0.74 |
| Transaminase increase | 1.15 (1.01-1.30) | 0.04 1.26 (0.69-2.29) 0.46 |
| Skin Rash | 9.42 (6.32-14.05) | 0.00 4.17 (0.60-28.93) 0.15 |
| Neurophathy | 1.01 (0.90-1.14) | 0.83 1.56 (0.88-2.76) 0.13 |
| Asthenia | 0.98 (0.84-1.15) | 0.84 1.28 (0.69-2.36) 0.43 |
| Anorexia | 0.99 (0.86-1.14) | 0.92 0.87 (0.36-2.09) 0.75 |
Risk Ratios (RRs) were calculated from the reported events in the population assessable for toxicity (n = 623); CI: confidence interval.
Many meta-analyses and systematic reviews have been conducted to evaluate different interventions. Eckel F. presented a pooled analysis of 104 trials containing 2810 evaluable patients with advanced BTCs and showed that the response rates and tumour control rates were higher for the patient subgroup receiving a combination of gemcitabine and platinum-based agents[30]. Tian-Tian Sun and Heng Liu performed a meta-analysis and systematic review in 2013 and 2014[31, 32] and reported the efficacy and safety of gemcitabine and platinum-based chemotherapy compared with gemcitabine alone. However, many clinical trials have conflicting outcomes. Therefore, Lawrence Chen recently conducted a meta-analysis and systematic review of RCTs to evaluate the efficacy of different chemotherapy regimens using the same four RCTs used for our meta-analysis, concluding that combining EGFR-targeted therapy with gemcitabine and platinum-based agents (GP) chemotherapy is an option for treating patients with advanced BTCs[33]. Although the OR and random effect models were applied to analyse RCTs in the previous study, we used the RR and fixed effect models. Furthermore, we conducted a subgroup analysis that revealed potentially different behaviours between cholangiocarcinoma and non-cholangiocarcinoma, and we calculated an OS indicating that drug treatment did not translate into a longer survival time.
Our meta-analysis specifically focused on the GEMOX and EGFR-targeted regimen, which appeared to fail in nearly every RCT but actually reduced progression risk (defined by PFS) by 20%. As different EGFR-targeted drugs inhibit the EGFR pathway via different mechanisms, we divided EGFR-targeted drugs into EGFR-TKI and EGFR-mAb groups and tested heterogeneity between these two groups. The results showed that inhibition of the EGFR pathway in both groups conferred a PFS benefit with tolerable side effects. Additionally, EGFR-TKI drugs might increase ORR, which agrees with other reported clinical data[34]. However, further analysis of OS indicated that the observed PFS did not translate into an increased OS (HR = 0.92, 95% CI 0.75-1.08, P = 0.39).
Forest plot of comparison between GEMOX-targeted and Gemox alone in common toxicity.
Analysis of the cholangiocarcinoma and non-cholangiocarcinoma subgroups showed that cholangiocarcinoma exhibited significantly greater benefits from targeted therapy, but only a 6% reduction was observed for non-cholangiocarcinoma, which was not significant. Therefore, patients with cholangiocarcinoma more than patients with gallbladder adenocarcinoma or ampulla of Vater cancer would benefit from targeted therapy. Targeted treatment generated a significant 44% reduction in cholangiocarcinoma risk, but only a 6% reduction in non-cholangiocarcinoma, which was not significant. This result was also supported by recent studies demonstrating that KRAS mutation and EGFR overexpression in BTC, especially in cholangiocarcinoma. Additionally, immunohistochemistry studies have shown that EGFR is overexpressed in human cholangiocarcinoma samples. Although the BINGO and TCOG T1210 trial demonstrated that neither KRAS mutations nor EGFR overexpression improved the selection of patients who respond to therapy, the patients were pooled in a mixed analysis. Therefore, this conclusion should be reconsidered in the subgroup selection and when choosing different targeted therapy drugs, which requires more studies and clinical trials. The above findings provide convincing evidence that might offer physicians a meaningful treatment option of EGFR pathway inhibition for groups that might experience a survival benefit.
Furthermore, GP treatments compared with the cisplatin and gemcitabine (GC) regimen, the GEMOX regimen is the first to demonstrate the survival benefit of systemic chemotherapy over best supportive care in BTC patients as well as better compliance with GEMOX than GC in clinical practice. Sharma et al. reported that modified GEMOX improved the PFS and OS compared to the best supportive care or fluorouracil. The median OS times were 4.5, 4.6, and 9.5 months, respectively, for best supportive care, fluorouracil, and modified GEMOX arms (P = 0.039). The corresponding PFS values were 2.8, 3.5, and 8.5 months (P < 0.001)[35]. Additionally, many studies and meta-analyses report a higher rate of adverse events in the group receiving GC. However, the GC regimen appears to provide extra benefits to PFS and OS in recent studies, especially in terms of the outcomes of ABC02 and BT22 for advanced BTC, which demonstrates that this combination improved both the OS and PFS by 30% over gemcitabine alone. The median OS values were 11.7 months and 8.1 months (HR = 0.64, 95% CI 0.52-0.80, P < 0.001), and the median PFS was 8.0 months vs. 5.0 months (HR = 0.63, 95% CI 0.51-0.77, P < 0.001), which favours the GC arm[10]. Therefore, acquiring more data comparing GC or the GEMOX chemotherapy combination with EGFR-TKIs to evaluate the benefit to quality of life and survival would be ideal, even though this has been evaluated in many prior clinical trials (Table 5).
Notably, EGFR-targeted therapy groups exhibited higher rates of adverse events, including significantly increased rates of diarrhoea, neutropaenia and transaminase elevation. However, further analysis comparing grade 3-4 adverse events did not reveal significant differences in either group. Therefore, GEMOX combined with EGFR-targeted therapy is a tolerable choice for advanced BTC patients. Skin rash was also a more common adverse event in the EGFR-targeted therapy group. The correlation between skin rash severity and EGFR-targeted therapy efficacy supports a predictive role for EGFR antagonists in patients with mCRC and NSCLC[36, 37]. Therefore, we calculated the rate for this reaction to occur in the included RCTs and demonstrated a significant difference in the GEMOX+EGFR-targeted therapy group. It would be interesting to explore whether the skin rash might be a predictive factor for BTC patients using EGFR-targeted therapy or be useful in selecting patient subgroups for different therapy regimens.
The limitation of our meta-analysis is that few clinical trials evaluating the benefit of GEMOX and EGFR inhibitors in BTC patients and performing detailed subgroup analyses to identify groups of patients with a benefit in PFS and OS were available. Therefore, we recognized the need for additional clinical trials that can be incorporated into a future meta-analysis.
Summary of Relative Ongoing Clinical Trial
| NCT No. | Intervention | Status | Country |
|---|---|---|---|
| NCT02170090 | Gemcitabine+Cisplatin vs Obervation | Recruiting | Germany |
| NCT00779454 | GEMOX + Panitumumab + Capecitabine | Active, not recruiting | France |
| NCT01320254 | Panitumumab + Cisplatin/Gemcitabine | Unknown (information has not been verified recently) | Denmark |
| NCT00033462 | Erlotinib | Unknown (information has not been verified recently) | Germany |
| NCT00747097 | Gemcitabine + Cetuximab | Completed, no results available | USA |
| NCT00948935 | Gemcitabine/Irinotecan + Panitumumab | Unknown (information has not been verified recently) | Belgium |
In conclusion, combination chemotherapy with GEMOX and EGFR-targeted therapy improves PFS with tolerable toxicity in advanced BTCs, but the activity of the drugs does not translate into an increase in OS. Patients with cholangiocarcinoma exhibited a better ORR with EGFR-targeted therapy. Additional randomized studies comparing different platinum therapies with different EGFR-targeted therapy combinations to treat specific original sites malignancies of the biliary tract are warranted. Furthermore, it could be interesting to determine whether a skin rash is a predictive factor for BTC patients using EGFR- targeted drugs.
Abbreviations
EGFR: Epidermal Growth Factor Receptor;
GEMOX: Gemcitabine and Oxaliplatin Regimen;
TKI: Tyrosine Kinase Inhibitor;
mAb: Monoclonal Antibody;
RCT: Randomized Controlled Trial;
CI: Confidence Interval;
HR: Hazard Ratio;
RR: Risk Ratio;
PFS: Progression-Free Survival;
OS: Overall Survival;
ORR: Objective Response Rate;
ITT: Intent to Treat;
GBCA: Gallbladder Cancer;
CCA: Cholangiocarcinoma;
ICC: Intrahepatic Cholangiocarcinoma;
ECC: Extrahepatic Cholangiocarcinoma;
GC: Cisplatin and Gemcitabine;
mCRC: Metastatic Colorectal Cancer;
NSCLC: Non-Small Cell Lung Cancer.
Acknowledgements
Research projects of public welfare of National Health and Family Planning Commission, China (No.201502014); Jiangsu Provincial Key Research and Development Special Fund (BE2015666).
Compliance with Ethical Standards
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nature reviews Gastroenterology & hepatology. 2016;13:261-80
2. Lazcano-Ponce EC, Miquel JF, Munoz N. et al. Epidemiology and molecular pathology of gallbladder cancer. CA: a cancer journal for clinicians. 2001;51:349-64
3. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. International journal of cancer. 2006;118:1591-602
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7-30
5. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD. et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Annals of surgery. 2007;245:755-62
6. Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer treatment reviews. 2009;35:322-7
7. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z. et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1188-95
8. Bridgewater J, Lopes A, Wasan H, Malka D, Jensen L, Okusaka T. et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27:134-40
9. Zhu GQ, Shi KQ, You J, Zou H, Lin YQ, Wang LR. et al. Systematic review with network meta-analysis: adjuvant therapy for resected biliary tract cancer. Alimentary pharmacology & therapeutics. 2014;40:759-70
10. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H. et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Annals of oncology: official journal of the European Society for Medical Oncology. 2014;25:391-8
11. Edeline J, Bonnetain F, Phelip JM, Watelet J. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017:35
12. Primrose JN, Fox R, H D, Palmer RP. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017:35
13. Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. The Journal of pathology. 2005;206:356-65
14. Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119:1669-74
15. Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS. et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:3156-63
16. Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E. et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. The Lancet Oncology. 2014;15:819-28
17. Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF. et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:943-9
18. Leone F, Marino D, Cereda S, Filippi R, Belli C, Spadi R. et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer. 2016;122:574-81
19. Kipp BR, Fritcher EG, Clayton AC, Gores GJ, Roberts LR, Zhang J. et al. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. The Journal of molecular diagnostics: JMD. 2010;12:780-6
20. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A. et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021-31
21. Ruys AT, Groot Koerkamp B, Wiggers JK, Klumpen HJ, ten Kate FJ, van Gulik TM. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Annals of surgical oncology. 2014;21:487-500
22. Vaquero J, Guedj N, Claperon A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. Journal of hepatology. 2017;66:424-41
23. Verlingue L, Hollebecque A, Boige V, Ducreux M, Malka D, Ferte C. Matching genomic molecular aberrations with molecular targeted agents: Are biliary tract cancers an ideal playground? European journal of cancer. 2017;81:161-73
24. Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G. et al. Phase II study of erlotinib in patients with advanced biliary cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:3069-74
25. Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K. et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. The Lancet Oncology. 2010;11:1142-8
26. Hezel AF, Noel MS, Allen JN, Abrams TA, Yurgelun M, Faris JE. et al. Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancer. British journal of cancer. 2014;111:430-6
27. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16
28. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719-48
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557-60
30. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. British journal of cancer. 2007;96:896-902
31. Sun TT, Wang JL, Fang JY. Gemcitabine alone or in combination with Cisplatin for advanced biliary tract carcinomas: an overview of clinical evidence. Asian Pacific journal of cancer prevention: APJCP. 2013;14:877-83
32. Liu H, Zhang QD, Li ZH, Zhang QQ, Lu LG. Efficacy and safety of gemcitabine-based chemotherapies in biliary tract cancer: a meta-analysis. World journal of gastroenterology. 2014;20:18001-12
33. Chen L, Chen C, Yen Y, Tam KW. Chemotherapy for advanced biliary tract carcinoma: A meta-analysis of randomized controlled trials. Medicine. 2016;95:e4584
34. Kim ST, Jang KT, Lee SJ, Jang HL, Lee J, Park SH. et al. Tumour shrinkage at 6 weeks predicts favorable clinical outcomes in a phase III study of gemcitabine and oxaliplatin with or without erlotinib for advanced biliary tract cancer. BMC cancer. 2015;15:530
35. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V. et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4581-6
36. Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung cancer. 2012;78:8-15
37. Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Targeted oncology. 2013;8:173-81
Author contact
![]() Corresponding author: Hanguang HU. Email: huhanguangedu.cn; Fax: +86 571 87767088; Tel: +86 13858110651
Corresponding author: Hanguang HU. Email: huhanguangedu.cn; Fax: +86 571 87767088; Tel: +86 13858110651

 Global reach, higher impact
Global reach, higher impact