Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(9):1560-1567. doi:10.7150/jca.24544 This issue Cite
Research Paper
Hepatitis B Virus Infection Predicts Better Survival In Patients With Colorectal Liver-only Metastases Undergoing Liver Resection
1. Department of Colorectal Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China.
2. Department of Public Health, School of Public Health, Sun Yat-Sen University, Guangzhou, China.
#Both authors equally contributed to this manuscript
Received 2017-12-25; Accepted 2018-2-10; Published 2018-4-12
Abstract
Objective: Hepatitis B virus (HBV) infection has been shown to decrease the risk of liver metastasis in patients with non-metastatic colorectal cancer (CRC). However, the prognostic value of HBV infection in long-term survival of patients with colorectal liver-only metastases (CRLM) after liver resection has not yet been evaluated. This study aims to explore the association between HBV infection and survival in CRLM patients.
Methods: A total of 289 CRLM patients undergoing liver resection were recruited at our center from September 1999 to August 2015. Patients were divided into an HBV infection group and a non-HBV infection group. Progression-free survival (PFS) and overall survival (OS) related to HBV infection were analyzed using both Kaplan-Meier and multivariate Cox regression methods.
Results: HBV infection was found in 12.1 %(35/289) of patients. Of these patients, 31.4 %(11/35) had chronic hepatitis B (CHB), 42.9 % (15/35) were inactive hepatitis B surface antigen (HBsAg) carriers (IC) and 25.7 % (9/35) did not undergo HBV DNA detection. HBV infection was associated with more liver metastases (P = 0.025) and larger-sized liver metastases (P = 0.049). The 3-year OS and PFS rates in the HBV infection group were higher than those in the HBV non-infected group (OS: 75.0 % vs 64.8 %, P = 0.031; PFS: 55.9 % vs 39.6 %, P = 0.034). In multivariate Cox analysis, HBV infection was identified as an independent factor for better 3-year OS (hazard ratio (HR), 0.446; 95 %confidence interval (CI), 0.206-0.966; P = 0.041) but not an independent factor for 3-year PFS.
Conclusions: HBV-infected CRLM patients survived longer than non-infected patients. In clinical work, therapeutic regimens and follow-up for HBsAg-positive patients may be different from that for HBsAg-negative patients, even though objective prospective studies are still needed.
Keywords: Colorectal cancer, Hepatitis B virus, Liver metastases, Liver resection, Prognosis
Introduction
With increasing incidence and mortality, colorectal cancer (CRC) has become the third most common cause of cancer death worldwide[1]. In China, mortality caused by CRC was estimated to reach 191,000 in 2015[2]. In all these deaths, the development of distant metastatic disease was the main cause of death, and the liver was the most common site of CRC metastasis. Approximately 20 - 25 % of CRC patients were initially diagnosed with synchronous liver metastases, and approximately half of the patients developed metachronous liver metastases after primary tumor resection[3, 4]. Liver metastases resection is the only known option for curing patients with colorectal liver metastases[5, 6]. However, approximately 75 % of patients will experience recurrence within 2 years[7]. Recurrence after colorectal liver metastases resection is common, with approximately 30 % of intrahepatic recurrence patients and more than 50 % extrahepatic recurrence[8, 9].Thus, clinical biomarkers are needed to find suitable patients for corresponding individual interventions.
Hepatitis B virus (HBV) is the most common cause of chronic liver disease in the world, with an estimated 250 - 400 million people chronically infected with HBV [10]. China has a high prevalence of HBV infection, with 97 million HBV carriers and at least 20 million individuals with an active chronic hepatitis B infection[11]. HBV infection was previously shown to play an important role in the progression of hepatocellular carcinoma(HCC) and to present with increased intrahepatic metastasis, thus suggesting the role of HBV infection in survival[12].
Several reports on the relationship between HBV infection and liver metastasis in CRC are available. Utsunomiya et al.[13] reported that CRC patients infected with HBV or hepatitis C virus (HCV)scarcely present with liver metastases. Another study reported that compared with non-infected patients, HBV-infected CRC patients exhibited a reduced liver metastasis rate and increased survival rate[14, 15]. Since HBV-infected CRC patients presented with a low possibility of liver metastases and better prognosis, we hypothesize that HBV infection is associated with better prognosis of CRLM patients undergoing liver metastases resection. In this study, we examined the association between HBV infection and the survival of CRLM patients undergoing liver metastases resection to investigate the predictive value of HBV infection in screening for high-risk patients for individual treatment.
Materials and Methods
Patients
A retrospective analysis was conducted by reviewing the clinicopathological data of 289 CRLM patients who underwent radical liver resection from September 1999 to August 2015 at Sun Yat-sen University Cancer Center (Guangzhou, China). Patients who met the following criteria were enrolled: 1) histologically confirmed colorectal adenocarcinoma, 2) metastases limited to the liver before liver resection, 3) R0 resection for primary lesion and metastases, and 4) no other cancer during follow-up. Patients were excluded if they had extrahepatic metastatic lesions, died in the perioperative period or underwent palliative liver resection. The primary tumor was completely removed, and liver metastases were radically resected in all eligible patients. Clinicopathological features of CRLM patients; tumor characteristics; and the time of hepatectomy, relapse and last follow-up were all reviewed from the medical records and the follow-up system of Sun Yat-sen University Cancer Center. The present study was undertaken according to the ethical standards of the 1964 Declaration of Helsinki. Institutional Review Board approval was obtained from independent ethics committees at the Sun Yat-sen University Cancer Center with a waiver of informed consent because this research was retrospective and did not involve accessing any patient identification data.
Serologic assay for CRLM patients
Routine peripheral blood biochemical tests and serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were acquired about one week before liver metastases resection. CEA levels above 5 ng/ml and CA19-9 levels above 35U/ml were considered to be elevated. Hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), hepatitis B e-antigen (HBeAg), hepatitis B e-antibody (anti-HBe), and hepatitis B core antibody (anti-HBc) levels were examined in all patients by enzyme-linked immunosorbent assay (ELISA), and HBV DNA was detected by polymerase chain reaction(PCR). Blood samples were collected from all CRLM patients at the first visit, and serum samples were separated for the HBV infection test. A serum HBV DNA result of >1000 copies/ml was considered positive. According to the HBsAg status at their first visit, patients who were HBsAg-positive or HBsAg-negative were divided into an HBV-infected or non-infected group. The infected group was further subdivided into two groups based on HBeAg and serum HBV DNA detection. Chronic hepatitis B (CHB) patients, defined as patients having active HBV replication, were characterized by being HBsAg-positive and positive for either HBeAg or HBV DNA. Inactive HBsAg carriers (IC), indicating HBV infection but without active replication, were identified as HBsAg-positive and negative for both HBeAg and HBV DNA[16, 17].
Follow-up of patients
All patients were followed up after hospital discharge. The patients were followed up every 3 months in the first 2 years after liver resection and then semi-annually until 3 years after liver resection. The follow-up evaluation included a routine blood test, tests for the tumor markers CEA and CA19-9, abdominal ultrasonography, and a chest radiograph. A chest computed tomography scan, abdominal/pelvic magnetic resonance imaging, and a colonoscopy were performed annually. The follow-up period was terminated in June 2017.
Baseline characteristics of 289 colorectal liver metastases patients
| Characteristics | n (%) | |
|---|---|---|
| Sex | Male | 185 (64.0) |
| Female | 104 (36.0) | |
| Age (years) | <=57 | 150 (51.9) |
| >57 | 139 (48.1) | |
| Primary tumor sitea | Colon | 179 (62.2) |
| Rectum | 109 (37.8 ) | |
| Primary tumor size (cm)b | <=4 | 126 (61.2) |
| >4 | 80 (38.8) | |
| T stage (T1-3/T4)c | T1-3 | 170 (64.2) |
| T4 | 95 (35.8) | |
| N stage (N0/N1-2)d | N0 | 110 (42.3) |
| N1-2 | 150 (57.7) | |
| Histological grade (/ poor)e | Well/moderate | 212 (86.2) |
| Poor | 34 (13.8) | |
| Albumin (g/dl) | <=35 | 272 (94.1) |
| >35 | 17 (5.9) | |
| CEA level (ng/ml)f | <=5 | 102 (37.1) |
| >5 | 173 (62.9) | |
| CA199 level (U/ml)g | <=35 | 185 (69.3) |
| >35 | 82 (30.7) | |
| Liver metastases tumor size (cm)h | <=2.5 | 151 (53.0) |
| >2.5 | 134 (47.0) | |
| Liver metastases number | Single | 147 (50.9) |
| Multiple | 142 (49.1) | |
| Liver metastases segments | Oligo | 136 (47.1) |
| Multiple | 153 (52.9) | |
| HBV infection statusi | Non infection | 254 (90.7) |
| CHB | 11 (3.9) | |
| IC | 15 (5.4) | |
| Liver metastases | Synchronous | 190 (65.7) |
| Metachronous | 99 (34.3) | |
| Hepatic resection timing | Synchronous | 131 (45.3) |
| Metachronous | 158 (54.7) |
Abbreviations: CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; HBV, hepatitis B virus; CHB, chronic hepatitis B; IC, inactive carriers.
aData of 288 patients were available
bData of 206 patients were available
cData of 265 patients were available
dData of 260 patients were available
eData of 246 patients were available
fData of 275 patients were available
gData of 267 patients were available
hData of 285 patients were available
iData of 280 patients were available
Statistical analysis
Values are presented as the median (range) or percentage, as appropriate. Differences in baseline clinicopathological characteristics between the two groups were assessed by chi-square test or Fisher's exact test. Progression-free survival (PFS) was defined as the time from the date of liver resection to the date of disease progression, date of death due to any cause or date of the last follow-up. Overall survival (OS) was defined from the date of liver resection until the date of death or last follow-up. OS and PFS curves were evaluated with the Kaplan-Meier method, and the significance differences between the HBsAg-positive and HBsAg-negative groups were compared by using log-rank test. Univariate and multivariate analyses with Cox proportional hazard regression models were performed to determine the hazard ratio (HR) of each prognostic factor. Covariates with P values<0.05 in univariate analysis were subjected to multivariate analysis. All statistical analyses were performed using SPSS 20.0 statistical software (IBM, NY, USA) and GraphPad Prism version 6.01 (GraphPad Software, Inc, La Jolla, CA, USA). A two-tailed P value < 0.05was interpreted as statistically significant.
Results
1. Clinicopathological characteristics of CRLM patients
A total of 289 cases were qualified for the analyses. Liver metastasis occurred in these 289 patients, including 190 cases with synchronous liver metastasis and 99 cases with metachronous liver metastasis. According to the HBsAg infection status, 35 patients (12.1 %) were distributed to the HBV infection group, and 254 patients (87.9 %) were distributed to the non-infected group. Among the 35 HBV-infected CRLM patients, except for 9 HBeAg-negative patients without HBV DNA detection, CHB was identified in 11 patients (3.8 %), and IC was identified in 15patients (5.2 %).
As shown in Table 1, the median age of the 289 patients was 57 years (range, 25 - 82 years), with 185 (64.0 %) males and 104 (36.0 %) females. HBV infection status was significantly associated with fewer liver metastases (P = 0.025) and smaller-sized liver metastases (P = 0.049) (Table 2).
2. Overall survival and progression-free survival difference according to HBV infection status
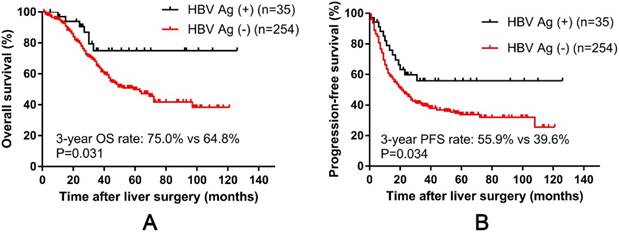
The median follow-up time after liver resection was 34 months (range 1 - 126) for all 289 patients. Overall, from the start of the study until the date of last follow-up, 115 patients (39.8 %) died of CRC, and 174 patients (60.2 %) had intrahepatic or extrahepatic recurrence. Postoperative recurrence in HBV-infected patients was significantly lower than that in non-infected patients (P = 0.009) (Table 3). The 1-, 3-, and the 5-year OS rates were94.0%, 66.0%, and 52.4%, respectively, and the 1-, 3-, 5-year PFS rates were 62.9 %, 41.5 %, and 36.1 %, respectively. The results showed that OS and PFS of the HBV-infected CRLM patients were significantly better than the rates in the non-infected patients (Fig. 1A and 1B). The 1-, 3-, and 5-year OS rates were 97.0 %, 75.0 %, and 75.0 % in the HBV-infected group and 93.6 %, 64.8 %, and 49.8 % in the non-infected group, respectively (log-rank test, P = 0.031). Similarly, the 1-, 3-, and 5-year PFS rates were 79.0 %, 55.9 %, and 55.9 % in the infected group and 60.8 %, 39.6 %, and 33.7 % in the non-infected group, respectively (log-rank test, P = 0.034).
Relationships between HBV infection and patient characteristics
| Clinicaopathological features | HBV infection (n=35) | HBV non-infection (n=254) | P value | |
|---|---|---|---|---|
| Age (years) | <=57 | 20 | 130 | 0.508 |
| >57 | 15 | 124 | ||
| Sex | Male | 22 | 163 | 0.879 |
| Female | 13 | 91 | ||
| T stagea | T1-3 | 25 | 145 | 0.079 |
| T4 | 7 | 88 | ||
| N stageb | N0 | 15 | 95 | 0.577 |
| N1-2 | 17 | 133 | ||
| Primary tumor locationc | Left-sided colon | 13 | 99 | 0.221 |
| Right-sided colon | 12 | 55 | ||
| Rectum | 10 | 99 | ||
| Primary tumor size (cm)d | <=4 | 13 | 113 | 0.085 |
| >4 | 15 | 65 | ||
| Histological gradee | Well/Moderate | 27 | 185 | 0.518 |
| Poor | 3 | 31 | ||
| Albumin (g/dL) | <=35 | 34 | 238 | 0.668 |
| >35 | 1 | 16 | ||
| Total bilirubin (mg/dL) | <=20.5 | 34 | 244 | 1.000 |
| >20.5 | 1 | 10 | ||
| LDH (IU/L) | <=245 | 32 | 222 | 0.683 |
| >245 | 3 | 32 | ||
| GGT (IU/L)c | <=50 | 27 | 188 | 0.718 |
| >50 | 8 | 65 | ||
| Liver matastases segments | Oligo | 19 | 117 | 0.361 |
| Multiple | 16 | 137 | ||
| Liver matastases numbers | Single | 24 | 123 | 0.025 |
| Multiple | 11 | 131 | ||
| Liver metastasis timing | Synchronous | 27 | 163 | 0.130 |
| Metachronous | 8 | 91 | ||
| Liver metastases largest size (cm)f | <=2.5 | 24 | 127 | 0.049 |
| >2.5 | 11 | 123 | ||
| Surgical margin (cm)g | <=0.5 | 9 | 84 | 0.778 |
| >0.5 | 9 | 73 | ||
| CEA level (ng/ml)h | <=5 | 16 | 86 | 0.149 |
| >5 | 17 | 156 | ||
| CA199 level (U/ml)i | <=35 | 21 | 164 | 0.843 |
| >35 | 10 | 72 | ||
| Neoadjuvant chemotherapy | Yes | 14 | 126 | 0.286 |
| No | 21 | 128 | ||
| Adjuvant chemotherapy | Yes | 7 | 81 | 0.152 |
| No | 28 | 173 |
Abbreviations: HBV, hepatitis B virus; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transpeptidase; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
aData of 265 patients were available
bData of 260 patients were available
cData of 288 patients were available
dData of 206 patients were available
eData of 246 patients were available
fData of 285 patients were available
gData of 175 patients were available
hData of 275 patients were available
iData of 267 patients were available
3. Prognostic factors for overall survival and progression-free survival
For patients with CRLM undergoing liver resection, univariate analysis revealed that older patients (HR,1.515; 95 % confidence interval(CI),1.048 - 2.192; P = 0.027), HBV non-infection (HR,2.258; 95 % CI,1.050 - 4.853; P = 0.037),lower albumin (ALB) level (g/dL) (HR,0.479; 95 % CI,0.250 - 0.918; P = 0.027), higher lactate dehydrogenase (LDH)level (IU/L) (HR,1.772; 95 % CI,1.092 - 2.876;P = 0.02), higher gamma-glutamyl transpeptidase (GGT) level (IU/L) (HR, 1.759; 95 % CI, 1.188 - 2.604; P = 0.005), multiple liver metastatic segments (HR, 1.815; 95 % CI, 1.241 - 2.655; P = 0.002), more liver metastases(HR, 1.827; 95 % CI,1.256 - 2.657; P = 0.002), and patients undergoing neoadjuvant chemotherapy (HR, 1.817; 95 % CI, 1.252 - 2.638; P = 0.002) were associated with worse OS (Table 4). For PFS, lymph node metastasis (HR, 1.800; 95 % CI, 1.295 - 2.503; P < 0.001), smaller-sized primary tumor (≤ 4 cm) (HR, 1.534; 95 % CI,1.062 - 2.216; P = 0.023), HBV non-infection (HR,1.779; 95 % CI,1.030 - 3.072; P = 0.039), higher GGT level (HR, 1.561; 95 % CI, 1.128 - 2.162; P = 0.007), multiple liver metastatic segments (HR, 1.847; 95 % CI, 1.360 - 2.507; P < 0.001), more liver metastases (HR, 1.94; 95 % CI, 1.432 - 2.628; P < 0.001),larger-sized metastases tumor (>2.5 cm) (HR, 1.355; 95 % CI, 1.002 - 1.831; P = 0.048), closer liver resection surgery margin(≤ 0.5 cm) (HR,1.755; 95 % CI,1.195 - 2.578; P = 0.004), higher CEA level (HR, 1.403; 95 % CI, 1.014 - 1.942; P = 0.041), and patients undergoing neoadjuvant chemotherapy (HR, 2.282; 95 % CI, 1.681 - 3.099; P < 0.001) were associated with worse PFS (Table 4).
Postoperative recurrence in patients with colorectal liver-only metastases after radical liver resection between HBV infection and non-infection group
| Parameters | HBV infection (n, %) | HBV non-infection (n, %) | P value |
|---|---|---|---|
| Median follow-up time (month, range) | 33.0 (1.0-126.0) | 35.0 (1.0-121.0) | 0.344 |
| Postoperative recurrence (n=289) | |||
| Yes | 14 (40.0) | 160(63.0) | 0.009 |
| No | 21(60.0) | 94(37.0) | |
| Recurrence period (n=173) | |||
| Early recurrence (≤ 6 months) | 3(21.4) | 45(28.3) | 0.811 |
| Latter recurrence (> 6 months) | 11(78.6) | 114(71.7) | |
| Recurrence pattern (n=142) | |||
| Intrahepatic metastases | 4(30.8) | 76(58.9) | 0.051 |
| Extrahepatic metastases | 9(69.2) | 53(41.1) |
Abbreviations: HBV, hepatitis B virus
A multivariate Cox proportional hazard model was used to further analyze the prognostic factors that were significantly associated with OS or PFS in univariate analysis including HBV infection status. As shown in Table 4, the multivariate analysis demonstrated that HBV non-infection (HR,2.242; 95 % CI,1.035 - 4.853; P = 0.041) as well as older age (HR,1.702; 95 % CI, 1.171-2.474; P = 0.005), higher blood GGT level (HR, 1.597; 95 % CI, 1.073 - 2.377; P = 0.021), multiple liver metastatic segments (HR, 1.538; 95 % CI, 1.034 - 2.286; P = 0.033) and neoadjuvant chemotherapy (HR, 1.632; 95 % CI, 1.103 - 2.416; P = 0.014) were all independent predictors of worse OS for patients with CRLM undergoing liver resection. Furthermore, lymph node metastasis (HR,2.023; 95 % CI,1.229 - 3.329; P = 0.006), blood GGT level (HR, 1.806; 95 % CI, 1.099 - 2.967; P = 0.02), and number of liver metastases (HR, 1.748; 95 % CI, 1.043 - 2.929; P = 0.034), but not HBV infection, were independent predictors of PFS.
Correlation of hepatitis B surface antigen (HBsAg) status with progression-free survival and overall survival in 289 colorectal liver metastases (CRLM) patients. Kaplan-Meier survival curve showing better progression-free survival (A) and overall survival (B) in HBsAg-positive CRLM patients.
Prognostic factors for OS and PFS in univariate and multivariate analysis
| Clinicaopathological features | OS univariate analysis | Multivariate analysis | PFS univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value | ||
| Age (years) | ≤ 57 | 1.515(1.048-2.192) | 0.027 | 1.702(1.171-2.474) | 0.005 | 1.199 (0.891-1.615) | 0.231 | ||
| > 57 | |||||||||
| Gender | Male | 1.305(0.882-1.932) | 0.183 | 1.365 (0.991-1.880) | 0.057 | ||||
| Female | |||||||||
| T stage | T1-3 | 1.154(0.772-1.725) | 0.484 | 1.607 (0.773-1.472) | 0.695 | ||||
| T4 | |||||||||
| N stage | N0 | 1.448(0.966-2.171) | 0.073 | 1.8 (1.295-2.503) | <0.001 | 2.023 (1.229-3.329) | 0.006 | ||
| N1-2 | |||||||||
| Primary tumor location | Left-sided colon | 1.235(0.966-1.580) | 0.092 | 1.164 (0.957-1.416) | 0.127 | ||||
| Right-sided colon | |||||||||
| Rectum | |||||||||
| Primary tumor size (cm) | > 4 | 1.537(0.960-2.460) | 0.074 | 1.534 (1.062-2.216) | 0.023 | ||||
| ≤ 4 | |||||||||
| Histological grade | Well/Moderate | 1.164(0.856-1.582) | 0.334 | 1.097 (0.851-1.414) | 0.477 | ||||
| Poor | |||||||||
| HBV infection | Yes | 2.258(1.050-4.853) | 0.037 | 2.242(1.035-4.853) | 0.041 | 1.779 (1.030-3.072) | 0.039 | ||
| No | |||||||||
| Albumin (g/dL) | ≤ 35 | 0.479(0.250-0.918) | 0.027 | 0.590 (0.335-1.040) | 0.068 | ||||
| > 35 | |||||||||
| Total bilirubin (mg/dL) | ≤ 20.5 | 1.062(0.431-2.616) | 0.897 | 1.179 (0.577-2.408) | 0.652 | ||||
| > 20.5 | |||||||||
| LDH (IU/L) | ≤ 245 | 1.772(1.092-2.876) | 0.02 | 1.454 (0.957-2.209) | 0.079 | ||||
| > 245 | |||||||||
| GGT (IU/L) | ≤ 50 | 1.759(1.188-2.604) | 0.005 | 1.597(1.076-2.377) | 0.021 | 1.561 (1.128-2.162) | 0.007 | 1.806 (1.099-2.967) | 0.020 |
| > 50 | |||||||||
| Liver matastases segments | Oligo | 1.815(1.241-2.655) | 0.002 | 1.538(1.034-2.286) | 0.033 | 1.847(1.360-2.507) | <0.001 | ||
| Multiple | |||||||||
| Liver matastases numbers | Single | 1.827 (1.256-2.657) | 0.002 | 1.940 (1.432-2.628) | <0.001 | 1.748( 1.043-2.929) | 0.034 | ||
| Multiple | |||||||||
| Liver metastasis timing | Synchronous | 0.920 (0.628-1.348) | 0.668 | 0.964 (0.704-1.319) | 0.818 | ||||
| Metachronous | |||||||||
| Liver metastases largest size (cm) | ≤ 2.5 | 1.329 (0.915-1.932) | 0.136 | 1.355 (1.002-1.831) | 0.048 | ||||
| > 2.5 | |||||||||
| Surgical margin of liver resection (cm) | > 0.5 | 1.583 (0.985-2.543) | 0.058 | 1.755(1.195-2.578) | 0.004 | ||||
| ≤ 0.5 | |||||||||
| CEA level (ng/ml) | ≤ 5 | 1.137 (0.769-1.681) | 0.519 | 1.403(1.014-1.942) | 0.041 | ||||
| > 5 | |||||||||
| CA199 level (U/ml) | ≤ 35 | 1.285 (0.864-1.912) | 0.216 | 1.125(0.809-1.566) | 0.483 | ||||
| > 35 | |||||||||
| Neoadjuvant chemotherapy | No | 1.817 (1.252-2.638) | 0.002 | 1.632(1.103-2.416) | 0.014 | 2.282(1.681-3.099) | <0.001 | ||
| Yes | |||||||||
| Adjuvant chemotherapy | No | 0.964 (0.650-1.430) | 0.854 | 0.943(0.681-1.305) | 0.723 | ||||
| Yes | |||||||||
Abbreviations: OS, overall survival; PFS, progression-free survival; HBV, hepatitis B virus; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transpeptidase; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Discussion
In this study, 289 colorectal liver metastases patients all underwent a radical liver resection. There were fewer and smaller-sized liver metastases in the HBV-infected group than in the non-infected group, indicating that HBV infection and clinicopathological features of CRLM patients were significantly correlated. In this study, the OS and PFS rates of CRLM patients in the HBV-infected group were higher than those in the non-infected group (P=0.031 and P = 0.034, respectively), and there was a significant difference in survival rate between these two groups, suggesting that HBV infection may have a strong correlation with survival of CRLM patients undergoing liver resection. We also found that HBV-infected CRLM patients had less postoperative recurrence than non-infected patients (P = 0.009). In addition, the recurrence pattern between these two groups differed. HBV-infected patients were more likely to have extrahepatic metastases than HBV non-infected patients, who had intrahepatic metastases; however, this result was not statistically significant (P = 0.051). In multivariate Cox analysis, HBV infection was identified as an independent factor for better OS but not an independent factor for PFS. We also analyzed the difference in the survival rate between patients in the CHB and IC groups, but there was no significant difference. (Figure S1.)
Reports are available on the relationship between HBV infection and liver metastasis in CRC patients. In a previous cohort study[15], HBV infection was demonstrated to decrease the risk of liver metastasis in patients with CRC and elevate the surgical resection rate of liver metastatic lesions. Utsunomiya et al. reported that CRC seldom metastasized to the liver in patients infected with HBV or HCV; however, most of the patients in the study were HCV-infected[13].
Song et al. suggested that chronic HBV infection with viral replication could reduce liver metastasis and prolong the survival of CRC patients[14]. In our study, we also demonstrated that HBV-negative CRLM patients are more likely to develop recurrence after liver resection than HBV-positive patients and that the former are more likely to develop intrahepatic recurrence than the latter. The reasons why HBV-positive patients scarcely metastasize to the liver or are not prone to intrahepatic recurrence are not completely understood, but some investigations have been conducted. Approximately 5241 consecutive autopsies in the Trieste area and 6511 consecutive autopsies in the Tokyo-Chiba area were analyzed to study the frequency of liver metastases in cirrhosis[18]. The results showing that metastases in cirrhotic liver are rare were similar in both areas, which implied that in HBV-infected patients, the scarce metastasis of CRC to the liver maybe due to the hostile “soil” in the liver caused by viral replication.
However, whether the liver-associated immunity in HBV-infected patients hindered liver metastasis of CRC and prolonged survival needs further exploration. Viral clearance and disease pathogenesis are largely mediated by the adaptive immune response in HBV infection [19]. Previous studies reported that the liver has a considerable number of innate immune cells, especially lymphocytes including natural killer cells[20]. Several cells with phagocytic and antigen-presenting properties, including Kupffer cells and dendritic cells, play an important role in the local innate immunity of the liver[21].HBV receptors are expressed in a variety of extrahepatic cells, and HBV can alter the biological characteristics of these cells through their receptors[22].In addition, it has been reported that HBV replication can activate specific lytic pathways of cell damage by cytotoxic lymphocytes (CTLs)and Kupffer cells[23]. It is widely believed that the HBV specific CD8+ T cell response plays a fundamental role in viral clearance. Investigation of experimentally induced hepatic metastasis of colon cancer indicated that changes in liver-related immunity may play an important role in the impediment of liver metastasis[24].
In HBV transgenic mice, the CTL response to HBV by inducing several inflammatory factors. Indeed, they purge HBV replication in the liver by secreting type 1 inflammatory cytokines to limite virus spread to uninfected cells [25]. Furthermore, HBV replication is inhibited by stimulus which could induce IFNγ or IFN α/β in the liver, including CD4+ T cells, NK and NKT cells, as well as toll-like receptor activation [26,27]. In studies of HBV-infected chimpanzees, intrahepatic gene expression profiling in these animals revealed that the early phase of HBV clearance was associated with CD3, CD8 and IFNγ mRNA and other T cell-derived and interferon gamma (IFNγ)-stimulated genes [28,29]. Furthermore, HBV replication can promote the production of tumor necrosis factor a (TNF-a) by residual immune cells as well as hepatocytes in the liver[30,31], which can exhibit an antitumor effect on tumor cells. A previous study showed that the liver microenvironment in patients with HBV-positive liver metastases can greatly alter their gene expression profiles, and two significant clusters in the profile showed significant changes associated with gene products involved in immune function. In fact, over 30% of the genes in these clusters are related to immunity[23]. Another study on the interaction of tumor and stroma showed that liver-prone metastatic tumors were inherent in tumor cells and affected by the local environment of metastatic sites[32]. In conclusion, the soil (liver microenvironment) and activation of liver-associated immunity due to HBV infection may partially explain the reduction in the incidence of liver metastasis in CRC patients and the prolonged survival rate of CRLM patients undergoing liver resection. In our study, we found that the survival rates in the CHB and IC groups are similar. Thus, it is reasonable to postulate that HBV infection with or without HBeAg infection or viral replication may affect survival of colorectal liver metastases in patients undergoing liver resection.
There are a number of limitations to our study. First, our study included a retrospective study with a relatively small sample size of patients from a single-institution experience. Second, the follow-up period was not long enough, which may have resulted in bias during analysis. Third, molecular detections are lacking. This retrospective result did not consider the status of KRAS, NRAS, or BRAF expression, which may have an influence on disease progression. Accordingly, further investigations of KRAS, NRAS, and BRAF expression in tumor tissues are needed to elucidate the precise contribution to the development of CRLM together with HBV. Finally, a previous study found that maintaining a relatively low level of HBV DNA plays a strong protective role in the overall and long-term recurrence-free survival of patients with HBV-related HCC[33]. In our study, we did not analyze the effect of HBV DNA copy number on survival of CRLM patients undergoing liver resection because of the small sample population of HBV-positive patients and the lack of HBV DNA detection for several cases. Future studies should increase the sample size and include the effect of HBV viral load on survival of those patients. Therefore, objective prospective studies with more comprehensive clinical data and a longer follow-up period are needed to confirm our results.
In conclusion, we first found that HBV infection correlates with a good prognosis in infected patients; however, the mechanisms still need to be further explored and clarified. In clinical work, when HBsAg-positive patients may have two viable options for therapeutic regimens, patients can choose the less aggressive option. In addition, these patients can consider undertaking a less strict follow-up. However, larger and more objective prospective studies are needed to guide clinical decision making.
Abbreviations
HBV: Hepatitis B virus; CRC: colorectal cancer; CRLM: colorectal liver metastases; PFS: progression-free survival; OS: overall survival; CHB: chronic hepatitis B; HBsAg: hepatitis B surface antigen; IC: inactive carriers; HR: hazard ratio; CI: confidence interval; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; CEA: carcinoembryonic antigen; CA199: carbohydrate antigen 19-9; anti-HBs: hepatitis B surface antibody; HBeAg: hepatitis B e-antigen; anti-HBe: hepatitis B e-antibody; anti-HBc: hepatitis B core antibody; ELISA: enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; ALB: albumin; LDH: lactate dehydrogenase; GGT: gamma-glutamyl transpeptidase; CTLs: cytotoxic lymphocytes; TNF-a: tumor necrosis factor a.
Supplementary Material
Supplementary figure.
Acknowledgements
We would like to acknowledge the patients who participated in this study. We also gratefully acknowledge the assistance of the colleagues at State Key Laboratory of Oncology in South China and the Department of Colorectal Surgery in Sun Yat-sen University Cancer Center.
Ethics Committee Approval and Patient Consent
All procedures performed in studies involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Institutional Review Board approval was also obtained from the independent ethics committee at Sun Yat-sen University Cancer Center. Informed consent was waived for this non-interventional, observational, and retrospective study, in which the patient data used were kept strictly confidential.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fedewa SA. et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177-193
2. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132
3. Lykoudis PM, O'Reilly D, Nastos K. et al. Systematic review of surgical management of synchronous colorectal liver metastases. Br J Surg. 2014;101(6):605-612
4. Adam R. Colorectal cancer with synchronous liver metastases. Br J Surg. 2007;94(2):129-131
5. Akgul O, Cetinkaya E, Ersoz S. et al. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20(20):6113-6122
6. Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102(9):1313-1318
7. Jones RP, Jackson R, Dunne DF. et al. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg. 2012;99(4):477-486
8. D'Angelica M, Kornprat P, Gonen M. et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. ANN SURG ONCOL. 2011;18(4):1096-1103
9. Vigano L, Russolillo N, Ferrero A. et al. Evolution of long-term outcome of liver resection for colorectal metastases: Analysis of actual 5-year survival rates over two decades. ANN SURG ONCOL. 2012;19(6):2035-2044
10. Protzer U, Maini MK, Knolle PA. Living in the liver: Hepatic infections. NAT REV IMMUNOL. 2012;12(3):201-213
11. Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7-10
12. Yu SJ, Kim YJ. Hepatitis B viral load affects prognosis of hepatocellular carcinoma. World J Gastroenterol. 2014;20(34):12039-12044
13. Utsunomiya T, Saitsu H, Saku M. et al. Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. AM J SURG. 1999;177(4):279-281
14. Song E, Chen J, Ou Q. et al. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. AM J SURG. 2001;181(6):529-533
15. Qiu HB, Zhang LY, Zeng ZL. et al. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: A cohort study. World J Gastroenterol. 2011;17(6):804-808
16. Lok AS, McMahon BJ. Chronic hepatitis B. HEPATOLOGY. 2007;45(2):507-539
17. Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: What exactly has REVEAL revealed? LIVER INT. 2012;32(9):1333-1341
18. Melato M, Laurino L, Mucli E. et al. Relationship between cirrhosis, liver cancer, and hepatic metastases. An autopsy study. CANCER-AM CANCER SOC. 1989;64(2):455-459
19. Guidotti L G, Chisari F V. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61
20. Doherty DG, Norris S, Madrigal-Estebas L. et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J IMMUNOL. 1999;163(4):2314-2321
21. McDonald B, Spicer J, Giannais B. et al. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. INT J CANCER. 2009;125(6):1298-1305
22. Neurath AR, Strick N, Sproul P. et al. Detection of receptors for hepatitis B virus on cells of extrahepatic origin. VIROLOGY. 1990;176(2):448-457
23. Budhu A, Forgues M, Ye QH. et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. CANCER CELL. 2006;10(2):99-111
24. Okuno K, Hirai N, Lee YS. et al. Involvement of liver-associated immunity in hepatic metastasis formation. J SURG RES. 1998;75(2):148-152
25. Wieland S F, Guidotti L G, Chisari F V. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74(9):4165-4173
26. Kakimi K, Guidotti L G, Koezuka Y. et al. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192(7):921-930
27. Isogawa M, Robek M D, Furuichi Y. et al. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79(11):7269-7272
28. Wieland S, Thimme R, Purcell R H. et al. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101(17):6669-6674
29. Wieland S F, Vega R G, Muller R. et al. Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes. J Virol. 2003;77(2):1227-1236
30. Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L. et al. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J EXP MED. 1994;179(3):841-848
31. Lara-Pezzi E, Majano PL, Gomez-Gonzalo M. et al. The hepatitis B virus X protein up-regulates tumor necrosis factor alpha gene expression in hepatocytes. HEPATOLOGY. 1998;28(4):1013-1021
32. Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. NAT REV CANCER. 2004;4(11):839-849
33. An HJ, Jang JW, Bae SH. et al. Sustained low hepatitis B viral load predicts good outcome after curative resection in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(12):1876-1882
Author contact
![]() Corresponding author: Zhizhong Pan, Department of Colorectal Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P.R.China. Phone:+86-20-87343124; Fax: +86-20-87343609; E-mail: panzhzhorg.cn
Corresponding author: Zhizhong Pan, Department of Colorectal Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P.R.China. Phone:+86-20-87343124; Fax: +86-20-87343609; E-mail: panzhzhorg.cn

 Global reach, higher impact
Global reach, higher impact