Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(16):2773-2777. doi:10.7150/jca.26376 This issue Cite
Research Paper
The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: a retrospective study
1. Department of Medical Oncology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
2. Department of Internal Medicine, Yuyao Traditional Chinese Medicine Hospital, Yuyao, Zhejiang, China
Received 2018-3-29; Accepted 2018-5-8; Published 2018-7-16
Abstract
Purpose: Liver cancer is insensitive to chemotherapy. Sorafenib is currently the standard treatment for patients with advanced diseases, with mild survival extension and several intolerable drug-related side effects. The establishment of new treatments is an unmet clinical need. The aim of our study was to assess the efficacy and safety of apatinib, a novel antiangiogenic drug, in the treatment of patients with liver cancer.
Materials and Methods: Patients with unresectable or relapsed liver cancer were included in a single center, retrospective, observational study and treated with apatinib until progressive disease or unacceptable toxicity.
Results: 32 patients were reviewed from January 2015 to March 2017. No complete response (CR) occurred, 5 patients (16%) showed partial response (PR), 14 patients (44%) had stable disease (SD), 13 patients (41%) had progressive disease (PD), with disease control rate of 60%. Median progression-free survival (PFS) was 5 months (95% confidence interval [CI]: 4.3-6.1 months) for hepatocellular carcinoma (HCC) and 3 months (95% CI: 2.5-4.2 months) for intrahepatic cholangiocarcinoma (ICC). The median overall survival (OS) was 13 months (95% CI: 12.4-14.1 months) for HCC and 5 months (95% CI: 4.5-6.2 months) for ICC, respectively. The most common adverse effects (AEs) were proteinuria (31%), secondary hypertension (28%) and liver dysfunction (13%).
Conclusion: Apatinib treatment was an effective for patients with liver cancer. The toxicities were mild and tolerable.
Keywords: Liver cancer, apatinib, angiogenesis, retrospective study
Introduction
Primary liver cancer is one of the most common malignancies. It is estimated that 42,220 people will be newly diagnosed and 30,200 people will die of liver cancer in United States in 2018[1]. The mortality of liver cancer is 422.1 per 100 thousand people in China, which ranked the top 3 malignant cancers [2]. Furthermore, the incidence of liver cancer is still increasing during the past 20 years [1].
Liver cancer is insensitive to chemotherapy. Hepatectomy is the priority treatment of early-diagnosed patients. For advanced patients who are not eligible to receiving surgical resection, transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) can be alternative means. Sorafenib is currently the standard therapy for advanced patients, which extended the overall survival for 2.8 months compared to placebo arm (10.7 vs 7.9 months), but some severe side effects, such as gastrointestinal events, fatigue, and liver dysfunction often lead to treatment discontinuation [3]. The establishment of new treatments is urgently needed. However, multiple novel drugs for liver cancer treatment failed [4, 5]. In recent years, anti-angiogenic therapies are emerging as effective therapeutic strategies. Regorafenib, which showed anti-angiogenic activity, was approved by FDA for liver cancer patients progressed on sorafenib treatment [6]. Two other anti-angiogenic drugs, Lenvatinib and Cabozantinib also showed promising results in clinical trials involving patients with liver cancer [7, 8].
Vascular endothelial growth factor receptor-2 (VEGFR-2), the primary molecule upon binding of VEGF (Vascular endothelial growth factor) in angiogenesis process, was the prominent target of angiogenesis therapy [9]. Apatinib is a novel anti-angiogenic small molecule that highly binds and inhibits VEGFR-2, thereby suppressing tumor growth by inhibiting tumor angiogenesis [10]. In a randomized, double blind, placebo-controlled phase III trial, patients with advanced gastric cancer treated with apatinib had 6.5 months OS and 2.6 months PFS benefits compared to placebo arm[11]. Therefore, apatinib was approved by China National Food and Drug Administration (CFDA) for treatment of advanced gastric adenocarcinoma or stomach-esophageal junction adenocarcinoma. Quantities of clinical trials on apatinib are in progress (NCT03365765, NCT03129698, NCT03020979 et al. on clinicaltrial.gov). Due to the essential role of VEGFR2 in liver cancer development and the safety and efficacy of apatinib in the treatment of gastric cancer, some patients with liver cancer were recommended for apatinib treatment in Sir Run Run Shaw Hospital with fully informed consents. Here, we reported a retrospective clinical study to investigate the efficacy and safety of apatinib in patients with unresectable or relapsed liver cancer.
Patients and Methods
Patients
Patients with unresectable or relapsed primary liver cancers were enrolled in this study from January 2015 to March 2017 in Sir Run Run Shaw Hospital. Eligibility criteria included age≥18 years; pathologically confirmed as hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC); no TACE or RFA was performed during apatinib treatment; at least one measurable lesion according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1; Eastern Cooperative Oncology Group performance status (ECOG) 0-1; Child-Pugh score A-B. Key exclusion criteria were: other malignancies that had been diagnosed or treated before this study; serious respiratory, cardiovascular or kidney disease; pregnant and lactating women. The study was conducted according to Good Clinical Practices and was approved by the ethics committee of Sir Run Run Shaw Hospital. All patients provided written informed consents prior to study procedures.
Treatment
Apatinib was provided by Jiangsu Hengrui Medicine Co., Ltd. as tablets to be administered orally daily. Patients were treated with apatinib at 250, 425 or 500 mg daily until disease progression or intolerable. Performance status, blood pressure, complete blood count, urine, liver and kidney function were monitored during the trial.
Efficacy and safety assessments
Clinical and radiologic assessments were conducted at baseline and every 2 months. Adverse events (AEs) were assessed all through the treatment cycles according to Common Terminology Criteria for Adverse Events (CTCAE) 4.03. The efficacy evaluation was conducted according to the RECIST 1.1 criteria, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Progression-free survival (PFS) was defined as the time from enrollment to investigator-assessed disease progression or death, whichever occurred first. Overall survival (OS) was defined as time from enrollment to death. Apatinib related toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0.
Data analysis
Statistical analyses were performed by SPSS version 24.0 (IBM, Chicago, IL). Median PFS and OS were calculated using the Kaplan-Meier method.
Results
Patients Characteristics
A total of 32 patients with refractory or relapsed liver cancer were included in this retrospective study. Patient characteristics at baseline were summarized in Table 1. 22 patients (69%) were male and 24 patients (75%) were older than 65. Most of the patients (78%) were diagnosed as hepatocellular carcinoma. The dosage of the apatinib was determined by the attending physician based on patient's age, body weight, general status and tolerance. Finally, 7 patients (22%) were assigned to 250 mg group, 14 patients (44%) to 425 mg group and 11 patients (34%) to 500 mg group.
Patient characteristics
| Characteristics | Results |
|---|---|
| Sex, n (%) | |
| Male | 22 (69) |
| Female | 10 (31) |
| Age, n (%) | |
| ≤60 | 8 (25) |
| >60 | 24 (75) |
| Pathology, n (%) | |
| Hepatocellular carcinoma | 25 (78) |
| Intrahepatic cholangiocarcinoma | 7 (22) |
| ECOG performance status, n (%) | |
| 0 | 10 (31) |
| 1 | 22 (69) |
| Child-Pugh score, n (%) | |
| A | 15 (47) |
| B | 17 (53) |
| Dosage of Apatinib (mg), n (%) | |
| 250 | 7 (22) |
| 425 | 14 (44) |
| 500 | 11 (34) |
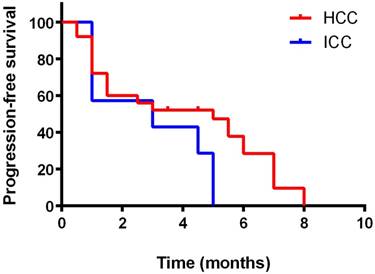
Kaplan-Meier curve for progression-free survival of the patients
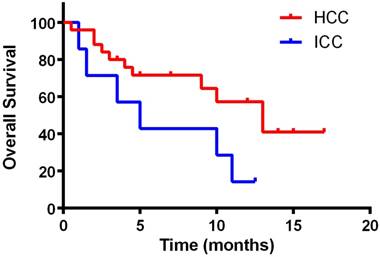
Kaplan-Meier curve for overall survival of the patients
Efficacy of Apatinib Treatment
All patients who were treated with at least one dose of apatinib were included in efficacy analyses. After 15 months follow-up, no CR occurred, 5 patients (16%) showed PR, 14 patients (44%) had SD, 13 patients (41%) had PD. The overall response rate was 16%. The disease control rate (CR plus PR plus SD) was 60%.
All 5 patients with PR were HCC, and 4 of them were advanced diseases upon diagnoses, for whom surgery could not be an option, the other one was relapsed cancer after surgery. 1 of them had previous failed with sorafenib. Their ages ranged from 44 to 78. The dosages of apatinib were between 250mg to 500mg.
Kaplan-Meier curves for PFS and OS were depicted in Figures 1 and 2, respectively. The median PFS was 5 months (95% confidence interval [CI]: 4.3-6.1 months) for HCC and 3 months (95% confidence interval [CI]: 2.5-4.2 months) for ICC. The median OS was 13 months (95% CI: 12.4-14.1 months) for HCC and 5 months (95% confidence interval [CI]: 4.5-6.2 months) for ICC, respectively. Kaplan-Meier curves for PFS and OS by gender and ages were shown in supplementary figure 1-4. No significant differences of efficacy were found between genders as well as ages (p>0.05).
Safety
The most frequently adverse effects of apatinib were summarized in Table 2. Generally, the treatment-related AEs were mild and no toxic induced death occurred. Liver dysfunction was the most common toxicity, manifested as elevated transaminases. Four of these patients (13%) developed liver damage at grade 3. One patient suffered from hand and foot syndrome, which caused treatment discontinuation. Secondary hypertension occurred in 9 patients (28%). 10 patients (31%) had proteinuria. 4 patients (12%) had symptoms of fatigue. There were no hematologic toxicities greater than grade 3. Other rare side effects were nausea, vomiting and dizziness, mainly as grade 1 to 2.
Treatment-related toxicities
| Side effects | Grade, n (%) | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| Secondary hypertension | 5 (15) | 4 (13) | - | - |
| Proteinuria | 8 (25) | 2 (6) | - | - |
| Liver dysfunction | 4 (13) | 8 (25) | 4 (13) | - |
| Hand and foot syndrome | 5 (16) | 2 (6) | 1 (3) | - |
| Fatigue | 2 (6) | 2 (6) | - | - |
| Anemia | 3 (9) | 1 (3) | - | - |
| Neutropenia | 5 (15) | 3 (9) | - | - |
| Thrombocytopenia | 2 (6) | 1 (3) | - | - |
Discussion
Liver cancer is not sensitive to conventional cytotoxic chemotherapy, which is a big obstacle for patients with refractory or relapsed disease. Sorafenib, a multiple kinases inhibitor approved by FDA as a standard of care for advanced HCC, extended the median OS for about 3 months (10.7 months in sorafenib arm vs 7.9 months in placebo arm). The disease control rate was 43% vs 32% (sorafenib vs placebo) [3]. In our study, patients treated with apatinib, a specific VEGFR-2 inhibitor, showed median media OS for 13 months, with mild drug-related toxicities. The disease control rate was 60%. A phase II randomized trial on apatinib as first line treatment in Chinese patients confirmed that apatinib had potential survival benefit in patients with advance HCC [12]. Our study also demonstrated that apatinib was effective in treating advanced liver cancer.
The most common treatment-related side effects in our study were proteinuria (31%), secondary hypertension (28%) and liver dysfunction (13%). Apatinib treatment was suspended when liver dysfunction reached grade 3 and continued after liver function recovered. No patients discontinued due to liver dysfunction. Only one patient discontinued permanently because of grade 3 hand and foot syndrome in this study. Hematologic toxicities were mild. No grade 3 or 4 anemia, neutropenia or thrombocytopenia occurred during treatment. In the trials of sorafenib, grade 3 drug-related adverse events were diarrhea (8%), hand-foot skin reaction (8%), hypertension (2%), and abdominal pain (2%). The frequent adverse events leading to sorafenib discontinuation were gastrointestinal events (6%), fatigue (5%), and liver dysfunction (5%) [3], which were different from apatinib. It suggests that the adverse effects of apatinib are mild and it might be an alternative treatment for patients intolerant to sorafenib.
A previous phase II study was carried out in 121 patients with advanced HCC to assess the efficacy of apatinib in treatment-naive Chinese patients [12]. The dosages in that study were 850 mg/qd or 750 mg/qd, much higher than ours. The median overall survival of the two groups was 9.7 months and 9.8 months respectively. The disease control rate was 48.57% and 37.25%, respectively. Patients were tolerable, most of the adverse event could be managed by dose interruptions or reductions, which were similar to our study. The differences of efficacy may be caused by different inclusion criteria. In our study, 16 patients (50%) had previous hepatectomy treatments, 10 of 25 HCC patients (40%) had been treated with sorafenib before enrollment. The tumor burden in our study may be smaller than naïve patients. However, only 32 patients were enrolled in our study and it was a single center trial, which might cause bias of the results. Also, due to the limited number of patients, we could not further analyze the clinical and molecular characteristics in each group with PR, SD or PD, which might be import for efficacy prediction.
Many previous clinical studies set the dosage of apatinib at 850 mg or 750 mg [11, 13, 14]. However, in clinical practice, we found that most liver cancer patients were intolerant to 850 mg due to severe hand-foot syndrome, proteinuria and hypertension. In this study, the dosage of the apatinib was determined by the attending physician based on patient's age, body weight, general status and tolerance, ranging from 250 mg to 500 mg. In a pharmacokinetic study of orally administrated apatinib, Yu etc. concluded that the pharmacokinetics of apatinib in gastric cancer patients were significantly different from those in patients with other cancer types and the dosage of apatinib in various cancer subpopulations may require adjustments to optimize efficacy and benefits to patients [15]. This point was confirmed by several other clinical studies [16, 17], within which the dosage of apatinib varied from 250mg to 500mg, consisted with our study.
Conclusion
This study demonstrated that apatinib was potentially an effective drug for patients with unresectable or recurrent liver cancers. The frequently observed AEs were proteinuria, secondary hypertension and liver dysfunction. The toxicities were tolerable and manageable.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81602635, 81772543), the Zhejiang Province Preeminence Youth Fund (LR16H160001), and the Zhejiang medical innovative discipline construction project-2016.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498 author reply -9
4. Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C. et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-16
5. Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y. et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172-9
6. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66
7. Oikonomopoulos G, Aravind P, Sarker D. Lenvatinib: a potential breakthrough in advanced hepatocellular carcinoma? Future Oncol. 2016;12:465-76
8. ML BP, Miksad RA. Cabozantinib in the treatment of hepatocellular carcinoma. Future Oncol. 2017
9. Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. 2015;20:660-73
10. Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y. et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-80
11. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y. et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-54
12. Qin S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. Journal of Clinical Oncology. 2014:32
13. Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y. et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-25
14. Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z. et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961-9
15. Yu M, Gao Z, Dai X, Gong H, Zhang L, Chen X. et al. Population Pharmacokinetic and Covariate Analysis of Apatinib, an Oral Tyrosine Kinase Inhibitor, in Healthy Volunteers and Patients with Solid Tumors. Clin Pharmacokinet. 2017;56:65-76
16. Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L. et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820
17. Zhang Y, Gou M, Han C, Li J, Wang L, Qiao Q. et al. Efficacy and safety of apatinib as second-line therapy for advanced gastric cancer: a single-center observational study. Anticancer Drugs. 2017
Author contact
![]() Corresponding authors: Weidong Han, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006926, E-mail: hanwdedu.cn; Hongming Pan, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006922, E-mail: panhongmingedu.cn.
Corresponding authors: Weidong Han, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006926, E-mail: hanwdedu.cn; Hongming Pan, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006922, E-mail: panhongmingedu.cn.

 Global reach, higher impact
Global reach, higher impact