Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(17):3101-3108. doi:10.7150/jca.27206 This issue Cite
Research Paper
Clinical Value of Combining 18F-FDG PET/CT and Routine Serum Tumor Markers in The Early Detection of Recurrence Among Follow-up Patients Treated for Cervical Squamous Cell Carcinoma
1. The Key Laboratory of Cancer Invasion and Metastasis of the Ministry of Education of China, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, P. R. China.
2. Department of Nuclear medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P. R. China.
Received 2018-5-11; Accepted 2018-6-28; Published 2018-8-6
Abstract
Objective: The purpose of this retrospective study was to investigate the role of 18F-Fluorodeoxyglucose Positron Emission Tomography (18F-FDG PET/CT) and evaluate if combined elevated serum tumor markers levels improve the accuracy of 18F-FDG PET/CT in detecting recurrence of cervical squamous cell carcinoma.
Methods: A total number of 42 patients who were treated for cervical squamous cell carcinoma and had underwent 18F-FDG PET/CT for suspected recurrence of cervical cancer were retrospectively reviewed in this study and their clinical, pathological and serological data were collected and analyzed. The clinical value of combining 18F-FDG PET/CT with serum tumor markers was investigated.
Results: Among the 42 patients, 18F-FDG PET/CT was true positive in 25 (59.5%), false positive in 5 (11.9%), true negative in 12 (28.5%) and false negative in none. The overall patient-based sensitivity, specificity, accuracy, positive predictive value and negative predictive value of 18F-FDG PET/CT in detecting recurrent cervical cancer were 100%, 70.6, 88.1%, 83.3%, and 100%, respectively. The accuracy of 18F-FDG PET/CT with combined squamous cell carcinoma antigen (SCC Ag) and carcinoembryonic antigen (CEA) elevation was 100% compared to only SCC Ag elevation and only CEA elevation, 90% and 33.3%, respectively. The positive predictive value of a positive 18F-FDG PET/CT with combined SCC Ag and CEA elevation was 100% for detection of recurrent cervical cancer. Also, the negative predictive value of a negative 18F-FDG PET/CT combined with normal SCC Ag and CEA levels was 100%.
Conclusion: 18F-FDG PET/CT is highly sensitive in the diagnosis of recurrent cervical cancer. When 18F-FDG PET/CT is associated with both SCC Ag and CEA elevation or only SCC Ag elevation, the accuracy is increased but not when associated with only CEA elevation. Positive 18F-FDG PET/CT associated with both tumor markers elevation can precisely predict recurrence. Moreover, normal levels of both tumor markers with a negative 18F-FDG PET/CT result may clinically reassure that a recurrence is absent.
Keywords: Cervical cancer, recurrence, 18F-FDG PET/CT, squamous cell carcinoma antigen, carcinoembryonic antigen
Introduction
Cervical cancer figures among the second most frequently diagnosed malignancy of the female genital tract and is the third leading cause of cancer-related death among females in developing countries [1]. In China, cervical cancer ranks sixth among the most commonly diagnosed female malignancy and is the eighth cause of cancer-related death among females [2]. The wide application of cytological screening for cervical cancer has reduced the incidence significantly in developed countries, but in developing countries, its incidence is still high [3, 4]. Cervical cancer is clinically staged by International Federation of Gynecology and Obstetrics system which has been revised in 2009 [5]. The current approach after diagnosis of cervical cancer is treatment guided by the clinical staging followed by tumor surveillance program with the aim for early recognition of recurrence [5, 6]. Although there have been great advances in the treatment of cervical cancer, detection of disease recurrence is still high during follow-up. About one-third of patients treated for cervical cancer develop recurrence within the first 2 years of completed therapy, ranging from 10-20% for stage IB1/IIA, up to 72% for stage IVA [7, 8]. The conventional follow-up procedures consist of systematic visits for patient history, complete physical examination, cytological investigations, tumor markers and imaging such as Computed Tomography and/or Magnetic Resonance Imaging. However, neither this methodology nor the tumor markers evaluated are part of a standardized protocol [9, 10]. Rising levels of tumor markers during follow-up can precede clinical detection of tumor recurrence [10]. Currently, Squamous cell carcinoma antigen (SCC Ag) and carcinoembryonic antigen (CEA) are the two serum tumor markers of choice by physicians for detection of recurrent cervical cancer. Raised levels of SCC Ag and CEA during follow-up of treated cervical cancer is observed in 62.5-92% and 29-64% of cases, respectively [11]. Although these tumor markers can predict recurrence before the lesions become clinically apparent, they cannot define the location of these lesions.
A review of the literature supports 18F-Fluorodeoxyglucose Positron Emission Tomography (18FDG PET) or 18F-Fluorodeoxyglucose Positron Emission Tomography/ Computed Tomography (18FDG PET/CT) as a promising tool for determination of metabolically active lesions during follow-up after therapy of various malignancies. These methods have the capacity to detect recurrent cervical cancer lesions, through pathologically increased tissue metabolism, which precedes the appearance of morphological changes detected by conventional imaging techniques [12, 13]. An intervention on 18FDG PET/CT using metabolic tumor parameter has been suggested to improve the diagnostic accuracy in several cancers. To date, semi-quantitative analysis is based on volume-of-interest 18FDG uptake where maximum standardized uptake value (SUVmax) is the backbone [14]. Previous reports have shown the clinical value 18FDG PET/CT in patients with elevated serum tumor markers during follow-up of cervical cancer. All these studies have evaluated the clinical significance of 18FDG PET/CT with elevated SCC Ag or CEA in the detection of cervical cancer recurrence, but currently, there are no reports on 18FDG PET/CT and combined elevation of SCC Ag and CEA for early recognition of recurrence in treated cervical cancer cases [15-18].
In this study, we evaluated the clinical value of combining 18F-FDG PET/CT and routine serum tumor markers in the early detection of recurrence among follow-up patients treated for cervical squamous cell carcinoma.
Materials and methods
Patients
We enrolled patients who underwent 18F-FDG PET/CT scan for detection of cervical cancer recurrence from February 2014 to June 2017 in our hospital, and their medical records were retrospectively reviewed. The inclusion criteria for the study were: 1) Patients whose histopathological examinations showed squamous cell carcinoma of the cervix; 2) Patients were completely treated for cervical carcinoma; 3) patients were in remission for at least 6 months' period before recurrent lesions were suspected; 4) patients had both serum SCC Ag and CEA measured during the tumor surveillance program; 5) patients had 18F-FDG PET/CT scan done within an interval of less than 2 weeks following suspicion of recurrent lesions; 6) patients received no treatment between the interval from suspicion of cervical carcinoma recurrence and 18F-FDG PET/CT scan. The exclusion criteria were: 1) histopathological examinations showed adenocarcinoma or adenosquamous carcinoma of the cervix; 2) treatment was not cure-intent. The methods of enrolling patients were shown in Figure 1. A total number of 42 patients met the above criteria and were enrolled in this study.
Suspicion of cervical carcinoma recurrence
Suspicion of cervical carcinoma recurrences were based on patients presented with symptoms of tumor recurrence, abnormal findings on physical and internal examinations, abnormal results of cytological investigations, elevated levels of serum tumor markers and conventional imaging showed suspected recurrent lesions.
Tumor markers measurements
Serum samples were collected on each follow-up visit. The serum SCC Ag level was estimated using an immunoradiometric assay kit (Imx, Abbott Diagnostics, Abbott Park, IL, USA). Serum CEA level was estimated using an automated chemiluminescence analyzer (ZT-480 Automated Chemiluminescence Analyzer, Beijing Savant Biotechnology Co., LTD., CHINA). The normal upper cut-off limit for SCC Ag and CEA were 1.5 ng/ml and 5.0 ng/ml, respectively.
Flowchart illustrating methods of enrolling patients in the study.
18F-FDG PET/CT scanning and image interpretation
Prior to 18F-FDG PET/CT scans, all patients were instructed to fast for at least 6 hours. 3.7MBq/kg body weight of 18F-FDG was then administered and the patients laid in a supine position for 1 hour in a dark and quiet room. Image acquisition was thus done using an integrated 18F-FDG PET/CT (Discovery PET/CT Elite, GE Medical Systems, USA). A whole body 18F-FDG PET/CT scanning consisted of CT scans prior to PET scans, in a supine position from the head to mid-thigh. The CT scan parameters were set to 120Kv, 28.5-150mA, 0.5s per rotation and 39.37mm per rotation. The data obtained from the CT scans were constructed to images with a matrix 512 x 512 and a slice thickness of 3.75mm. The PET scan was acquainted in a 3D imaging mode and the data obtained were reconstructed to image with a matrix of 192 x 192.
The CT and PET images were fused using Advanced workstation 4.5 (GE Medical Systems, Waukesha, Wisconsin, USA). The generated images were analyzed by two experienced nuclear medicine physicians, being aware of the patients' clinical history and indication for 18F-FDG PET/CT. A semi-quantitative analysis was done by placing a VOI (Volume of Interest) over the suspected lesions to measure the SUVmax. The SUVmax was calculated by the formula: SUVmax=maximum voxel activity/ (injected dose/body weight).
A region was defined as malignant if the focal uptake of 18F-FDG was higher (SUVmax ≥2.5) than the surrounding tissue and were not associated with physiological uptake.
Confirmation of recurrences
The confirmation of recurrence for a positive PET/CT case was determined either by tissue biopsy or imaging studies or with second look surgery. As for the negative PET/CT cases, the patients were followed up for a short period by physical examination, cytological investigations, routine tumor markers as well as imaging modalities.
Data and statistical analysis
The following patients' characteristics were retrospectively collected: age at first presentation, histopathology of cervical cancer, initial FIGO stage, treatment modalities, date of last treatment, date of suspicion of recurrence, date of PET/CT done, tumor markers (SCC Ag and CEA) during follow-up, means of suspecting recurrence, PET/CT reports, median SUVmax values and evidence of recurrence.
The PET/CT reports were classified as recurrence and no recurrence groups. Upon confirmation of the diagnosis, the cases were further classified into true-positive, false-positive, true-negative and false-negative. The overall sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of PET/CT for detection of recurrence of cervical carcinoma were determined. Fisher's extract test or Pearson Chi-square test and Mann-Whitney U test were used for categorical and numerical data, respectively. Absolute and percentage frequencies were used to describe categorical data while means, median, standard deviation, and range were used to express continuous data. Statistical analyses were performed using SPSS software package (Version 23.0.0, SPSS, Chicago, IL, USA) and GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. A p value < 0.05 was considered as statistically significant.
Results
A total of 42 patients with treated histopathologically proven squamous cell carcinoma of cervix underwent 18F-FDG PET/CT due to suspicion of recurrence by either symptoms, abnormal findings on physical and internal examinations, abnormal cytological reports, elevated levels of serum tumor markers or abnormal conventional imaging. The characteristics of patients enrolled in this study is summarized in Table 1.
Characteristics of patients enrolled in the study.
| Characteristics | n |
|---|---|
| Total patients | 42 |
| Age (years) | 47.62 (26-67) |
| Pathology | |
| Squamous cell carcinoma | 42 |
| Stage | |
| IA | 2 |
| IB | 13 |
| IIA | 5 |
| IIB | 15 |
| IIIA | 1 |
| IIIB | 5 |
| IVA | 1 |
| Primary treatment | |
| Conization | 1 |
| Radical hysterectomy | 5 |
| Radical hysterectomy + Chemotherapy | 2 |
| Radical hysterectomy + Radiotherapy | 8 |
| Radical hysterectomy + Concurrent chemoradiotherapy | 2 |
| Neoadjuvant chemotherapy + Radical hysterectomy + concurrent chemoradiotherapy | 17 |
| Concurrent chemoradiotherapy | 7 |
| Interval | |
| Last treatment to suspicion of recurrence (months) | 40.6 (6-150) |
| Suspicion of recurrence to PET/CT (days) | 4.1 (0-12) |
| Elevated tumor markers | |
| SCC Ag only | 10 |
| CEA only | 6 |
| SCC Ag and CEA | 8 |
| None | 18 |
| Confirmation of diagnosis | |
| Biopsy | 18 |
| Imaging (CT/MRI) | 16 |
| Tumor markers | 1 |
| Follow-up | 4 |
| Second look surgery | 3 |
SCC Ag, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen
CT, computed tomography; MRI, magnetic resonance imaging
Diagnostic efficiency of 18F-FDG PET/CT to detect cervical cancer recurrence
Of the 42 cervical cancer patients, 18F-FDG PET/CT detected recurrent lesions in 30 patients and ruled it out in 12 patients. The 18F-FDG PET/CT was true positive in 25 patients, false positive in 5 patients, true negative in 12 patients and false negative in none (Table 2). The common localization of 18F-FDG uptake was in pelvic lymph nodes. 18F-FDG PET/CT also found a second disease which was primary adenocarcinoma in lung. The distribution of pathological uptake of 18F-FDG are summarized in Table 3. In this study, the overall patient-based sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of 18F-FDG PET/CT for detecting cervical cancer recurrence were 100%, 70.6%, 88.1%, 83.3% and 100%, respectively.
Comparative analysis of 18F-FDG PET/CT patient-based performance of 42 treated cervical cancer patients in comparison with tumor markers.
| Elevated tumor markers | |||||
|---|---|---|---|---|---|
| SCC Ag | CEA | SCC Ag + CEA | None | Total | |
| TP | 9 | 2 | 8 | 6 | 25 |
| TN | 0 | 0 | 0 | 12 | 12 |
| FP | 1 | 4 | 0 | 0 | 5 |
| FN | 0 | 0 | 0 | 0 | 0 |
| Total | 10 | 6 | 8 | 18 | 42 |
| Sensitivity (%) | 100 | ||||
| Specificity (%) | 70.6 | ||||
| Accuracy (%) | 88.1 | ||||
| PPV (%) | 83.3 | ||||
| NPV (%) | 100 | ||||
| Positive likelihood ratio | 3.4 | ||||
| Negative likelihood ratio | 0 | ||||
SCC Ag, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen
TP, true positive; TN, true negative; FP, false positive; FN, false negative
PPV, positive predictive value; NPV, negative predictive value
*1 primary adenocarcinoma of lung included
Location of pathological 18F-FDG uptake in true positive cases.
| Sites of abnormal 18F-FDG uptake | n |
|---|---|
| Vaginal stump | 3 |
| Pelvic lymph nodes | 10 |
| Inguinal lymph nodes | 2 |
| Parametrium | 2 |
| Para-aortic lymph nodes | 1 |
| Peritoneal deposits | 2 |
| Colonic metastases | 1 |
| Liver metastases | 5 |
| Mediastinal lymph nodes | 4 |
| Pulmonary metastases | 2 |
18F-FDG, 18F-Fluorodeoxyglucose
Diagnostic efficiencies of 18F-FDG PET/CT with each tumor marker and in combination of both tumor markers in detection of cervical cancer recurrence
Among the patients (n=42), 24 had elevated levels of SCC Ag and/or CEA and 18 had normal levels of both tumor markers. Of the elevated tumor markers (n=24), 10 had elevated levels of SCC Ag only, 6 had elevated levels of CEA only and 8 had elevated levels of both tumor markers. All 8 patients with both tumor markers elevated had developed recurrence. 9 out of 10 patients with elevated SCC Ag only and 2 out of 6 patients with elevated CEA only developed recurrence during the follow-up period. One patient with only increased SCC and 4 patients with only increased CEA levels were false positive, details of which are shown in Table 4. 6 patients developed recurrence and 12 did not within the normal levels of serum tumor markers group.
Characteristics of false positive 18F-FDG PET/CT report.
| Tumor marker | Value (ng/ml) | Diagnosis |
|---|---|---|
| SCC Ag | 4.30 | Radiation induced fibrosis |
| CEA | 61.2 | Granulation tissue following neck surgery |
| CEA | 5.50 | Abscess |
| CEA | 7.45 | Radiation induced fibrosis |
| CEA | 31.65 | Abscess |
SCC Ag, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen
The accuracy of 18F-FDG PET/CT with elevated SCC Ag levels (90%; 9/10) was higher than 18F-FDG PET/CT with elevated CEA levels (33.3%; 2/6). The accuracy of 18F-FDG PET/CT in detecting recurrence was significantly different between elevated SCC Ag levels group and elevated CEA levels group (p=0.036). 18F-FDG PET/CT with combined SCC Ag and CEA elevation was more accurate (100%; 8/8) than 18F-FDG PET/CT with elevated SCC Ag levels, but there was no statistically significant difference between them (p=1.00). The PPV of a positive 18F-FDG PET/CT result with elevated levels of both tumor markers for the detection of recurrent cervical cancer was 100%. The NPV of a negative 18F-FDG PET/CT associated with normal levels of both tumor markers was 100%.
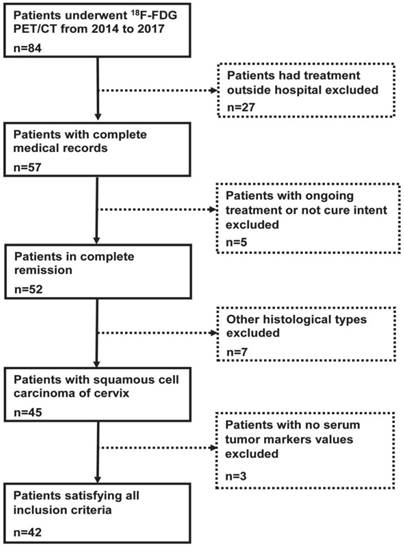
Correlation between tumor markers and SUVmax values
SUVmax showed a reasonable correlation with SCC Ag levels but not with CEA levels as shown in Figure 2A and 2B, respectively. The correlation between SUVmax and SCC Ag levels was statistically significant (p=0.03). No statistically significant correlation between SUVmax and CEA levels was observed (p=0.08). Moreover, there was so statistically significant correlation between the two tumor markers levels (p=0.23) as shown in Figure 2C.
Differences in tumor markers and SUVmax values among the recurrence and no recurrence group
The mean values of SCC Ag levels, CEA levels and SUVmax values were 11.3 ± 17.9 ng/ml, 14.6 ± 30.6 ng/ml and 7.3 ± 2.4, respectively in the recurrence group and 0.9 ± 0.9 ng/ml, 7.3 ± 15.7 ng/ml and 3.4 ± 3.7, respectively in the non-recurrence group. The differences of SCC Ag levels and SUVmax values were statistically significant (p<0.05 and p<0.05, respectively) between the recurrence and non-recurrence group. There was no statistically significant difference in CEA levels (p=0.05) between the recurrence and non-recurrence group. The distributions of SCC Ag, CEA, and SUVmax, respectively in recurrence versus non-recurrence group are illustrated in box-plot graphs (Figure 3).
Discussion
The aim of this retrospective study was to evaluate the role of 18F-FDG PET/CT in the diagnosis of recurrent squamous cell carcinoma of cervix in patients with elevated levels of SCC Ag and CEA. To our best knowledge, there is no previous study that reports the clinical value of 18F-FDG PET/CT in the recurrence of cervical cancer based on both tumor markers. Previous studies have reported the clinical value of 18F-FDG PET and 18F-FDG PET/CT to detect recurrence in patients treated for cervical cancer. However, those studies prioritized the use of 18F-FDG PET for detection of recurrence or included only patients with elevated tumor markers. Moreover, those studies showed the complementary role of 18F-FDG PET/CT and serum SCC Ag only for the diagnosis of recurrent cervical cancer [9, 15-28].
Scatter-plot graphs illustrating the correlation value between various measured parameters. (A) There was a reasonable correlation between SUVmax and SCC Ag levels (r= 0.44). This correlation between SUVmax and SCC Ag levels was statistically significant (p< 0.05). (B) There was a very low correlation between SUVmax and CEA levels (r= 0.23). This correlation between SUVmax and CEA levels was not statistically significant (p= 0.08). (C) There was a meaningless correlation between SCC Ag and CEA levels (r= 0.19). No statistically significant correlation between the two tumor markers was observed (p= 0.23). r, correlation coefficient; SCC Ag, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value.
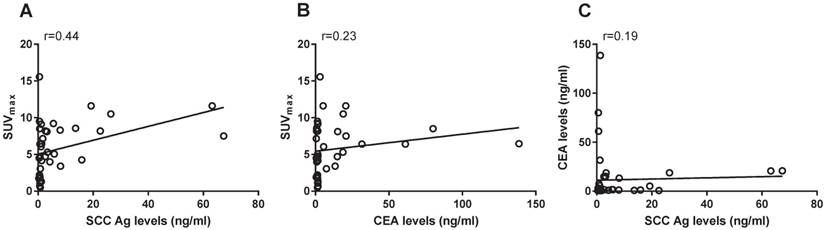
Box-plot graphs illustrating the distribution of SCC Ag, CEA and SUVmax through recurrence and non-recurrence group. (A) There was statistically significant difference in SCC Ag levels through the recurrence and non-recurrence group (p< 0.05). (B) There was no statistically significant difference in CEA levels through recurrence and non-recurrence group (p=0.55). (C) There was statistically significant difference in SUVmax values through the recurrence and non-recurrence group (p<0.05). SCC Ag, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value.
The principal finding of our study is that combining elevated SCC Ag and CEA levels with 18F-FDG PET/CT predicts the presence of recurrence and localization of relapse with 100% accuracy. The second major finding of this study is the high negative predictive value of 100% with negative 18F-FDG PET/CT scan combined with normal tumor markers levels. Thus, a negative 18F-FDG PET/CT scan with normal SCC Ag and CEA levels may be clinically reassuring of no cervical cancer recurrence.
This study has also found that there exists a reasonable correlation between SCC Ag levels and tumor volumes based on a SUVmax value of 2.5 to determine tumor boundaries and volumes on 18F-FDG PET/CT. However, no such correlation was observed with CEA levels suggesting that cervical cancer recurrence with normal CEA levels would be demonstrated with 18F-FDG PET/CT or elevated CEA levels might be associated with negative 18F-FDG PET/CT. As such, the combination of only CEA levels with 18F-FDG PET/CT to detect cervical cancer recurrence during follow-up might not be appropriate for clinical practice.
Mike et al. and Forni et al. showed that elevated levels of SCC Ag can predict recurrence before the lesions become clinically apparent [29, 30]. However, these tumor markers cannot define the location of these lesions. In a comparative study between 18F-FDG PET and CT/MRI, it was reported that 18F-FDG PET was superior to CT in detecting small metastatic lymph nodes, whose principle is based on increased glycolysis levels in tumor cells [31]. Our study demonstrated a good overall patient-based sensitivity, accuracy, PPV and NPV of 18F-FDG PET/CT in detecting recurrent cervical cancer that are consistent with previous studies [16-18, 22]. However, the specificity is lower (70.6%) compared to those studies. The reason might be due to the fact that both elevated and non-elevated levels of tumor markers were considered in the study. Another reason that could explain this is the inclusion of CEA in the study that accounted for most of the false positive results. False positive results have also been reported in several previous reports and these results are explained by physiological hypermetabolism and reactive lymph nodes [8, 17, 18].
In the study, we also found six patients with normal levels of serum tumors markers who developed recurrences. As such, we are unable to say that normal levels of serum tumor markers can rule out recurrence. However, normal levels of both tumor markers associated with negative 18F-FDG PET/CT result could clinically reassure that a recurrence is absent.
In a retrospective study, Chong et al. [17] evaluated the clinical usefulness of 18F-FDG PET/CT in the detection of early recurrence in treated cervical cancer patients with unexplained elevation of serum tumor markers and the authors reported a similar finding that 18F-FDG PET/CT combined with elevated SCC Ag levels is more accurate than 18F-FDG PET/CT combined with elevated CEA levels which is supported by no correlation between these tumor markers. However, the discrepancy in accuracy values might be due to a difference in sample size and patients in their study measured either SCC Ag or CEA but not both.
Hu et al. [18] reported three cases of metachronous tumors that were considered as true positive. In our study, one metachronous tumor of adenocarcinoma of lung was observed that was regarded as true negative as 18F-FDG PET/CT distribution is well known for detecting of other diseases and/or metachronous tumor [32].
This study had certain limitations. Despite the combination of both tumor markers with 18F-FDG PET/CT is superior to 18F-FDG PET/CT with SCC Ag, the associated increase in diagnostic accuracy is uncertain due to small sample size. Other limitations were this was a retrospective study and the diagnosis of cervical carcinoma recurrence was based on follow-up.
Conclusions
Our study indicated that 18F-FDG PET/CT is highly sensitive for the diagnosis of recurrent cervical cancer regardless to type and level of tumor marker. The combination of elevated SCC Ag and CEA levels with 18F-FDG PET/CT improves the accuracy and localization of recurrent lesions to 100% thus offering maximum information for restaging and selecting appropriate salvage therapy for cervical cancer recurrence. Also, normal levels of both tumor markers with a negative 18F-FDG PET/CT result may clinically reassure that a recurrence is absent.
Abbreviations
18FDG PET/CT: 18F-Fluorodeoxyglucose Positron Emission Tomography/ Computed Tomography, SCC Ag: Squamous cell carcinoma antigen, CEA: Carcinoembryonic antigen, SUVmax: maximum standardized uptake value, PPV: positive predictive value, NPV: negative predictive value.
Acknowledgements
This work was supported by the National Science Foundation of China (Grants 81372806, 81472783, 81630060); the National 973 Project of China (2015CB553903); the National Key Research & Development Program of China (2016YFC0902901). We thank all participants recruited for this study. We would also like to thank Shixuan Wang and Ding Ma. We also appreciate the cooperation of all participating institutions.
Ethics committee approval
This retrospective study was approved by the Ethics Committee of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, P. R. China.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global Cancer Statistics, 2012. CA: a cancer journal of clinicians. 2015;65:87-108
2. Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chinese journal of cancer. 2016;35:73
3. Vaccarella S, Lortet-tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. European Journal of Cancer. 2013;49:3262-73
4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136:E359-E86
5. Pecorelli S. Corrigendum to "Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium". [International Journal of Gynecology and Obstetrics (2009) 105: 103-104] (DOI:10.1016/j.ijgo.2009.02.012). International Journal of Gynecology and Obstetrics. 2010;108:176
6. Burkman RT. Berek & Novak's Gynecology. JAMA. 2012;308:516
7. Perez CA, Grigsby PW, Camel HM, Galakatos AE, Mutch D, Lockett MA. Irradiation alone or combined with surgery in stage IB, IIA, and IIB carcinoma of uterine cervix: update for a nonrandomized comparison. International Journal of Radiation Oncology, Biology, Physics. 1995;31:703-16
8. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. International journal of radiation oncology, biology, physics. 1992;24:197-204
9. Sakurai H, Suzuki Y, Nonaka T, Ishikawa H, Shioya M, Kiyohara H. et al. FDG-PET in the detection of recurrence of uterine cervical carcinoma following radiation therapy — tumor volume and FDG uptake value. Gynecol Oncol. 2006;100:601-7
10. Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Critical Reviews in Oncology/Hematology. 2008 p. 10-20
11. Gadducci A, Cosio S, Carpi A, Nicolini A, Genazzani AR. Serum tumor markers in the management of ovarian, endometrial and cervical cancer. Biomedicine and Pharmacotherapy. 2004;58:24-38
12. Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738-44
13. Nakamura K, Okumura Y, Kodama J, Hongo A, Kanazawa S, Hiramatsu Y. The predictive value of measurement of SUVmax and SCC-antigen in patients with pretreatment of primary squamous cell carcinoma of cervix. Gynecologic Oncology. 2010;119:81-6
14. Basu S, Alavi A. Partial volume correction of standardized uptake values and the dual time point in FDG-PET imaging: Should these be routinely employed in assessing patients with cancer?. European Journal of Nuclear Medicine and Molecular Imaging. 2007 p. 1527-9
15. Chang T-C, Law K-S, Hong J-H, Lai C-H, Ng K-K, Hsueh S. et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004;101:164-71
16. Chang WC, Hung YC, Lin CC, Shen YY, Kao CH. Usefulness of FDG-PET to detect recurrent cervical cancer based on asymptomatically elevated tumor marker serum levels - A preliminary report. Cancer Investigation. 2004;22:180-4
17. Chong A, Ha JM, Jeong SY, Song HC, Min JJ, Bom HS. et al. Clinical Usefulness of (18)F-FDG PET/CT in the Detection of Early Recurrence in Treated Cervical Cancer Patients with Unexplained Elevation of Serum Tumor Markers. Chonnam Med J. 2013;49:20-6
18. Hu Y-Y, Fan W, Zhang X, Liang P-Y, Lin X-P, Zhang Y-R. et al. Complementary roles of squamous cell carcinoma antigen and 18F-FDG PET/CT in suspected recurrence of cervical squamous cell cancer. Journal of Cancer. 2015;6:287-91
19. Chung HH, Jo H, Kang WJ, Kim JW, Park NH, Song YS. et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecologic Oncology. 2007;104:529-34
20. van der Veldt AAM, Buist MR, van Baal MW, Comans EF, Hoekstra OS, Molthoff CFM. Clarifying the Diagnosis of Clinically Suspected Recurrence of Cervical Cancer: Impact of 18F-FDG PET. Journal of Nuclear Medicine. 2008;49:1936-43
21. Grigsby PW, Siegel BA, Dehdashti F, Mutch DG. Posttherapy surveillance monitoring of cervical cancer by FDG-PET. International Journal of Radiation Oncology Biology Physics. 2003;55:907-13
22. Hoon Chung H, Kim JW, Kang KW, Park N-H, Song Y-S, Chung J-K. et al. Predictive role of post-treatment [18F]FDG PET/CT in patients with uterine cervical cancer. European Journal of Radiology. 2012;81:e817-e22
23. Brooks RA, Rader JS, Dehdashti F, Mutch DG, Powell MA, Thaker PH. et al. Surveillance FDG-PET detection of asymptomatic recurrences in patients with cervical cancer. Gynecologic Oncology. 2009;112:104-9
24. Pallardy A, Bodet-Milin C, Oudoux A, Campion L, Bourbouloux E, Sagan C. et al. Clinical and survival impact of FDG PET in patients with suspicion of recurrent cervical carcinoma. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37:1270-8
25. Chu Y, Zheng A, Wang F, Lin W, Yang X, Han L. et al. Diagnostic value of 18F-FDG-PET or PET-CT in recurrent cervical cancer: a systematic review and meta-analysis. Nuclear medicine communications. 2014;35:144-50
26. Unger JB, Ivy JJ, Connor P, Charrier A, Ramaswamy MR, Ampil FL. et al. Detection of recurrent cervical cancer by whole-body FDG PET scan in asymptomatic and symptomatic women. Gynecologic Oncology. 2004 p. 212-6
27. Havrilesky LJ, Wong TZ, Secord AA, Berchuck A, Clarke-Pearson DL, Jones EL. The role of PET scanning in the detection of recurrent cervical cancer. Gynecologic Oncology. 2003;90:186-90
28. Yen TC, See LC, Chang TC, Huang KG, Ng KK, Tang SG. et al. Defining the priority of using 18F-FDG PET for recurrent cervical cancer. Journal of Nuclear Medicine. 2004;45:1632
29. Micke O, Bruns F, Schäfer U, Prott F-J, Willich N. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-)radiotherapy. Anticancer research. 2005;25:1663-6
30. Forni F, Ferrandina G, Deodato F, Macchia G, Morganti AG, Smaniotto D. et al. Squamous Cell Carcinoma Antigen in Follow-Up of Cervical Cancer Treated With Radiotherapy: Evaluation of Cost-Effectiveness. International Journal of Radiation Oncology Biology Physics. 2007;69:1145-9
31. Park DH, Kim KH, Park SY, Lee BH, Choi CW, Chin SY. Diagnosis of recurrent uterine cervical cancer: computed tomography versus positron emission tomography. Korean journal of radiology. 2000;1:51-5
32. Gődény M, Lengyel Z, Polony G, Nagy ZT, Léránt G, Zámbó O. et al. Impact of 3T multiparametric MRI and FDG-PET-CT in the evaluation of occult primary cancer with cervical node metastasis. Cancer imaging: the official publication of the International Cancer Imaging Society. 2016;16:38
Author contact
![]() Corresponding author: pengwu8626com
Corresponding author: pengwu8626com

 Global reach, higher impact
Global reach, higher impact