Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(19):3489-3499. doi:10.7150/jca.26155 This issue Cite
Research Paper
Prognostic value of PD-L1 expression in resected lung adenocarcinoma and potential molecular mechanisms
1. Department of Radiation Oncology, Harbin Medical University Cancer Hospital, Harbin, 150081, China
2. Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin150081, China
3. Department of Medical oncology, Heilongjiang Provincial Hospital, Harbin, 150000, China
*These authors contributed equally to this work
Received 2018-3-19; Accepted 2018-7-17; Published 2018-9-8
Abstract
Background: The prognostic role of PD-L1 expression in surgically resected lung adenocarcinoma (ADC) remains controversial. The present study was aimed to clarify the role of PD-L1 expression in predicting prognosis and to investigate its biological function in ADC.
Materials and Methods: The association between PD-L1 expression and clinical outcomes in patients with resected ADC was analyzed using immunohistochemistry (IHC) in our cohort (n=104), externally validated by a meta-analysis of 13 published studies. The biological role of PD-L1 in ADC was explored using gene set enrichment analysis (GSEA).
Results: Positive PD-L1 expression in tumor cells was observed in 38.5% (40/104). High PD-L1 expression levels were significantly correlated with poor overall survival (P=0.008). Furthermore, the meta-analysis also showed that positive PD-L1 expression was associated with shorter OS than negative PD-L1 expression (HR= 1.75, 95% CI: 1.26-2.42; P<0.001). In subgroup analysis stratified according to ethnicity, the pooled results demonstrated that increased PD-L1 expression was an unfavorable prognostic factor for Asian populations (HR= 2.11, 95% CI: 1.48-3.02; P<0.001), but not for non-Asian populations (HR=1.16, 95% CI: 0.63-2.11, P=0.64). The pooled odds ratios (ORs) indicated that PD-L1 expression was associated with positive lymph node metastasis (OR=1.74, 95% CI: 1.23-2.46; P=0.002) and male (OR=1.56, 95% CI: 1.02-2.37; P=0.04). GSEA revealed PD-L1 expression levels positively correlated with immune process or immune-related pathways.
Conclusion: PD-L1 expression is an important negative prognostic factor in resected ADC. This finding has important implications for immunotherapy targeting the PD-1/PD-L1 pathway in patients with resected ADC.
Keywords: programmed cell death-ligand 1, lung adenocarcinoma, prognosis, GSEA
Introduction
Lung cancer, especially non-small cell lung cancer (NSCLC), is the most prevalent cancer worldwide [1]. Among NSCLC; adenocarcinoma is the most common type of NSCLC. Despite recent advances in screening, minimally invasive techniques for surgery, radiation therapy, targeted therapies, and immunotherapies, the prognosis of NSCLC remains poor [2].Complete surgical resection is the preferred treatment modality for patients with early stage NSCLC. Although patients with early stage NSCLC underwent complete resections, they are not cured, and the 5-year survival rate varies from 73% in stage IA to 9% in stage IIIB [3]. Adenocarcinoma is the most frequently diagnosed form of NSCLC. To improve prognosis, it is of great importance to identify effective biomarker to predict the progression of resected ADC patients.
More recently, the blockade of programmed death 1 (PD-1)/ programmed death ligand 1 (PD-L1) immune checkpoint has been demonstrated a remarkable clinical efficacy through increasing host antitumor immunity [4-7]. PD-1/PD-L1 pathway inhibitors were approval for the treatment of metastatic NSCLC patients [2]. Unfortunately; PD-1/PD-L1 pathway inhibitors are only effective in some patients with NSCLC. It is critically important to effectively screen out patients who may benefit most from PD-1/PD-L1-targeted therapy. A meta-analysis indicated that PD-L1 expression level on tumor cells might be a predictive biomarker of therapeutic response to PD-1/PD-L1-targeted therapy [8]. Therefore, it is essential to fully understand PD-L1 expression in NSCLC and the relationship between PD-L1 expression and prognosis. PD-L1 expression has been found in several cancers, including breast cancer [9], lung cancer [10], gastric cancer [11], colorectal cancer [12], ovarian cancer [13], and prostate cancer [14]. Our previous study showed that positive PD-L1 expression was associated with poor prognosis in gastric cancer [15], breast cancer [16] and surgical lung squamous cell carcinoma [17]. However, data on the prognostic role of PD-L1 expression and the mechanism of progression for resected ADC remains controversial.
In the present study, we explored the prognostic significance of PD-L1 expression by the IHC evaluation in patients with resected ADC, externally validated by a meta-analysis of 13 published studies. Furthermore, we elucidated the molecular pathways associated with PD-L1 expression by gene set enrichment analysis (GSEA) on RNA-sequencing data from The Cancer Genome Atlas (TCGA)
Materials and Methods
Clinical specimen analysis
One hundred and four patients who underwent complete surgical resection lung adenocarcinoma were enrolled at Harbin Medical University Cancer Hospital from January 2009 to December 2012. None of the patients received preoperative chemotherapy, target therapy or radiotherapy. Clinicopathological variables were obtained from medical records. Sixty-three patients (60.6%) were male, and the median age was 62.9 years (range 32-81). This population included 49 smokers (47.1%) and 55 non-smokers (52.9%). Sixty-nine patients (66.3%) presented with pathological stage I-II disease, 35 patients (33.7%) with stage III-IV. This study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital.
Immunohistochemical analysis of PD‑L1 expression
Immunohistochemical (IHC) analysis was performed on formalin‑fixed paraffin‑embedded (FFPE) blocks. Briefly, 4μm-thick sections were dewaxed with xylene, and rehydrated with a graded series of ethanol solutions, and treated with H2O2 in methanol to inhibit endogenous peroxidase activity. Each slide was incubated with rabbit monoclonal antibodies to human PD-L1 (Abcam, Cambridge, UK). The PD-L1 immunostaining results were divided into two groups based on staining intensity and the percentage of tumor cell positivity. Patients with weak staining or less than 5% of tumor cells were considered negative. Patients with moderate or strong staining and more than 5% of tumor cells were considered positive. The 5% cutoff value was chosen based on the result of a previous clinical trial [18]. The detailed protocol used in this study was described in our previous study [17].
Meta-analysis analysis
We conducted a comprehensive electronic database search for published articles using the PubMed, Embase, and Cochrane library databases (up to 31 May, 2017).The following text words were used: (PD-L1 OR B7-H1 OR CD274 OR programmed cell death 1 ligand 1 protein OR CD274 Antigen OR PD-L1 costimulatory protein OR B7H1 Antigen) AND (lung cancer OR non-small cell lung cancer OR lung adenocarcinoma). The inclusion criteria for the present study were as follows: (1) all patients underwent complete pulmonary resection and were histologically confirmed as lung adenocarcinoma; (2) PD-L1 expression was detected by IHC in primary lung adenocarcinoma tissue; (3) Studies provided the correlation between PD-L1 expression and clinicopathological features. (4) Studies provided sufficient information to extract hazard ratio (HR) and 95% confidence interval (CI) date for OS; and (5) Studies were written in English. When duplicate publications were identified, only the most recent article was included in the analysis.
Two independent investigators extracted the relevant data, and any discrepancy was resolved by consensus involving a third investigators. The following data was collected: name of the first author, year of publication, country, number of ADC patients, TNM stage, PD-L1-positive expression, endpoint, HR estimation and outcome.
Public datasets analysis
In order to further investigate the prognostic impact of PD-L1 mRNA gene expression data in lung adenocarcinoma. KM plotter was used to analyze the correlation of PD-L1 mRNA expression to OS (http://kmplot.com/analysis/index.php?p=service&cancer=lung).
Gene set enrichment analysis (GSEA)
Gene expression profile of Lung adenocarcinoma was from The Cancer Genome Atlas (TCGA) database (https://tcga-data.nci.nih.gov/tcga/). The association between gene expression and biological processes was analyzed using GSEA. We used lung adenocarcinoma RNA-seq data generated by TCGA and sorted the samples into the top and bottom quartiles of PD-L1 expression (high and low PD-L1 expression, respectively). Default settings were used and thresholds for significance were determined by permutation analysis (1000 permutations). The gene sets showing FDR of 0.25, a well-established cutoff for the identification of biologically relevant gene, were considered enriched between classes under comparison. The nominal P value and normalized enrichment score (NES) were used to sort the pathways enriched in each phenotype. The KEGG gene sets, GO gene sets biological process database and Canonical pathways from the Molecular Signatures Database-MsigDB were used for enrichment analysis.
Statistical methods
The correlation of PD-L1 expression with clinicopathological characteristics was evaluated using chi-square tests or Fisher's exact test. DFS and OS were assessed by the Kaplan-Meier method, and comparison was conducted using the log-rank test. Prognostic factors of OS were calculated by univariate and multivariate analysis. Statistical analyses were performed using SPSS software (version 17.0; SPSS, Chicago, Illinois, USA).
In the meta-analysis, RevMan 5.3 software and STATA version 12.0 was used for all of the meta-analysis data. The odds ratio (OR) was pooled to measure the correlation of PD-L1 expression with clinicopathological parameters. HR was combined to obtain the association between PD-L1 expression and OS. If HR was not available, we calculated these data points from Kaplan-Meier survival curves using Engauge Digitizer version 4.1.The heterogeneity was assessed using the Chi2 test and I2. If Chi2 P value< 0.1 or an I2 statistic >50%, indicating the presence of heterogeneity; In these cases, a random-effects model was used. Otherwise, a fixed-effects model was used. The potential publication bias was assessed by Egger's and Begg's tests.
Results
Correlations between PD-L1 expression and clinicopathologic features
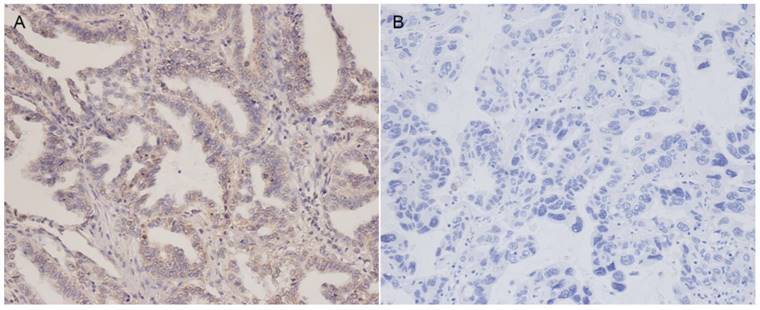
Staining for PD-L1 was mainly observed in the membranes of tumor cells. Representative examples of PD-L1 staining patterns are shown in Figure 1. Positive PD-L1 protein expression was noted in 40 of 104 patients (38.5%). The relationship between PD-L1 expression and clinicopathological features are presented in Table 1. Positive lymph node metastasis tended to show high PD-L1 expression in ADC, but this was not statistically significant (P = 0.081). There were no significant correlations between PD-L1 expression levels and age, gender, smoking history, tumor size, TNM stage.
Representative immunohistochemical staining of PD-L1 in lung adenocarcinoma patients. (A) Positive PD-L1 expression (Magnification 200×). (B) Negative PD-L1 expression (Magnification 200×).
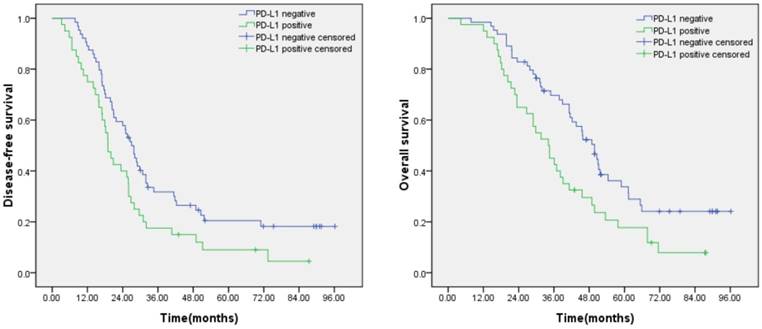
Prognostic significance of PD-L1 expression in lung adenocarcinoma. (A) Disease free survival curves for patients with positive PD-L1 expression and negative PD-L1 expression (P=0.018). (B) Overall survival curves for patients with positive PD-L1 expression and negative PD-L1 expression (P=0.008).
Associations between clinicopathologic parameters and PD-L1 expression
| Clinicopathologic characteristics | All patientsn (%) | PD-L1 expression | P-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Age | 0.779 | |||
| ≤65 | 58(55.8) | 35 | 23 | |
| >65 | 46(44.2) | 29 | 17 | |
| Gender | 0.253 | |||
| Male | 63(60.6) | 36 | 27 | |
| Female | 41(39.4) | 28 | 13 | |
| Smoking history | 0.641 | |||
| Smoker | 49(47.1) | 29 | 20 | |
| Non-Smoker | 55(52.9) | 35 | 20 | |
| Tumor size | 0.361 | |||
| ≤3 cm | 68(65.4) | 44 | 24 | |
| > 3 cm | 36(34.6) | 20 | 16 | |
| Lymph node metastasis | 0.081 | |||
| Negative | 63(60.6) | 43 | 20 | |
| Positive | 41(39.4) | 21 | 20 | |
| TNM stage | 0.164 | |||
| I-II | 69(66.3) | 46 | 23 | |
| III-IV | 35(33.7) | 18 | 17 | |
Univariate and multivariate analyses of prognostic factors for overall survival
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age(>65 vs ≤65) | 1.353 (0.862-2.123) | 0.188 | ||
| Gender (Male vs Female) | 1.231 (0.780-1.942) | 0.373 | ||
| Smoking status (Yes vs No) | 1.184 (0.757-1.852) | 0.460 | ||
| Tumor size (>3cm vs ≤3cm) | 1.553 (0.975-2.475) | 0.064 | 1.283(0.787-2.09) | 0.318 |
| Lymph node metastasis (Yes vs No) | 1.51 (0.963-2.370) | 0.073 | 1.538(0.955-2.475) | 0.077 |
| TNM stage (III-IV vs I-II) * | 1.902 (1.19-3.038) | 0.007 | 1.922(1.182-3.124) | 0.008 |
| PD-L1 (Positive vs Negative) * | 1.811 (1.154-2.842) | 0.01 | 1.571(0.982-2.513) | 0.06 |
*P<0.05
PD-L1 expression was associated with clinical outcomes
Kaplan-Meier analysis revealed that patients with positive PD-L1 expression was significantly correlated with poor disease-free survival (DFS) (P=0.018) and overall survival (OS) (P=0.008) (Figure 2).The univariate Cox regression model showed that TNM stage and PD-L1 expression were correlated with OS, whereas age, gender, smoking status, tumor size, and lymph node metastasis status were not significantly correlated with OS. Further multivariate analyses demonstrated that TNM stage was significant independent predictors of OS (Table 2).
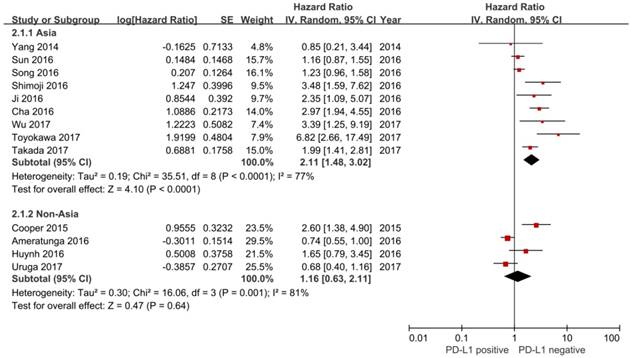
Meta-analysis confirmed the prognostic value of PD-L1 expression
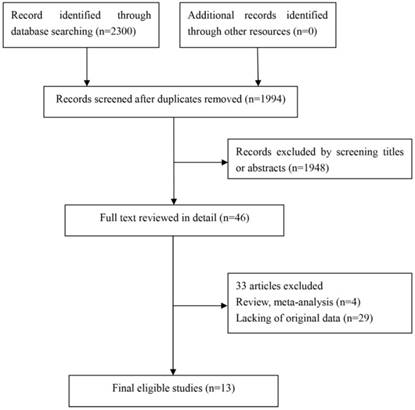
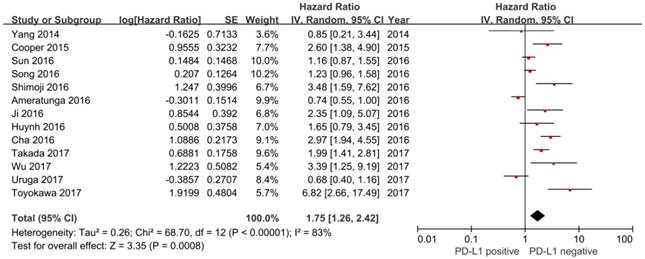
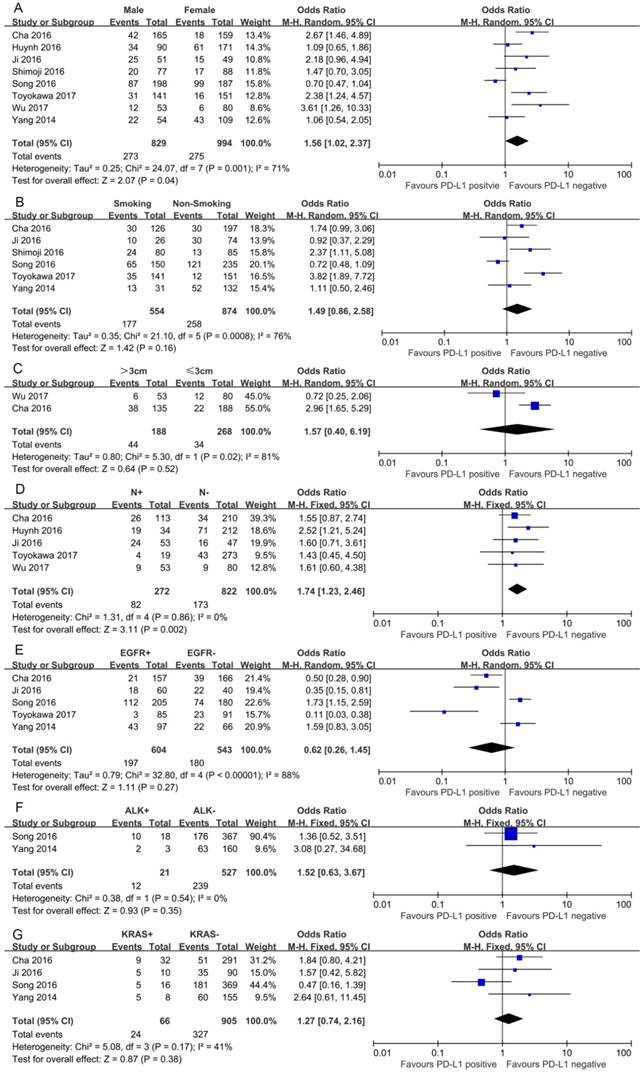
In this study, we identified a total of 2300 potentially relevant articles with our initial search strategy. After screening these articles, we determined that 13 trials met our inclusion criteria and thus included these articles in the final analysis. A detailed flowchart depicting the study selection is presented in Figure 3. The characteristics of the included studies are shown in Table 3. The meta-analysis showed that positive PD-L1 expression was associated with shorter OS than negative PD-L1 expression (HR= 1.75, 95% CI: 1.26-2.42; P<0.001) (Figure 4). Significant heterogeneity was observed (I2 = 83%, P <0.001), therefore, a random effects model was used for the analysis. In addition; we performed subgroup analyses according to ethnicity. The results showed that the combined HRs of Asian studies and non-Asian studies were 2.11 (95% CI: 1.48-3.02, P<0.001) and 1.16 (95% CI: 0.63-2.11, P=0.64), respectively, indicating that PD-L1 was an indicator of the poor prognosis in Asian populations, but not in non-Asian populations (Figure 5). In meta-analysis study, we investigated the association between PD-L1 expression and clinicopathological characteristics. The pooled results showed that PD-L1 expression was increased in patients with male (OR=1.56, 95% CI 1.02-2.37; P=0.04) and positive lymph node metastasis (OR=1.74, 95% CI 1.23-2.46; P=0.002). However, we detected no significant relationships between PD-L1 expression and smoking status (OR=1.49, 95% CI 0.86-2.58; P=0.16), tumor size (OR=1.57, 95% CI 0.40-6.19; P=0.52), EGFR status (OR=0.62, 95% CI 0.26-1.45; P=0.27), ALK status (OR=1.52, 95% CI 0.63-3.67; P=0.35) and KRAS status (OR=1.27, 95% CI 0.74-2.16; P=0.38) (Figure 6). Heterogeneity was not observed in the analysis of the relationships between PD-L1 expression and lymph node metastasis, ALK status and KRAS status; thus, a fixed effect model was used. The other analyses were performed using the random effects model.
Begg's funnel plot and the Egger's linear regression were performed to evaluate the publication bias of the inclusion studies. The P values for these tests were 0.081 and 0.3, respectively, indicating that there was no significant publication bias in the meta-analysis (Supplementary Figure 1).
Flow chart for this meta-analysis
Characteristics of the studies included in the meta-analysis
| First author | Year | Region | No. of ADC patients | TNM stage | PD-L1 positive rate | Endpoint | HR estimation | Outcome |
|---|---|---|---|---|---|---|---|---|
| Yang et al | 2014 | Asia | 163 | I | 39.9% (65/163) | OS | K-M | NR |
| Cooper et al | 2015 | Non-Asia | 276 | I-III | 5.1%(14/276) | OS | K-M | NR |
| Ameratunga et al | 2016 | Non-Asia | 288 | I-III | 48.6%(140/288) | OS | K-M | NR |
| Cha et al | 2016 | Asia | 323 | I-IV | 18.6%(60/323) | OS | K-M | Poor |
| Huynh et al | 2016 | Non-Asia | 261 | I-IV | 36.5%(95/261) | OS | K-M | Poor |
| Ji et al | 2016 | Asia | 100 | I-IV | 40%(40/100) | OS | K-M | Poor |
| Shimoji et al | 2016 | Asia | 165 | I-IV | 22.4%(37/165) | OS | K-M | Poor |
| Song et al | 2016 | Asia | 385 | I-III | 48.3%(186/385) | OS | K-M | NR |
| Sun et al | 2016 | Asia | 664 | I-IV | 36.6%(243/664) | OS | HR | NR |
| Toyokawa et al | 2017 | Asia | 292 | I | 16.1%(47/292) | OS | K-M | NR |
| Uruga et al | 2017 | Non-Asia | 109 | II-III | 51%(56/109) | OS | HR | NR |
| Takada et al | 2017 | Asia | 417 | I-III | 20.4%(85/417) | OS | HR | Poor |
| Wu et al | 2017 | Asia | 133 | I-IV | 13.5%(18/133) | OS | HR | Poor |
Abbreviations: OS=overall survival, HR= hazard ratio, K-M= Kaplan-Meier curve, NR= not relevant.
Forest plot of hazard ratio (HR) for the association between PD-L1 expression and overall survival in patients with lung adenocarcinoma
Forest plot describing subgroup analysis of the association between PD-L1 expression and overall survival stratified by patient source.
The prognostic value of PD-L1 mRNA expression in public datasets
We used K-M plotter and determined the prognostic value of PD-L1 mRNA expression in the database. The Affymetrix IDs is valid: 227458_at (PD-L1). Survival curves are drafted in www.kmplot.com for only surgical margins negative adenocacinoma of lung (n =204).PD-L1 mRNA high expression was significantly associated with worse OS (P=0.018) (Supplementary Figure 2).
The molecular mechanisms of PD-L1 in ADC
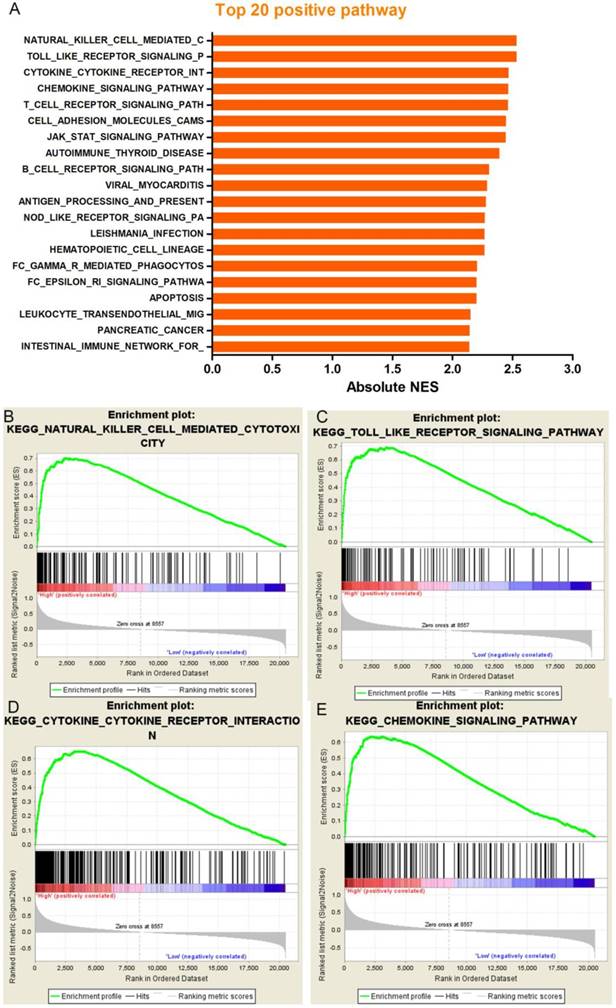
Our results noted that increased PD-L1 expression was associated with poor prognosis in patients with resected ADC; however. The molecular mechanisms were not clear. In order to assess whether the expression levels of PD-L1 were associated with known gene signatures, we used lung adenocarcinoma RNA-seq data generated by TCGA and sorted the samples into the top and bottom quartiles of PD-L1 expression. According to the results of the GSEA, we can see that the 20 most prominent pathways are immune-related gene sets which indicate PD-L1 expression levels positively correlated with immune process or immune-related pathways (Figure 7). Some typical pathways were listed below: natural killer cell mediated cytotoxicity, toll like receptor signaling pathway, cytokine-cytokine receptor interaction and chemokine signaling pathway. In order to further confirm our findings, we also analyzed a background set of Canonical pathways, as well as the background set with GO biological process, we found that GSEA analysis based on the gene set of Canonical pathways was quite similar with the result of KEGG, Immune-related signaling pathways occupied the most significant of the first four pathways. In addition, the GO biological process analysis also get a similar result, the most significant enrichment of the first four processes, were closely related with the immune (Supplementary Figure 3). By comparing the results of the three background sets, we found that immune-related signaling pathways were significantly enriched in different background sets and were ranked very well, suggesting that the function of PD-L1 in ADC may have a link between immune-associated factors.
Discussion
The PD-1/PD-L1 pathway plays an important role in immune escape. Previous studies already revealed that high PD-L1 expression is associated with the poor prognosis of many tumors [9,11-14].However, the function of PD-L1 in resected ADC is still disputed. Some studies showed that high PD-L1 expression was associated with poor prognosis [19-24]; however, other studies did not confirm this result [25-31].The following aspects might be possible reasons causing these different results: (1) PD-L1 protein expression was determined using different antibodies in the different studies; (2) the criteria for determining positive PD-L1 expression in different studies were not consistent; (3) the stages and intervention factors of enrolled patients in different studies were different; and (4) the different specimen collection times affected PD-L1 detection. Therefore, establishment of a unified PD-L1 detection platform and standardization of the determination criteria for positive PD-L1 expression have high significance for future PD-L1 detection. The current commonly used PD-L1 antibodies include clone 28-8, clone 22c3, clone SP142, and clone SP163. Our previous studies showed that clones 28-8, 22c3, and SP163 had higher consistency [32].
Forest plots for the association between PD-L1 expression and clinicopathologic features
Correlations between PD-L1 expression and predefined gene signatures by Gene set enrichment analysis in The Cancer Genome Atlas (TCGA) dataset. (A), GSEA analysis showed that PD-L1 expression levels positively correlated with immune process or immune-related pathways. Such as (B) natural killer cell mediated cytotoxicity, (C) toll like receptor signaling pathway, (D) cytokine-cytokine receptor interaction, (E) chemokine signaling pathway.
We found 104 cases of patients with resected ADC in our center using IHC. The study results showed that positive PD-L1 expression was associated with poor prognosis of the patients. To further validate the association between PD-L1 expression and prognosis in ADC, the PubMed, Embase, and Cochrane databases were searched to identify all relevant studies evaluating the PD-L1 expression and overall survival of resected ADC. The combined analytic results also showed that high PD-L1 expression was associated with poor prognosis for patients with resected ADC. The results of subgroup analyses based on populations with different races showed that high PD-L1 expression was associated with poor prognosis for ADC in the Asian population, whereas PD-L1 expression was not associated with the prognosis in non-Asian populations. A recent meta-analysis also showed that PD-L1 overexpression was closely associated with the prognosis in NSCLC in the Asian population [33]. In addition, our previous meta-analysis showed that PD-L1 expression was associated with poor prognosis (HR= 1.40, 95% CI: 1.19-1.65, P< 0.001). In subgroup analysis stratified according to histology types, the pooled results demonstrated that PD-L1 expression was an unfavorable prognostic factor for NSCLC and pulmonary lymphoepithelioma-like carcinoma (LELC) rather than small cell lung cancer (SCLC) [34]. However, the previous meta-analysis did not investigate the correlation between PD-L1 expression and prognosis in resected lung adenocarcinoma. Currently, the largest study analyzed 1,070 cases of operable NSCLC [29].The results showed that the PD-L1 positive expression group was more prone to relapse than the PD-L1 negative expression group, which was consistent with our results. In addition, our results from analyzing public databases showed that high PD-L1 mRNA expression is associated with poor prognosis. These results indicate that high PD-L1 expression could promote tumor relapse and metastasis.
Clinical trial has confirmed that PD-1/PD-L1 inhibitors have better efficacy in the treatment of lung adenocarcinoma [35]. Studies also showed that the PD-L1 protein expression level in tumor cells is closely associated with efficacy and is a predictive factor of efficacy [36]. Therefore, understanding the expression of PD-L1 in ADC and its association with clinical parameters can allow better screening to identify the population that is more suitable for PD-1/PD-L1 inhibitor treatment. Our study showed that the proportion of patients with ADC who were positive for PD-L1 expression was 38.5% (40/104). This result of our study was similar to that of Huynh et al [20], who showed that the proportion of patients who were positive for PD-L1 expression was 36.5%. The determination criterion of positive PD-L1 expression in that study was consistent with that in our study; both studies required the percentage of tumor cells to be greater than 5% for the determination criterion. Our previous studies showed that the positive rate of PD-L1 in patients with lung squamous cell carcinoma who were positive for PD-L1 was 58.3% (49/84), which was higher than that for patients with adenocarcinoma [17]. Tsao et al also showed that the proportion of patients with ADC who were positive for PD-L1 was lower than that of patients with lung squamous cell carcinoma [37]. Our analyses on combined data showed that PD-L1 expression was associated with gender and lymph node metastasis and that the proportion of patients who were positive for PD-L1 expression was higher in male and lymph node-positive patients. The results of our validation using clinical specimens also showed that the PD-L1 expression in patients positive for lymph node metastasis was higher; however, the difference was not statistically significant. The association between PD-L1 expression and lymph node metastasis indicates that the activation of the PD-1/PD-L1 pathway allows tumor cells to escape immune system surveillance; thus, metastasis was more likely to occur.
Increasing amounts of evidence have already shown that high PD-L1 protein expression in tumor cells is associated with poor prognosis for patients with ADC. However, the specific molecular mechanism is still not clear. It is currently thought that several possible action mechanisms exist for the PD-1/PD-L1 pathway in tumors. PD-L1 induces apoptosis in activated T cells through binding to the PD-1 expressed in activated T cells. Blocking the PD-1/PD-L1 signaling pathway can reduce apoptosis in tumor-specific T cells to exert anti-tumor effects [38]. The activation of the PD-1/PD-L1 signaling pathway can inhibit signaling pathways, such as RAS/MEK/ERK and PI3K/AKT, to suppress T cell proliferation [39]. In the tumor microenvironment, PD-L1 expression can induce depletion of infiltrating T lymphocytes to cause infiltrating T lymphocytes to lose the immune surveillance function [40]. PD-L1 induces the production of regulatory T cells (Treg), maintains and strengthens their negative regulation functions, and inhibits the activity of effector T cells [41]. PD-L1 can induce the epithelial-mesenchymal transition (EMT) to cause tumor cell invasion and metastasis [42]. The increase in HIF-1 expression can increase PD-L1 expression to downregulate the functions of activated T cells [43]. The activation of the JAK/STAT3, NF-κB, PI3K/AKT, EGFR/HER2, and KRAS pathways can increase PD-L1 expression to induce immune tolerance [44-48]. To comprehensively understand the association between high PD-L1 expression and signaling pathways, we considered the results of GSEA, which showed that the PD-L1 expression was mainly associated with immune signaling, such as natural killer cell-mediated cytotoxicity, “Toll-like receptor signaling pathway”, “cytokine receptor interaction”, “chemokine receptor interaction”, and “T cell receptor signaling pathway”. In addition to immune-related signaling, we also discovered that PD-L1 expression was associated with signaling pathways, such as apoptosis and JAK/STAT3. To further validate these results, we also analyzed a background set of canonical pathways, as well as the background set, with GO biological process, and the analytic results were similar. The discovery of these pathways can provide certain theoretical support for subsequent basic research studies.
We admit that our study has many limitations. First, the amount of clinical resected ADC specimens included was relatively small. To obtain more convincing results, we performed combined analyses on relevant published studies of the association between PD-L1 expression and the prognosis of patients with resected ADC. Second, this study mainly analyzed patients with ADC at the early stage and did not analyze patients with advanced ADC. The main reason for this choice was that the amount of advanced lung adenocarcinoma specimens was limited and that these specimens might provide useful information for subsequent treatment of patients; therefore, our center strictly restricted the use of specimens from patients with advanced ADC. Third, we discovered pathways associated with high PD-L1 expression using GSEA; however, validation was not performed. We will perform mechanism validation in future studies.
In summary, our study showed that high PD-L1 expression was a predictive indicator of poor prognosis for patients with resected ADC. PD-L1 expression was closely associated with gender and lymph node metastasis. This population may have a relative advantage in PD-1/PD-L1 treatment, and their treatment results may generate references for clinical drug selection. Furthermore, we found that PD-L1 expression was mainly associated with immune pathways. The underlying mechanism should be confirmed in basic studies.
Supplementary Material
Supplementary figures.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81372907), Health and Family Planning commission of Heilongjiang Province (2016-102), innovation fund of Harbin Medical University (2017LCZX95).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A. et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-93
2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR. et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:504-35
3. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R. et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706-14
4. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-65
5. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-46
6. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-35
7. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-50
8. Aguiar PN Jr, Santoro IL, Tadokoro H, de Lima Lopes G, Filardi BA, Oliveira P. et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy. 2016;8:1011-9
9. Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N. et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584-92
10. Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9:499-506
11. Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M. et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric cancer. 2017;20:407-15
12. Lee KS, Kwak Y, Ahn S, Shin E, Oh HK, Kim DW. et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother. 2017;66:927-39
13. Zhu J, Wen H, Ju X, Bi R, Zuo W, Wu X. Clinical Significance of Programmed Death Ligand1 and Intra-Tumoral CD8+ T Lymphocytes in Ovarian Carcinosarcoma. PloS one. 2017;12:e0170879
14. Ness N, Andersen S, Khanehkenari MR, Nordbakken CV, Valkov A, Paulsen EE. et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget. 2017;8:26789-801
15. Zhang M, Dong Y, Liu H, Wang Y, Zhao S, Xuan Q. et al. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6:37933
16. Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y. et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8:31347-54
17. Zhang M, Wang D, Sun Q, Pu H, Wang Y, Zhao S. et al. Prognostic significance of PD-L1 expression and 18F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget. 2017
18. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-75
19. Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung cancer. 2016;97:73-80
20. Huynh TG, Morales-Oyarvide V, Campo MJ, Gainor JF, Bozkurtlar E, Uruga H. et al. Programmed Cell Death Ligand 1 Expression in Resected Lung Adenocarcinomas: Association with Immune Microenvironment. J Thorac Oncol. 2016;11:1869-78
21. Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W. et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016;17:407-13
22. Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K. et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung cancer. 2016;98:69-75
23. Takada K, Toyokawa G, Okamoto T, Shimokawa M, Kozuma Y, Matsubara T. et al. A Comprehensive Analysis of Programmed Cell Death Ligand-1 Expression With the Clone SP142 Antibody in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer. 2017;18:572-82
24. Wu S, Shi X, Sun J, Liu Y, Luo Y, Liang Z. et al. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget. 2017;8:16421-9
25. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361-9
26. Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M. et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung cancer. 2015;89:181-8
27. Ameratunga M, Asadi K, Lin X, Walkiewicz M, Murone C, Knight S. et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PloS one. 2016;11:e0153954
28. Song Z, Yu X, Cheng G, Zhang Y. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med. 2016;14:188
29. Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z. et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol. 2016;11:1003-11
30. Toyokawa G, Takada K, Okamoto T, Kawanami S, Kozuma Y, Matsubara T. et al. Relevance Between Programmed Death Ligand 1 and Radiologic Invasiveness in Pathologic Stage I Lung Adenocarcinoma. Ann Thorac Surg. 2017;103:1750-7
31. Uruga H, Bozkurtlar E, Huynh TG, Muzikansky A, Goto Y, Gomez-Caraballo M. et al. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol. 2017;12:458-66
32. Zhang M, Feng D, Jing J, Liu H, Zhao S, Zhang Q. PD-L1 protein expression in non-small cell lung cancer based on different immunohistochemical antibodies. J Thorac Dis. 2017;9:E470-e3
33. Xia H, Shen J, Hu F, Chen S, Huang H, Xu Y. et al. PD-L1 over-expression is associated with a poor prognosis in Asian non-small cell lung cancer patients. Clin Chim Acta. 2017;469:191-4
34. Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P. et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7:10255
35. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-39
36. Maleki Vareki S, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017;116:116-24
37. Tsao MS, Le Teuff G, Shepherd FA, Landais C, Hainaut P, Filipits M. et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017;28:882-9
38. Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47
39. Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46
40. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900-12
41. Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ. et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012;278:76-83
42. Tsutsumi S, Saeki H. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017;108:1119-27
43. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P. et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781-90
44. Doi T, Ishikawa T, Okayama T, Oka K, Mizushima K, Yasuda T. et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37:1545-54
45. Xue J, Chen C, Qi M, Huang Y, Wang L, Gao Y. et al. Type Igamma phosphatidylinositol phosphate kinase regulates PD-L1 expression by activating NF-kappaB. Oncotarget. 2017;8:42414-27
46. Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR. et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76:227-38
47. Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2017;66:865-76
48. Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J. et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer immunology, immunotherapy: CII. 2017
Author contact
![]() Corresponding author: Minghui Zhang, Department of Medical Oncology, Harbin Medical University Cancer Hospital, No.150, Haping Road, Harbin, 150081, China. E-mail: zhangminghuiedu.cn; Tel: +86-0451-86298258; Fax-+86-0451-86298258.
Corresponding author: Minghui Zhang, Department of Medical Oncology, Harbin Medical University Cancer Hospital, No.150, Haping Road, Harbin, 150081, China. E-mail: zhangminghuiedu.cn; Tel: +86-0451-86298258; Fax-+86-0451-86298258.

 Global reach, higher impact
Global reach, higher impact