Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(21):3923-3928. doi:10.7150/jca.26220 This issue Cite
Research Paper
A Prognostic Nomogram for Cervical Cancer after Surgery from SEER Database
1. Department of Epidemiology and Biostatistics, School of Public Health, Harbin Medical University, Harbin, 150086, China;
2. Department of Gynecology Oncology, the Tumor Hospital, Harbin Medical University, Harbin 150086, China.
3. Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Cardiovascular Institute, Harbin Medical University, Harbin, China
*These authors contributed equally to this work.
Received 2018-3-21; Accepted 2018-8-1; Published 2018-10-10
Abstract
Background: To develop and validate a nomogram based on the conventional measurements and log of odds between the number of positive lymph node and the number of negative lymph node (LODDS) in predicting prognosis for cervical cancer patients after surgery.
Methods: A total of 8202 cervical cancer patients with pathologically confirmed between 2004 and 2014 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. All the patients were divided into training (n=3603) and validation (n=4599) cohorts based on consecutive age of diagnosis. Demographic and clinical pathological factors were evaluated the association with overall survival (OS). Parameters significantly correlating with OS were used to create a nomogram. An independent external validation cohort was subsequently used to assess the predictive performance of the model.
Results: In the training set, age at diagnosis, race, marital status, tumor grade, FIGO stage, histology, size and LODDS were correlated significantly with outcome and used to develop a nomogram. The calibration curve for probability of survival showed excellent agreement between prediction by nomogram and actual observation in the training cohort, with a bootstrap-corrected concordance index of 0.749(95% CI, 0.731-0.767). Importantly, our nomogram performed favorably compared to the currently utilized FIGO model, with concordance indices of 0.786 (95% CI, 0.764 to 0.808) vs 0.685 (95%CI, 0.660 to 0.710) for OS in the validation cohort, respectively.
Conclusions: By incorporating LODDS, our nomogram may be superior to the currently utilized FIGO staging system in predicting OS in cervical cancer patients after surgery.
Keywords: prognostic model, nomogram, cervical cancer, SEER database
Introduction
Cervical cancer is one of the most common types of gynecological malignancies around the world, which includes uncontrolled cell division and tissue of the female uterine cervix. In less developed countries, cervical cancer among females is the leading cause of cancer death. There were an estimated 527,600 new cervical cancer cases and 265,700 deaths worldwide in 2012[1]. It is the second most commonly diagnosed cancer and the third leading cause of cancer death among females in less developed countries[1]. Nearly 90% of cervical cancer deaths occurred in developing countries [1]. More advances in the treatment methods have been improved in less developed countries.
Traditionally, lymph node status is considered as one of the most important clinical parameters in the treatment determination and prognosis for cervical cancer[2]. The number of positive lymph node (PLNs) is the main factor affecting the 5-year overall survival rate of cervical cancer patients[3, 4]. The current International Federation of Gynecology and Obstetrics(FIGO) staging system does not assess lymph node status, though this feature is a prognostic factor and the 7th edition of the American Joint Committee on Cancer(AJCC)/Union for International Cancer Control(UICC) staging system only classifies the lymph nodes as N0 (negative) or N1 (positive)[5, 6].Currently, lymph node status is always based on PLN regardless of the number of resected lymph node (RLNs) in cervical cancer patients[7, 8]. In early stage cervical carcinoma, the 5-year overall survival rate of patients with lymph node positive was reported to be around 50%, while that of non-lymph node metastasis was more than 90%[9]. However, in the clinical practice clinicians demonstrate that lymph node status might not sufficiently be reflected by the number of the positive lymph nodes.
Recently, the lymph node ratio (LNR) that is the ratio of PLNs to RLNs, and log of odds between PLNs and the number of negative lymph node (LODDS) have emerged as alternative predictive factors for outcomes and showed superiority to lymph node status-based assessment in pancreatic cancer[10, 11], oral cancer [12, 13], non-small cell lung cancer [13],colorectal cancer[14], ovarian cancer[15], breast cancer[16]. LNR was also studied in ovarian cancer and found to be superior to both PLN and RLN in predicting survival [17, 18]. LODDS is defined as log ((the number of PLNs +0.05)/ (the number of negative nodes +0.05)). While this parameter has been validated in predicting survival for breast cancer, its utility in cervical cancer still remain unclear [19-21].
In this study, we hypothesized that LODDS might be an important prognostic predictor and has better predictive performance than LNR in cervical cancer patients. In addition, a nomogram incorporating LODDS may be superior in this respect to the currently-utilized FIGO score staging system.
Material and Methods
Data source and eligibility criteria
We identified cervical cancer cases from the SEER program of the National Cancer Institute (http://seer.cancer.gov/). Inclusion criterion is that all patients who were pathologically confirmed with cervical cancer from the SEER database from 2004 to 2014. Exclusion criteria are as follows: unknown age of diagnosis; uncertain race; unknown marriage status; undetermined grade; unknown stage; unspecified neoplasms; unknown tumor size; unknown or incomplete lymph node status. Finally, a total of 8202 cervical cancer patients were included in this study. These patients were divided into training and validation cohorts based on consecutive age of diagnosis (training cohort between 2004 and 2008, N=3603; validation cohort between 2009 and 2014, N=4599). In our study, 'married' was recorded as married and unmarried but having domestic partner; single was recorded as 'single'; separated, divorced and widowed were classified as “others”. Except squamous cell neoplasm and adenomas and adenocarcinomas, other histology types were recorded as “other”.
Statistical analysis
Categorical measurements were described as count and percentage, while continuous measurements were presented as mean and range. The chi-square was used to compare the categorical measurements, while t test for continuous ones. The optimal cutoff point for LODDS was determined by ROC curve in training cohort with the package of survivalROC in R. The nomogram was built with potential risk factors (P <0.05) based on multivariate Cox analysis in the training cohort with the package of rmsin in R. The predictive performance of the nomogram was measured by concordance index (C-index) and it compared nomogram-predicted survival probability with observed Kaplan survival probability. C-index comparisons between the nomogram and FIGO staging system were performed using thercorrp.cens package in Hmisc in R. The larger the C-index, the more accurate for the prognostic prediction. The predictive performance was validated by an independent external cohort. All statistical analyses were performed in R version 3.3.3 (http://www.r-project.org/).
Results
Patients Characteristics
In the training cohort, a total of 3603 cervical cancer patients between 2004 and 2008 were involved. A total of 4599 consecutive patients between 2009 and 2014 were involved in the validation cohort. The demographic and clinicopathologic characteristics of patients in the training and validation cohorts were listed in Table 1.
Optimal Cutoff for LODDS in Predicting OS
In order to facilitate the application of LODDS in clinical practice, we dichotomized LODDS into high LODDS and low LODDS based on the optimal cutoff from ROC curve. In our training cohort, the optimal cutoff was -4.394. We further validated the predicting performance of binary LODDS in an independent external dataset.
Demographic and clinicopathological characteristics of patients with cervical cancer between 2004 and 2014 in SEER database
| Demographic or Characteristics | All subjects (N=8202) | Training cohort (N=3603) | Validation cohort (N=4599) | P value |
|---|---|---|---|---|
| Age at diagnosis (year) | 0.002 | |||
| x<30 | 521(6.35) | 228(6.33) | 293(6.37) | |
| 30≤x<50 | 4757(58.00) | 2169(60.20) | 2588(56.27) | |
| 50≤x<70 | 2442(29.77) | 996(27.64) | 1446(31.44) | |
| x≥70 | 482(5.88) | 210(5.83) | 272(5.91) | |
| Race | 0.016 | |||
| White | 6554(79.91) | 2879(79.91) | 3675(79.91) | |
| Black | 687(8.38) | 331(9.19) | 356(7.74) | |
| Asian | 824(10.05) | 344(9.55) | 480(10.44) | |
| Others | 137(1.67) | 49(1.36) | 88(1.91) | |
| Marital Status | 0.054 | |||
| Single | 2194(26.75) | 917(25.45) | 1277(27.77) | |
| Married | 4417(53.85) | 1984(55.07) | 2433(52.90) | |
| Other | 1591(19.40) | 702(19.48) | 889(19.33) | |
| Grade | <0.001 | |||
| High | 1250(15.24) | 496(13.77) | 754(16.39) | |
| Medium | 3602(43.92) | 1546(42.91) | 2056(44.71) | |
| Low | 3110(37.92) | 1459(40.49) | 1651(35.90) | |
| Undifferentiated | 240(2.93) | 102(2.83) | 138(3.00) | |
| Stage | 0.874 | |||
| I | 6704(81.74) | 2953(81.96) | 3751(81.56) | |
| II | 1005(12.25) | 430(11.93) | 575(12.50) | |
| III | 170(2.07) | 75(2.08) | 95(2.07) | |
| IV | 323(3.94) | 145(4.02) | 178(3.87) | |
| Histology | 0.015 | |||
| squamous cell neoplams | 4557(55.56) | 2060(57.17) | 2497(54.29) | |
| adenomas and adenocarcinomas | 2673(32.59) | 1115(30.95) | 1558(33.88) | |
| Other | 972(11.85) | 428(11.88) | 544(11.83) | |
| Tumor size(cm) | 0.042 | |||
| x<4 | 6030(73.52) | 2608(0.7238) | 3422(0.7441) | |
| x≥4 | 2172(26.48) | 995(0.2762) | 1177(0.2559) | |
| LODDS* | 0.032 | |||
| mean(range) | -4.870 (-7.496 -7.245) | -4.818 (-7.496-5.638) | -4.910 (-7.496-7.245) | |
| RLNs* | 0.113 | |||
| mean(range) | 19.287(1-90) | 19.517(1-90) | 19.107(1-90) | |
| PLNs* | 0.304 | |||
| mean(range) | 0.618(0-70) | 0.644(0-37) | 0.597(0-70) | |
| LNR* | 0.099 | |||
| mean(range) | 0.044(0-1) | 0.047(0-1) | 0.042(0-1) | |
*LODDS: log((the number of PLNs +0.05)/ (the number of negative nodes +0.05)); RLNs: the number of resected lymph node; PLNs: the number of positive lymph node; LNR: the lymph node ratio.
Independent Prognostic Factors of OS in the Training Cohort
The results of the univariate and multivariate analysis were listed in Table 2. Univariate analyses demonstrated that age at diagnosis, race, marital status, grade, stage, histology, PLN, LNR, RLN and LODDS were associated with OS. Multivariate analysis demonstrated that age at diagnosis, race, marital status, grade, stage, histology, tumor size and LODDS were independent risk factors for OS (Table 2).
Univariate analysis and multivariate analysis in the training cohort
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age at diagnosis | |||||
| x<30 | reference | reference | |||
| 30≤x<50 | 0.868(0.620-1.214) | 0.407 | 0.945(0.672-1.329) | 0.744 | |
| 50≤x<70 | 1.594(1.134-2.239) | 0.007 | 1.321(0.931-1.874) | 0.119 | |
| x≥70 | 3.490(2.408-5.058) | <0.001 | 2.941(1.987-4.354) | <0.001 | |
| Race | |||||
| White | reference | reference | |||
| Black | 0.899(0.691-1.170) | 0.021 | 1.347(1.070-1.696) | 0.011 | |
| Asian | 1.305(1.042-1.635) | 0.049 | 0.791(0.606-1.032) | 0.083 | |
| Others | 1.134(0.625-2.060) | 0.679 | 0.879(0.483-1.602) | 0.674 | |
| Marital status | |||||
| Single | reference | reference | |||
| Married | 0.945(0.788-1.133) | 0.544 | 1.053(0.873-1.271) | 0.590 | |
| Other | 1.621(1.324-1.984) | <0.001 | 1.402(1.126-1.746) | 0.003 | |
| Grade | |||||
| High | reference | reference | |||
| Medium | 1.709(1.274-2.294) | <0.001 | 1.358(1.001-1.842) | 0.049 | |
| Low | 2.668(2.001-3.556) | <0.001 | 1.637(1.202-2.229) | 0.002 | |
| Undifferentiated | 3.646(2.351-5.655) | <0.001 | 1.604(1.017-2.532) | 0.042 | |
| Stage | |||||
| I | reference | reference | |||
| II | 2.711(2.259-3.255) | <0.001 | 1.562(1.283-1.901) | <0.001 | |
| III | 4.769(3.450-6.591) | <0.001 | 2.608(1.859-3.57) | <0.001 | |
| IV | 6.721(5.361-8.425) | <0.001 | 3.557(2.767-4.571) | <0.001 | |
| Histology | |||||
| squamous cell neoplams | reference | reference | |||
| adenomas and adenocarcinomas | 0.697(0.594-0.833) | <0.001 | 0.962(0.794-1.164) | 0.690 | |
| Other | 1.695(1.400-2.053) | <0.001 | 1.682(1.381-2.049) | <0.001 | |
| Tumor size(cm) | |||||
| x<4 | reference | reference | |||
| x≥4 | 2.734(2.356-3.160) | <0.001 | 1.706(1.454-2.000) | <0.001 | |
| RLN | 0.957(0.980-0.993) | <0.001 | 0.995(0.989-1.001) | 0.089 | |
| LNR | 15.75(11.85-20.90) | <0.001 | |||
| PLN | 1.131(1.115-1.147) | <0.001 | |||
| LODDS | |||||
| <-4.394 | reference | reference | |||
| ≥-4.394 | 2.943(2.547-3.402) | <0.001 | 1.809(1.541-2.125) | <0.001 | |
Prognostic Nomogram for OS
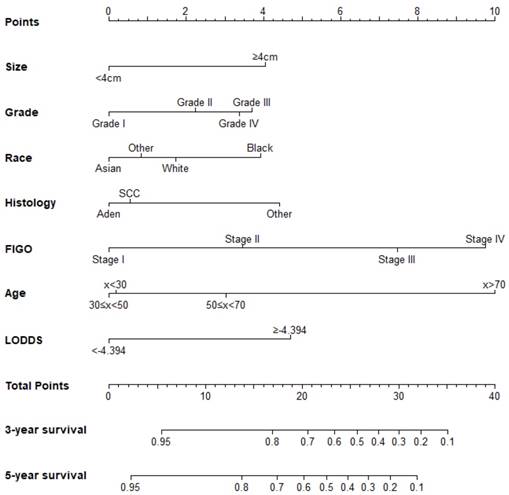
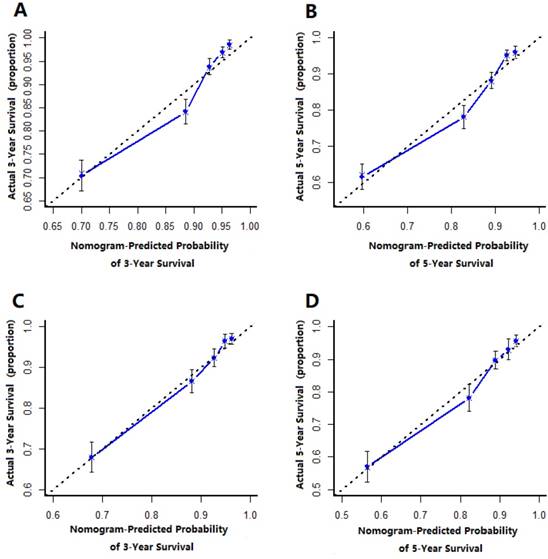
The prognostic nomogram that integrated all significant independent factors from multivariate analysis for OS in the training cohort was shown in Figure 1. The C-index of prognostic nomogram for OS prediction was 0.749 (95%CI, 0.731 to 0.767) in the training cohort and 0.786 (95%CI, 0.746 to 0.808) in the validation cohort. The calibration plot for the probability of survival at 3 or 5-year after surgery showed an optimal agreement between the prediction by nomogram and actual observation in the training cohort (Figure 2A and 2B) and in the validation cohort (Figure 2C and 2D), respectively.
Comparison with FIGO scoring system
The C-indices for FIGO staging and nomogram were 0.633 (95%CI: 0.615 to 0.651) and 0.749 (95%CI: 0.731 to 0.767) in the training cohort (P<0.01), respectively, and 0.685 (95%CI: 0.660 to 0.710) and 0.786 (95%CI, 0.746 to 0.808) in the validation cohort (P<0.01), respectively.
Cervical cancer after surgery survival nomogram.
Discussion
The present study suggested that age at diagnosis, race, grade, FIGO stage, histology, tumor size, LODDS were independent prognostic factors of OS in cervical cancer after surgery. The nomogram was created based on these prognostic factors and was used to predict the 3-year and 5-year OS after surgery in the cervical cancer patients. In the training and validation cohorts, the nomogram showed better predictive performance than the FIGO staging system for OS.
As far as we know, the nomogram has been applied to predict the survival status of various cancers [22-24]. Since the nomogram quantifies risk by combining and illustrating the relative importance of various prognostic factors and has been used in clinical oncology assessment. Although LODDS has been described as a predictor of cervical cancer, the nomograms with LODDS to predict the prognosis of cervical cancer after surgery has not been described[25].
To our knowledge, PLNs have been reported as a prognostic factor for cervical cancer. However, PLNs, part of RLNs, do not fully reflect the disease state in all situations[26]. Therefore, it is important to take simultaneously PLNs and the number of negative lymph nodes consideration for predicting the prongosis of cervical cancer. LODDS is an intuitive indicator that is reflective of both PLNs and the number of negative lymph nodes and it has been used to predict the prognosis of survival in other cancers. From out study, it is found that LODDS is an independent prognostic factor of cervical cancer and we take -4.394 as the optimal cutoff point for the LODDS based on ROC curve analysis from the training cohort. Meanwhile, we fully verify the nomogram accuracy with this optimal cutoff in an independent external cohort. From the created nomogram, females younger than 30 years old have higher score than older than 30 but younger than 50 years old, though this former group have no significance.
FIGO staging is the most common staging system for cervical cancer, so we compared C-index of the nomogram with that of FIGO stage and found that the C-index for the nomogram to predict OS were 0.749, 0.786 respectively in the training cohort and the validation cohort and higher than FIGO. In addition, the calibration plots represented that a perfect match between the nomogram predicted probability and the actual probability calculated by Kaplan-Meier analysis, especially for 3-year survival. These results showed that our nomogram could be used in practical work with less bias and better accuracy. In addition, we used the nomogram to calculate 3-year and 5-year survival rate individually so that the nomogram can help clinical make a more reasonable follow-up plan.
There are still several limitations that must be considered though the nomogram model demonstrated good accuracy for predicting OS. Firstly, the C-index of the nomogram is good but not excellent. Secondly, there are many factors may influence that may influence prognosis of postoperative cervical cancer patients so that further research should be carried to improve the nomogram.
In summary, our study demonstrated that age at diagnosis, race, grade, FIGO stage, histology, tumor size, LODDS are independent prognostic factors of OS in cervical cancer. In addition, we created the nomogram based on LODDS to predict estimate 3- and 5-year OS among postoperative cervical cancer patients which had good accuracy.
The calibration curve for predicting patient survival at (A) 3 year and (B) 5 years in the training cohort and at (C) 3 years and (D) 5 years in the validation cohort. Nonogram-predicted probability of overall survival is plotted on the x-axis, actual overall survival is plotted on the y-axis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81573256, 81773550, 81773551).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Ca A Cancer Journal for Clinicians. 2015;65:87-108
2. Zhou Z, Li W, Zhang F, Hu K. The value of squamous cell carcinoma antigen (SCCa) to determine the lymph nodal metastasis in cervical cancer: A meta-analysis and literature review. PloS one. 2017;12:e0186165
3. JJ E, MG N, KA tH, M K, H H, GH dB. et al. The epidermal growth factor receptor pathway in relation to pelvic lymph node metastasis and survival in early-stage cervical cancer. Human Pathology. 2010;41:1735-41
4. Cao S, Sun J, Lin S, Zhao L, Wu D, Liang T. et al. HPIP: a predictor of lymph node metastasis and poor survival in cervical cancer. Oncotargets & Therapy. 2017;10:4205
5. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105:107-8
6. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471-4
7. Kotowicz B, Fuksiewicz M, Kowalska M, Jonska-Gmyrek J, Bidzinski M, Kaminska J. The value of tumor marker and cytokine analysis for the assessment of regional lymph node status in cervical cancer patients. International Journal of Gynecological Cancer Official Journal of the International Gynecological Cancer Society. 2008;18:1279-84
8. Strnad P, Robova H, Skapa P, Pluta M, Hrehorcak M, Halaska M. et al. A prospective study of sentinel lymph node status and parametrial involvement in patients with small tumour volume cervical cancer. Gynecologic oncology. 2008;109:280-4
9. Aoki Y, Sasaki M, Watanabe M, Sato T, Tsuneki I, Aida H. et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecologic oncology. 2000;77:305-9
10. Ramacciato G, Nigri G, Petrucciani N, Pinna AD, Ravaioli M, Jovine E. et al. Prognostic role of nodal ratio, LODDS, pN in patients with pancreatic cancer with venous involvement. BMC surgery. 2017;17:109
11. Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, Bui JL, Yuan C. et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. British journal of cancer. 2017;117:1874-82
12. Lee CC, Su YC, Hung SK, Chen PC, Huang CI, Huang WL. et al. Recommendation for incorporation of a different lymph node scoring system in future AJCC N category for oral cancer. Scientific reports. 2017;7:14117
13. Safi AF, Kauke M, Grandoch A, Nickenig HJ, Drebber U, Zoller J. et al. The importance of log odds of positive lymph nodes for locoregional recurrence in oral squamous cell carcinoma. Oral oncology. 2017;72:48-55
14. Parnaby CN, Scott NW, Ramsay G, MacKay C, Samuel L, Murray GI. et al. Prognostic value of lymph node ratio and extramural vascular invasion on survival for patients undergoing curative colon cancer resection. Br J Cancer. 2015;113:212-9
15. Xu XL, Cheng H, Tang MS, Zhang HL, Wu RY, Yu Y. et al. A novel nomogram based on LODDS to predict the prognosis of epithelial ovarian cancer. Oncotarget. 2017;8:8120-30
16. Wen J, Ye F, He X, Li S, Huang X, Xiao X. et al. Development and validation of a prognostic nomogram based on the log odds of positive lymph nodes (LODDS) for breast cancer. Oncotarget. 2016;7:21046-53
17. Ataseven B, Grimm C, Harter P, Prader S, Traut A, Heitz F. et al. Prognostic value of lymph node ratio in patients with advanced epithelial ovarian cancer. Gynecologic oncology. 2014;135:435
18. Zhou J, He ZY, Li FY, Sun JY, Lin HX, Wu SG. et al. Prognostic value of lymph node ratio in stage IIIC epithelial ovarian cancer with node-positive in a SEER population-based study. Oncotarget. 2016;7:7952-9
19. Chen LJ, Chung KP, Chang YJ, Chang YJ. Ratio and log odds of positive lymph nodes in breast cancer patients with mastectomy. Surgical Oncology. 2015;24:239-47
20. Wen J, Feng Y, He X, Li S, Huang X, Xiao X. et al. Development and validation of a prognostic nomogram based on the log odds of positive lymph nodes (LODDS) for breast cancer. Oncotarget. 2016;7:21046-53
21. Wu SG, Wang Y, Zhou J, Sun JY, Li FY, Lin HX. et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. American Journal of Cancer Research. 2015;5:844
22. Sun F, Ma K, Yang X, Li M, Shi Y, Zhan C. et al. A nomogram to predict prognosis after surgery in early stage non-small cell lung cancer in elderly patients. International Journal of Surgery. 2017;42:11
23. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z. et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2013;31:1188-95
24. Kawai K, Sunami E, Yamaguchi H, Ishihara S, Kazama S, Nozawa H. et al. Nomograms for colorectal cancer: A systematic review. World Journal of Gastroenterology. 2015;21:11877
25. Kwon J, Eom KY, Kim IA, Kim JS, Kim YB, No JH. et al. Prognostic Value of Log Odds of Positive Lymph Nodes after Radical Surgery Followed by Adjuvant Treatment in High-Risk Cervical Cancer. International Journal of Radiation Oncology Biology Physics. 2015;93:E268-E
26. Ataseven B, Harter P, Grimm C, Heitz F, Heikaus S, Traut A. et al. The revised 2014 FIGO staging system for epithelial ovarian cancer: Is a subclassification into FIGO stage IVA and IVB justified? Gynecologic oncology. 2016;142:243
Author contact
![]() Corresponding authors: Yan Hou, Department of Epidemiology and Biostatistics, School of Public Health, Harbin Medical University, Harbin, 150086, China; telephone: 86-451-87502645; Fax: 86-451-87202885; Email: houyanhrbmu.edu.cn; Fengyu Sun, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Cardiovascular Institute, Harbin Medical University, Harbin, China. Telephone: 86-451-85555943; E-mail: sun.129.comcom
Corresponding authors: Yan Hou, Department of Epidemiology and Biostatistics, School of Public Health, Harbin Medical University, Harbin, 150086, China; telephone: 86-451-87502645; Fax: 86-451-87202885; Email: houyanhrbmu.edu.cn; Fengyu Sun, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Cardiovascular Institute, Harbin Medical University, Harbin, China. Telephone: 86-451-85555943; E-mail: sun.129.comcom

 Global reach, higher impact
Global reach, higher impact