Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(21):4087-4091. doi:10.7150/jca.26631 This issue Cite
Research Paper
Impact of the Time Interval from Neoadjuvant Chemotherapy to Surgery in Primary Ovarian, Tubal, and Peritoneal Cancer Patients
1. Department of Obstetrics and Gynecology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
2. Department of Orthopedics, Shantou Central Hospital, Shantou, China
Received 2018-4-12; Accepted 2018-8-2; Published 2018-10-18
Abstract
Neoadjuvant chemotherapy (NACT) plays an important role in ovarian cancer. The appropriate time interval from the completion of NACT to interval debulking surgery (TTS) in ovarian cancer is still unknown. The aim of this retrospective study was to evaluate the effect of the time interval between the end of NACT and surgery (TTS ≤ 4 weeks vs TTS > 4 weeks) on the survival outcomes among patients with advanced-stage ovarian, tubal, and peritoneal cancers. 152 patients with stage III or IV ovarian, tubal, and peritoneal cancers were included in this retrospective cohort study: 115 in the TTS ≤4 weeks and 37 in the TTS >4 weeks groups. The Kaplan-Meier analysis showed that the progression-free survival in the TTS ≤4 weeks group was longer than that in the TTS >4 weeks group (26 vs 14 months, P=0.04). However, the overall survival was not different between the two groups (66 vs 36 months, P=0.105). The multivariate analysis presented that delay in surgery after NACT (TTS >4 weeks) was associated with a shorter progression-free (P=0.002) but not overall survival (P=0.231). Our findings demonstrated no relationship between the NACT to surgery interval and OS, while a detrimental effect of TTS >4 weeks on PFS was observed.
Keywords: ovarian cancer, neoadjuvant chemotherapy, time to surgery
Introduction
Ovarian cancer is a highly lethal gynecologic cancer, and its incidence and mortality rates in China are increasing[1]. Primary cytoreductive surgery (PDS) followed by adjuvant chemotherapy has historically been the standard of care for women with advanced ovarian cancer. Recently, several phase 3 clinical trials involving women with stage III-IV ovarian cancer have demonstrated that survival and postoperative morbidity and mortality rates after neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) are not inferior to those following PDS[2,3]. However, the appropriate time interval from the completion of NACT to surgery (TTS) is still unknown. While general consensus supports avoiding unnecessary delays, no studies have examined the impact of the TTS on survival outcomes.
Clinical practice guidelines on the maximum TTS and the results from numerous studies are conflicting. While some studies found no correlation between survival and the TTS in various solid tumors[4,5], other studies showed that surgery should be performed within 3-8 weeks after NACT[6-8]. Moreover, in ovarian cancer, few large randomized clinical trials on NACT have thoroughly addressed the TTS, and many do not even specify a recommended time interval. The commonly accepted practice is to perform surgery when neutropenia has resolved, normally resulting in a 3- to 4-week time interval. However, whether or not a longer time interval has any detrimental effects on survival has not been determined.
The aim of this retrospective study was to evaluate the effect of the time interval between the end of NACT and surgery (TTS ≤ 4 weeks vs TTS > 4 weeks) on the survival outcomes among patients with advanced-stage ovarian, tubal, and peritoneal cancers.
Methods
Approval to conduct this study was obtained from the medical ethics committees at the First Affiliated Hospital of Sun Yat-sen University in China. A retrospective review of our ovarian cancer research database at the First Affiliated Hospital of Sun Yat-sen University identified consecutive primary ovarian cancer patients between Jan 2006 and Dec 2016. Only women with pathologically confirmed Federation of Obstetrics and Gynecology (FIGO) stage IIIC-IV (2014 edition) epithelial ovarian, tubal, and peritoneal cancers who were treated with platinum- or taxane-based NACT were included[9].
Patients were clinically diagnosed with FIGO stage III or IV by initial imaging workup, comprising abdominal and pelvic computed tomography (CT) and 18F-fluorodeoxyglucose- positron emission tomography/CT. The diagnoses were histologically confirmed by examination of the biopsy specimens removed during diagnostic laparoscopy, or by cytological assessment of ascites or pleural effusion.
The exclusion criteria were as follows: 1) patients whose last NACT treatment or cytoreductive surgery were performed outside of our institution (n = 6); 2) patients who did not undergo cytoreductive surgery or complete adjuvant chemotherapy that was indicated or recommended (n = 11); 3) patients who had tumors with low malignant potential, additional synchronous primary tumors, non-epithelial histology or a non-primary ovarian malignancy (n = 6); and 4) patients who were lost to follow-up after surgery (n = 13).
Patients were divided into the following two groups based on the TTS after completing NACT: (a) TTS ≤4 weeks and (b) TTS >4 weeks. Patient and clinical characteristics, including age, FIGO stage, Eastern Cooperative Oncology Group (ECOG) score, histology, type of surgery, date of NACT completion and date of surgery were collected. NACT dose delay or dose reduction and chemotherapy toxicity were also noted.
After surgery, all the patients were treated with adjuvant chemotherapy with a platinum- or taxane-based regimen. The median follow-up time was 36 months (range, 8 - 120 months). The patients were examined every 3 months for the first 2 years, every 6 months for the next 3 years, and yearly thereafter. The dates of recurrence were determined based on clinical examinations, imaging studies, and CA 125 levels. The endpoints included the progression-free survival (PFS) and the overall survival (OS). PFS was defined as the interval between the date of diagnosis and the date of first recurrence. OS was defined as the interval between the date of diagnosis and the date of death. Survival was censored by a closeout date (Jan 1, 2018).
The associations between the variables and the different TTS groups were analyzed using the chi-square test. Survival curves were constructed using the Kaplan-Meier method. The prognostic values of the clinicopathological parameters with respect to PFS and OS were evaluated via a multivariate analysis (Cox proportional hazard regression test), with a conditional forward method if applicable, and the results are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical analyses were performed using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). All the tests were two-tailed, and results with P <0.05 were considered statistically significant.
Results
From Jan 2006 to Dec 2016, 152 patients were enrolled. All the patients were assigned to NACT and then underwent IDS as a protocol treatment. According to the TTS, patients were divided into two groups (TTS ≤4 weeks and TTS >4 weeks). The median TTS was 24 days and ranged from 13 to 74 days. In both groups, the median number of cycles of NACT and total chemotherapy were 3 and 8 cycles, respectively. The patients' clinical characteristics are listed in Table 1.
Of the 152 patients, 67 (44.1%) participants used IP/IV chemotherapy, compared with the other patients receiving IV chemotherapy. 126 (82.9%) were treated with carboplatin/paclitaxel (TC regimen), 12 patients received single-agent platinum therapy, and 14 patients received a platinum-based doublet or triplet protocol. The regimens for those 14 patients were as follows: carboplatin/fluorouracil (n =6), carboplatin/cyclophosphamide/doxorubicin (n =5), and carboplatin/gemcitabine (n =3). Additionally, among those 126 patients treated with carboplatin/paclitaxel, 7 patients were switched to complete chemotherapy with single-agent paclitaxel due to various adverse effects due to platinum. After NACT, most patients (n = 129, 84.9%) in our study exhibited a partial response.
Regular surgical procedures included the sampling of free fluid or peritoneal washings for cytology; a thorough inspection of the abdomen and pelvis, including the upper abdominal viscera, diaphragm, and retroperitoneal spaces; and a hysterectomy, bilateral oophorectomy and omentectomy, pelvic/para-aortic lymph node sampling or dissection, and appendectomy. There were 98 (64.5%) patients who underwent pelvic lymphadenectomy, and 71 patients who underwent para-aortic lymphadenectomy. Other radical surgeries included the following: bowel resection (n = 14), diaphragm or other peritoneal surface stripping (n =5), splenectomy (n =4), partial hepatectomy (n =3), partial gastrectomy (n =1), and ureteroneocystostomy (n =2). There were 97 (63.8%) patients who underwent an optimal debulking operation. There were no significant differences in the patient characteristics between the two groups, except there was a lower pelvic/para-aortic lymphadenectomy rate in the TTS >4 weeks group.
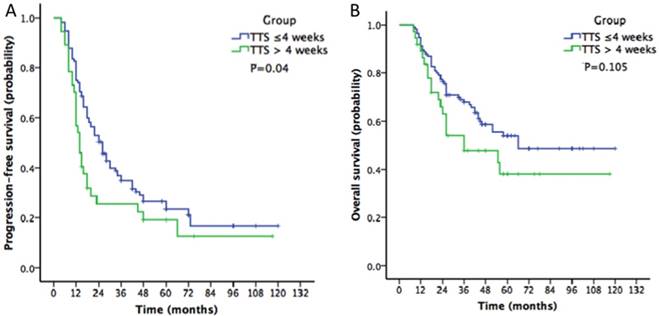
The median PFS and OS for the entire cohort were 20 and 66 months, respectively. The PFS in the TTS ≤ 4 weeks group was longer than that in the TTS >4 weeks group based on the Kaplan-Meier analysis (26 vs 14 months, P=0.04, Figure 1A). However, the OS was not different between the two groups (66 vs 36 months, P=0.105, Figure 1B). In the multivariate analysis, using patients with a TTS ≤ 4 weeks as a reference, patients who underwent surgery > 4 weeks after NACT had a poorer PFS (OR: 1.81, 95% confidence interval [CI]: 1.35-2.52, P=0.002). However, the TTS was not a significant variable affecting the OS of ovarian cancer patients (OR: 1.24, 95% CI: 0.79-1.89, P=0.231) (Table 2). Moreover, the multivariate analysis revealed that advanced stage (IV) and debulking status were important prognostic factors associated with both PFS and OS.
We analyzed the reasons for delay in IDS in 37 patients (Table 3). The most common reasons for surgery delays were chemotherapy-related toxicities, such as neutropenia or thrombocytopenia, poor performance status and gastrointestinal symptoms. Other reasons included patients' choice, due to economic reasons, venous thromboembolism and infection.
Discussion
Current guidelines recommend that NACT should be reserved for patients who are not candidates for primary surgery because they have an unacceptable surgical risk or unresectable disease[9], and in recent years, the use of NACT has gradually increased in the United States[10]. However, few studies have evaluated at the impact of the time to surgical staging after completing NACT in ovarian cancer. To this end, our data showed that delays in surgery of up to 4 weeks were significantly associated with poorer PFS, without an effect on OS.
Previous studies have investigated the importance of timeliness in initiating and administering adjuvant chemotherapy in ovarian cancer. Singh conducted a retrospective study and found that delays in initiating chemotherapy (>6 weeks from the primary debulking surgery) were associated with a shorter progression-free survival (P =0.014) but not with overall survival (P =0.19)[11]. Joseph confirmed that dose delays were an independent factor associated with decreased OS in elderly patients with epithelial ovarian cancer (P =0.02). In addition, chemotherapy dose reductions seemed to have no effect on survival[12].
Kaplan-Meier survival curves of progression-free survival and overall survival according to the time interval from the end of neoadjuvant chemotherapy to surgery (A: progression-free survival curve; B: overall survival curve).
The current evidence shows that treatment delays after NACT for breast cancer are lengthening over time [13]. As the incidence and mortality rates of ovarian cancer in China are increasing[1], the need for investigating the impact of optimal time intervals is an increasingly important consideration. Studies on various solid tumors have showed that this time interval might be related to survival. Sanford et al. analyzed data from 1,101 breast cancer patients who were treated with neoadjuvant chemotherapy at a single institution (the University of Texas MD Anderson Cancer Center). The sensitivity analysis revealed worse outcomes when surgery was performed after longer than 8 weeks[14]. A longer interval did not increase the pathological complete response (pCR) rate or survival benefit. A multicenter, randomized controlled trial (GRECCAR-6) on rectal cancer also showed that waiting over 11 weeks after neoadjuvant radiochemotherapy did not increase the pCR rate after surgical resection. Instead, a longer time interval might be associated with higher morbidity and a more difficult surgical resection[15]. In ovarian cancer, a recent study investigated the relationship between the time interval from the completion of NACT to the initiation of postoperative adjuvant chemotherapy and survival outcomes in patients at FIGO stage III or IV. The multivariate analysis revealed that patients with longer time intervals (>6 weeks) had significantly poorer PFS and OS. The longer time intervals were associated with higher risks of recurrence and death (P=0.006 and P <0.001, respectively)[16]. Our study revealed that a TTS >4 weeks was a risk factor for poor PFS but not for OS. This result is worth surgeons' attention. Animal models have shown that shrinkage of the primary tumor can stimulate residual tumor growth[17]. A prompt cytoreduction following the preoperative administration of effective systemic therapy might help disease control. Thus, unnecessary delays in the TTS in ovarian cancer might be avoided.
In clinical practice, the TTS is influenced by multiple factors. Lee YJ et al found that there was difficulty in scheduling a multidisciplinary team to perform surgery in a timely fashion after NACT[16]. However, compiling a multidisciplinary surgeon team quickly is not an issue in our hospital, because it is a comprehensive tertiary hospital. We analyzed reasons for delayed TTS in our cohort. The most common reasons for surgery delays were chemotherapy-related toxicities, such as neutropenia or thrombocytopenia, poor performance status and gastrointestinal symptoms. These findings imply a potential benefit of reinforcing hematologic supportive agents, such as myeloid growth factors and blood transfusion, during NACT so that IDS delays can be avoided in this population. Nonetheless, risk assessment should be made in order to reduce adverse events before the applications of hematologic support[18,19]. Greater investigation into the relationship between the hematologic support and survival is warranted.
Patient and clinical characteristics by interval from neoadjuvant therapy to surgery
| All patients | TTS ≤ 4 weeks | TTS > 4 weeks | P | |
|---|---|---|---|---|
| (N=152) No. of cases (%) | (N=115) No. of cases (%) | (N=37) No. of cases (%) | ||
| Age (years) | 0.872 | |||
| ≤ 60 | 64 (42.1%) | 48 (41.7%) | 16 (43.2%) | |
| > 60 | 88 (57.9%) | 67 (58.3%) | 21 (56.8%) | |
| FIGO stage | 0.852 | |||
| III | 109 (71.7%) | 84 (73.0%) | 25 (67.6.6%) | |
| IV | 43 (28.3%) | 31 (27.0%) | 12 (32.4%) | |
| ECOG score | 0.752 | |||
| 0-1 | 129 (84.9%) | 97 (84.3%) | 32 (86.5%) | |
| 2-3 | 23 (15.1%) | 18 (15.7%) | 5 (13.5%) | |
| Histology | 0.610 | |||
| Serous | 123 (80.9%) | 92 (80.0%) | 31 (83.8%) | |
| Clear cell | 17 (11.2%) | 14 (12.2%) | 3 (8.1%) | |
| Endometrioid | 6 (3.9%) | 4 (3.5%) | 2 (5.4%) | |
| Mucinous | 1 (0.7%) | 1 (0.9%) | 0 | |
| Mixed/other | 5 (3.3%) | 4 (3.5%) | 1 (2.7%) | |
| Diagnostic surgery before NACT | 0.897 | |||
| Yes | 11 (7.24%) | 9 (7.8%) | 2 (5.4%) | |
| No | 141 (92.8%) | 106 (92.2%) | 35 (94.6%) | |
| NACT dose reduction | 0.265 | |||
| Yes | 35 (23.0%) | 24 (20.9%) | 11 (29.7%) | |
| No | 117 (77.0%) | 91 (79.1%) | 26 (70.3%) | |
| Lymphadenectomy | 0.032 | |||
| pelvic | 98 (64.5%) | 84 (73.0%) | 14 (37.8%) | |
| pelvic+para-aortic | 71 (46.7%) | 68 (59.1%) | 3 (8.1%) | |
| Debulking status | 0.585 | |||
| Optimal | 97 (63.8%) | 72 (62.6%) | 25 (67.6%) | |
| Sub-optimal | 55 (36.2%) | 43 (37.4%) | 12 (32.4%) | |
Abbreviations: TTS= time interval from the completion of neoadjuvant therapy to surgery. Bold values indicate statistically significant differences.
Prognostic factors for PFS and OS in ovarian cancer patients (multivariate analysis)
| Characteristics | Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |
| Age | ||||||
| ≤ 60 | 1 | - | - | 1 | - | - |
| > 60 | 1.56 | 0.80-3.92 | 0.367 | 1.24 | 0.74-3.14 | 0.415 |
| FIGO stage | ||||||
| III | 1 | - | - | 1 | - | - |
| IV | 2.13 | 1.54-2.96 | <0.001 | 1.94 | 1.35-2.78 | 0.003 |
| Histology | ||||||
| serous | 1 | - | - | 1 | - | - |
| non-serous | 1.75 | 0.68-2.48 | 0.354 | 1.96 | 0.75-2.93 | 0.286 |
| Debulking status | ||||||
| Optimal | 0.43 | 0.23-0.68 | <0.001 | 0.57 | 0.31-0.76 | <0.001 |
| Sub-optimal | 1 | - | - | 1 | - | - |
| TTS | ||||||
| ≤ 4 weeks | 1 | - | - | 1 | - | - |
| > 4 weeks | 1.81 | 1.35-2.52 | 0.002 | 1.24 | 0.79-1.89 | 0.231 |
Abbreviations: HR=Hazard ratio; CI=confidence interval; TTS= time interval from the completion of neoadjuvant therapy to surgery. Bold values indicate statistically significant differences.
Indications for surgery delay in 37 patients.
| Number of patients | |
|---|---|
| Hematologic toxicity | 13 |
| ECOG status | 7 |
| Gastrointestinal symptoms | 4 |
| Ascites | 2 |
| Patient request | 5 |
| Venous thromboembolism | 3 |
| Infection | 3 |
This study is unique because it is the first to examine the impact of the TTS on the survival of ovarian cancer patients receiving NACT in Chinese patients. A limitation of this study was its retrospective and its dependency on medical records, as well as the fact that it was based on a single institution experience. In the future, prospective, multicenter studies are warranted to validate our findings and to provide additional information on the relationships between NACT, the initiation of IDS, and the survival outcomes of patients.
In conclusion, our findings demonstrated no relationship between the NACT to surgery interval and OS, while a detrimental effect of TTS >4 weeks on PFS was observed. Therefore, prolonged delay to surgical treatment might be avoided. Further investigation on TTS will be needed.
Acknowledgements and Funding
This study was funded by the National Natural Science Foundation of China 81602261. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
2. Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I. et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008;38:74-7
3. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-53
4. Liu Y, Zhang KC, Huang XH, Xi HQ, Gao YH, Liang WQ. et al. Timing of surgery after neoadjuvant chemotherapy for gastric cancer: Impact on outcomes. World J Gastroenterol. 2018;24:257-65
5. Rombouts AJM, Hugen N, Elferink MAG, Nagtegaal ID, de Wilt JHW. Treatment Interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients: A population-based study. Ann Surg Oncol. 2016;23:3593-601
6. Gao SJ, Corso CD, Wang EH, Blasberg JD, Detterbeck FC, Boffa DJ. et al. Timing of surgery after neoadjuvant chemoradiation in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:314-22
7. Lin G, Han SY, Xu YP, Mao WM. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus. 2016;29:1107-14
8. Omarini C, Guaitoli G, Noventa S, Andreotti A, Gambini A, Palma E. et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur J Surg Oncol. 2017;43:613-8
9. National comprehensive cancer network group. NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer (Version 1.2018). NCCN.org. http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed on 28 Feb. 2018
10. Melamed A, Hinchcli EM, Clemmer JT. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143:241-5
11. Singh S, Guetzko M, Resnick K. Preoperative predictors of delay in initiation of adjuvant chemotherapy in patients undergoing primary debulking surgery for ovarian cancer. Gynecologic oncology. 2016;143:241-45
12. Joseph N, Clark RM, Dizon DS, Lee MS, Goodman A, Boruta D Jr. et al. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol Oncol. 2015;137:401-5
13. Bleicher RJ, Ruth K, Sigurdson ER, Ross E, Wong YN, Patel SA. et al. Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol. 2012;30:4485-92
14. Sanford RA, Lei X, Barcenas CH, Mittendorf EA, Caudle AS, Valero V. et al. Impact of yime from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol. 2016;23:1515-21
15. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C. et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: A multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016;34:3773-80
16. Lee YJ, Chung YS, Lee JY, Nam EJ, Kim SW, Kim S. et al. Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer. Gynecol Oncol. 2018;148:62-7
17. Bell RS, Roth YF, Gebhardt MC, Bell DF, Rosenberg AE, Mankin HJ. et al. Timing of chemotherapy and surgery in a murine osteosarcoma model. Cancer Res. 1988;48:5533-8
18. National comprehensive cancer network group. NCCN Clinical Practice Guidelines in Oncology. Myeloid growth factors (Version 1.2018). https://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed on Mar 2. 2018
19. National comprehensive cancer network group. NCCN Clinical Practice Guidelines in Oncology. Cancer- and chemotherapy-induced anemia (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/anemia.pdf. Accessed on Nov 21. 2017
Author contact
![]() Corresponding author: Shuzhong Yao, MD. Department of Obstetrics and Gynecology, The First Affiliated Hospital, Sun Yat-sen University, 58 2nd Zhongshan Road, Guangzhou, Guangdong 510080, China. TEL: 86-20-28823388; FAX: 86-20-87332200; E-mail: yszlfycom
Corresponding author: Shuzhong Yao, MD. Department of Obstetrics and Gynecology, The First Affiliated Hospital, Sun Yat-sen University, 58 2nd Zhongshan Road, Guangzhou, Guangdong 510080, China. TEL: 86-20-28823388; FAX: 86-20-87332200; E-mail: yszlfycom

 Global reach, higher impact
Global reach, higher impact