3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(22):4179-4186. doi:10.7150/jca.27483 This issue Cite
Research Paper
Down-Regulated microRNA-34a Expression as a Prognostic Marker for Poor Osteosarcoma in Mice: A Systematic Review and Meta-Analysis
1. Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032China;
2. Key laboratory of theory and therapy of muscles and bones, Ministry of Education, Shanghai200032, China.
# Wenyi Wang and Shaopu Hu contributed equally to this work.
Received 2018-5-26; Accepted 2018-7-21; Published 2018-10-18
Abstract
Background: In children and adolescents, osteosarcomais the most common malignant bone tumor with a high mortality rate. New therapeutic strategies are urgent to be explored. Studies have proven that microRNAs (miRNAs) in malignant tumors often appear dysregulation, this provides a direction for exploring the new therapeutic strategies for cancers. The aim of this meta-analysis is to summarize and analyze whethermicroRNA-34a(miRNA-34a) could be a prognostic marker for osteosarcoma in mice.
Methods: We searched PubMed, Web of Science, Embase, Wan Fang Database, China Knowledge Resource Integrated Database, VIP Database, and SinoMed since their initiation date to January 24, 2018. After screening based on inclusion and exclusion criteria, eight articles were included for the final analysis.
Results: Our results showed that tumor volume and tumor weight were inhibited by restoring the down-regulated expression of miRNA-34a in the xenograft mouse models.
Conclusions: Down-regulated miRNA-34a expression is a prognostic marker for poor osteosarcoma. We should be more committed to investigate the clinical significance of miRNA-34a in osteosarcoma patients.
Keywords: microRNA-34a, osteosarcoma, prognosis, meta-analysis, mice
Introduction
Osteosarcoma is the most common primary bone cancer arising from metaphysis of the long bones, which mainly comes from young people and adolescents, accounting for about five percent of pediatric cancers. Despite the fact that there are aggressive tumor resection, combinatorial chemotherapy and radiotherapy treatments, the five-year survival rate of osteosarcoma patient without metastasis is only about sixty percent to seventy percent [1, 2]. The survival rate of patients with metastasis or relapse is still below 20%[3].Therefore, it is essential to develop new therapeutic approaches for osteosarcoma treatment.
MicroRNAs (miRNAs) are a class of non-coding RNAs, including 19 to 25 nucleotides, and regulate the translation and degradation of mRNAs through imperfect base pairing with 3′-untranslated region (3′-UTR) of the target mRNAs at post-transcriptional level[4, 5]. MiRNAs play important role in cellular physiologic processes, such as proliferation, differentiation, apoptosis, thus can act as oncogenesis or tumor suppressor genes in different tumors[6]. There are three members in microRNA-34 (miRNA-34) family (miRNA-34a, miRNA-34b and miRNA-34c). They are part of the p53 network and whose expression is directly induced by p53 in response to DNA damage or oncogenic stress. In the recent years, some studies indicate that miRNA-34a may act as a tumor suppressor by regulating apoptosis in several tumors[7, 8].
The purpose of this study is to evaluate the potential function of miRNA-34a as an independent prognostic marker for osteosarcoma based on the published literatures, and to investigate the methodological quality of the existing studies, thus to guide the preclinical experimental design and the future clinical trials.
Materials and Methods
Literature search methods and selection criteria
Two reviewers (W.Y.W and S.P.H) independently searched literatures out of PubMed, Web of Science, Embase, Wan Fang Database, China Knowledge Resource Integrated Database, VIP Database, and SinoMed since their initiation date to January 24, 2018, without restrictions of the languages, publication status or publication dates. Based on the issue to be resolved, the following key words were used: (miRNA-34a OR microRNA-34a) AND (Osteosarcoma).
According to the inclusion and exclusion criteria, two reviewers (J.L.C and H.F.R independently screened the literatures by reviewing the titles, abstract and full texts. Disagreements were determined by a third author (Y.P.Y.). Only literatures conformed to the standard were included in this meta-analysis.
Eligibility criteria
Types of studies
Studies that estimate the therapeutic effects on osteosarcoma in mouse models by restoring the abnormally expressed miRNA-34a were searched. Records only having in vitro experimental data and the clinical cases were excluded.
Types of participants
Any strains of mice and osteosarcoma cell lines used to produce osteosarcoma xenograft models were included.
Types of intervention
Any intervention methods to restore the altered miRNA-34a expression in mouse osteosarcoma models were collected.
Type of outcome measure
Tumor volume and tumor weight are the most common outcome measures to evaluate the anticancer efficacy of any anticancer interventions in the preclinical studies. In this meta-analysis, tumor volume and tumor weight were included, regardless the methods used to establish osteosarcoma xenograft models.
Tumor volume
The formula of tumor volume calculation [9]:
tumor volume=0.5× (width2 × length)
Tumor weight
Tumors were removed and weighed when mice were sacrificed at the end of experiments.
Statistical analysis
We conducted pair-wise meta-analysis for studies, which directly compared the influence on tumor growth between restored miRNA-34a expression and the control (abnormally expressed miRNA-34a) to determine the pooled relative effect of each intervention for the measurement outcomes (tumor volume and tumor weight), and the mean difference (MD) of the post-intervention value was determined.
In this meta-analysis, tumor volume and tumor weight were compared respectively. Meta-analysis was performed using Review Manager Software version 5.3 (software update; The Nordic Cochrane Centre, Copenhagen, Denmark). I2 and p value were calculated to evaluate the heterogeneity. Heterogeneity was existed if p value<0.10 by the chi-square (x2) test. The results indicated a high level of heterogeneity when I2 >50%, the data from studies should be pooled using the random-effects model. When I2<50%, data from studies should be pooled using the fixed-effects model. When same outcomes were measured using different instruments across studies, we used a standardized mean difference (SMD) in the meta-analysis to combine continuous data.
Results
Literature screening
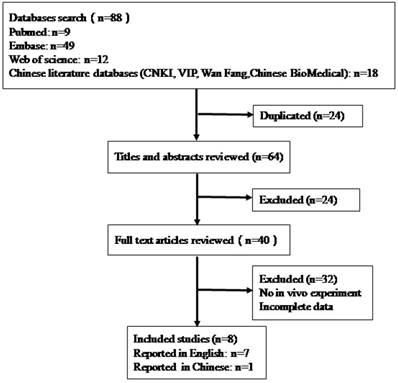
As shown in figure 1, the process of document retrieval is as follows. Firstly, we identified88 articles in the databases using the search strategy described in the section of the method, and excluded 24 duplicates from the initial articles. Twenty-four articles were further excluded after screening the titles, abstracts, publication types. Then, according to reading the full texts, 32 articles were excluded. Finally, eight articles were included in our final analysis, seven of the articles were reported in English, and one of them was reported in Chinese [10-17].
The flow diagram of the literature identification and selection process.
Study characteristics
Among the eight included studies, five studies used nude mice, one study used SCID mice, and two studies did not report the species of mice. Four studies used female mice, three studies used male mice, one study did not describe the gender of mice. The sample size in the eight articles was between 10 and 18.The main feeding situations of the mice were not reported in the included studies. Among the eight studies, three studies used subcutaneous injection to produce osteosarcoma xenograft models, and five studies used intratibial injection.
In this meta-analysis, tumor volume and tumor weight were used as the outcomes. Various osteosarcoma cell lines were used to produce osteosarcoma xenograft models (MG-63, Saos-2, 143B, SOSP-9607). Methods to produce xenograft models were also different (subcutaneous injection or intratibial injection) (Table 1).
Quality evaluations of the included studies
No study has described sample-size calculation, allocation concealment, blinded assessment of outcomes, or reported animals excluded from the analysis. All included studies reported inclusion and exclusion criteria, three studies reported randomization, and six studies reported potential conflicts of interest and study funding. No study reported blinded assessment of outcome. So, the methodological quality of studies included here was low (Table 2).
Inhibitory effects on the tumor growth (tumor weight/tumor volume) in osteosarcoma xenograft models by restoring the abnormally expressed miRNA-34a
All the eight included literatures reported that miRNA-34a was the tumor suppressor, however, different outcome measures (tumor volume or tumor weight) and different osteosarcoma cell lines (MG-63, Saos-2, 143B, SOSP-9607)and different inoculation sites to produce xenograft models (intratibial inoculation or subcutaneous inoculation) were reported. Therefore, a high heterogeneity could be produced. To minimize the heterogeneity, stratifications were performed based on these factors. Meanwhile, random-effects models or fixed-effects models were used for the analysis.
When all included studies used tumor volume as the major outcome was pooled for analysis
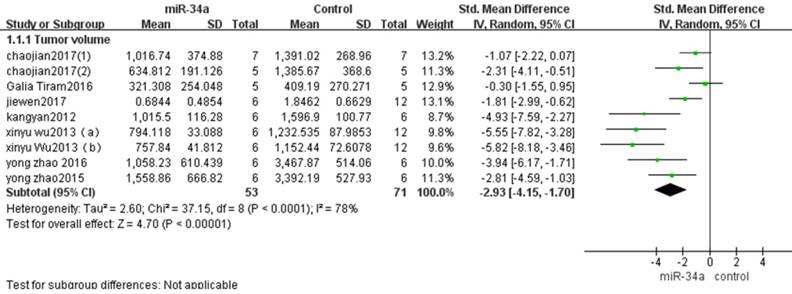
There were eight studies that used tumor volume as the major outcome measure in this meta-analysis, and all of them reported that miRNA-34a functioned as tumor suppressor [10-17].Therefore, all the data from these eight studies were extracted and pooled for analysis. There are 53 mice in the experimental group and 71 mice in the control group. The result of the forest plot using the random-effects model showed that the tumor volume in osteosarcoma xenograft was decreased after up-regulation of miRNA-34a expression, thus recovery of miRNA-34a expression to the normal level could inhibit osteosarcoma growth in vivo. The pooled MD = [-2.93]; confidence interval [CI]: [-4.15, -1.70]; P<0.00001 (Figure 2).
When above included studies that used tumor volume as the major outcome measure were stratified respectively by the following factors
Methods for producing xenograft models
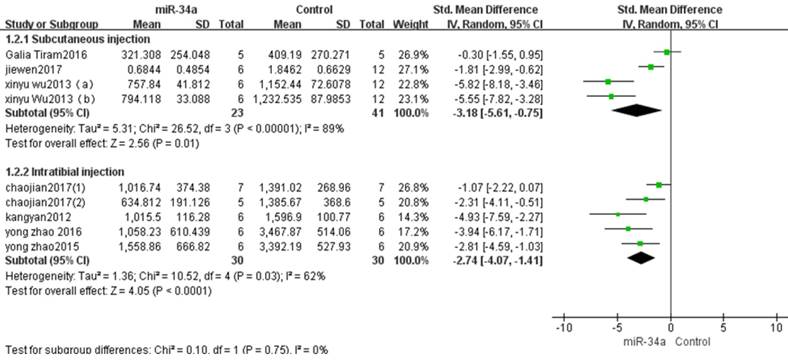
In these eight studies, three of them applied osteosarcoma xenograft models produced by subcutaneous inoculation of osteosarcoma cells[13, 14, 16].There were 23 mice in the experimental group and 41 mice in the control group. The result of the forest plot using the random-effects model suggested that tumor volume was significantly decreased by up-regulation of miRNA-34aexpression.The pooled MD = [-3.18]; 95% confidence interval [CI]: [-5.61, -0.75]; p =0.01(Figure 3, upper part).
The characteristics of studies included in this meta-analysis.
| Studies | Characteristics of animals | Animal groups | Osteosarcoma xenograft methods | Experimental groups | Control groups | Outcomes |
|---|---|---|---|---|---|---|
| Jie Wen 2017[16] | 18 nude mice (6 weeks) | 6/6/6 | Subcutaneous | MG-63+miR-34a mimic | A:MG-63+Normal B:MG-63+NC | Tumor volume |
| XinyuWu2013(a)[14] | 18 female BALB/c nude mice(4-6 weeks) | 6/6/6 | Subcutaneous | MG-63+pcDNA3.1-miR-34a | A:MG-63+blank B:MG-63+control | Tumor volume |
| Xinyu Wu 2013(b)[14] | 18 female BALB/c nude mice(4-6 weeks) | 6/6/6 | Subcutaneous | Saos-2+pcDNA3.1-miR-34a | A:Saos-2+blank B:Saos-2+control | Tumor volume |
| Kang Yan 2012[15] | 12 female BALB/c nude mice (4 weeks) | 6/6 | Intratibial | SOSP-9607+pcDNA-miR-34a | SOSP-9607+control | Tumor volume Tumor weight |
| Yong Zhao 2015[10] | 12 male athymic nude mice (4-6weeks) | 6/6 | Intratibial | 143B+ miR-34a | Vehicle | Tumor volume Tumor weight |
| Chao Jian 2017(1) [11] | 14 female mice (7weeks) | 7/7 | Intratibial | 143B+ miR-34a | Vehicle | Tumor volume Tumor weight |
| YongZhao2016[12] | 12 male athymic nude mice (5-6weeks) | 6/6 | Intratibial | 143B+ miR-34a | Vehicle | Tumor volume |
| Chaojian 2017(2)[17] | 10 female mice (5 weeks) | 5/5 | Intratibial | 143B+tRNA/ miR-34a | Vehicle | Tumor volume |
| GaliaTiram2016[13] | 10 male SCIDmice (6-8 weeks) | 5/5 | Subcutaneous | Saos-2+PG-NH2/miR-34a | NC | Tumor volume |
Note: (a),(b): different osteosarcoma cell lines used in same literature;(1),(2): different literatures from same first author;NC = negative control
Quality evaluation of the included literatures.
| Studies | Sample-size calculation | Inclusion and exclusion criteria | Randomization | Allocation concealment | Reporting animals excluded from analysis | Blinded assessment of outcomes | Reporting potential conflicts of interest and study funding |
|---|---|---|---|---|---|---|---|
| Chao Jian2017(1) [11] | No | Yes | Yes | No | No | No | Yes |
| Jie Wen 2017[16] | No | Yes | No | No | No | No | Yes |
| Kang Yan 2012[15] | No | Yes | No | No | No | No | No |
| Xinyu Wu 2013 [14] | No | Yes | No | No | No | No | Yes |
| Yong Zhao 2015[ 10] | No | Yes | Yes | No | No | No | Yes |
| Yong Zhao 2016 [12] | No | Yes | No | No | No | No | Yes |
| GaliaTiram 2016 [13] | No | Yes | No | No | No | No | Yes |
| Chao Jian 2017(2) [17] | No | Yes | Yes | No | No | No | No |
All included studies that used tumor volume as the major outcome were pooled for analysis. SD, standard deviation; CI, confidence interval.
Osteosarcoma xenograft models induced by intratibial injection of osteosarcoma cells were used in other five studies[10-12, 15, 17]. There were 30 mice in the experimental group and 30 mice in the control group. A random-effects model was used also due to the high heterogeneity among the included studies, and the tumor volume was significantly decreased after the decreased tumor suppressor miRNA-34a was restored (pooled MD = [-2.74]; 95% confidence interval [CI]: [-4.07, -1.41]; p<0.0001; Figure 3 lower part).
From the comparison in the figure 3, the overall effects on inhibiting tumor volume by upregulation of miRNA-34a expression was better when the osteosarcoma xenograft models were produced by subcutaneous injection than by intratibial injection.
Osteosarcoma cell lines used to produce osteosarcoma xenograft models
Among all eight studies that used tumor volume as the major outcome measure in this meta-analysis. Two studies used MG-63 to produce osteosarcoma xenograft models[14, 16]. There were 12 mice in the experimental group and 24 mice in the control group. The pooled MD = [-3.68]; 95% confidence interval [CI]: [-7.60, 0.25]; p =0.07 (Figure 4, part 1).The result demonstrated that up-regulation of miRNA-34a expression could not inhibit the tumor volume produced by MG-63.
Four studies used 143B to produce osteosarcoma xenograft models[10-12, 17].There were 24 mice in the experimental group and 24 mice in the control group. The result of the forest plot using the random-effects model suggested that tumor volume was significantly decreased by up-regulation of miRNA-34aexpression. The pooled MD = [-2.31]; 95% confidence interval [CI]: [-3.54, -1.09]; p = 0.0002(Figure 4, part 2).
All studies that used tumor volume as the major outcome measure were stratified by injection sites of osteosarcoma cells. SD, standard deviation; CI, confidence interval.
All studies that used tumor volume as the major outcome measure were stratified by osteosarcoma cell lines used to produce osteosarcoma xenograft models. SD, standard deviation; CI, confidence interval.
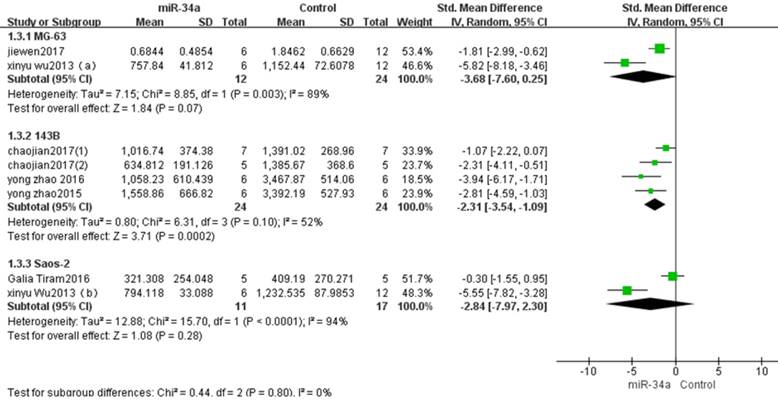
Two studies used Saos-2 to produce xenograft models[13, 14]. There were 11 mice in the experimental group and 17 mice in the control group. The pooled MD = [-2.84]; 95% confidence interval [CI]: [-7.97, 2.30]; p=0.28 (Figure 4, part 3). The result demonstrated that up-regulation of miRNA-34a expression could not inhibit the tumor volume produced by Saos-2.
When all the included studies used tumor weight as the major outcome was pooled for analysis
There were 19 mice in the experimental group and 19 mice in the control group from the 3 studies that used tumor weight as the major outcome measure in this meta-analysis[10, 11, 15].The result suggested that up-regulation of the decreased miRNA-34a was able to restrain the progression of osteosarcoma in vivo when a fixed-effects model was applied. And the pooled MD = [-2.52]; 95% confidence interval [CI]: [-3.46, -1.58]; p <0.00001(Figure 5).
When above included studies that used tumor weight as the major outcome measure were stratified respectively by the following factors
Methods for producing xenograft models
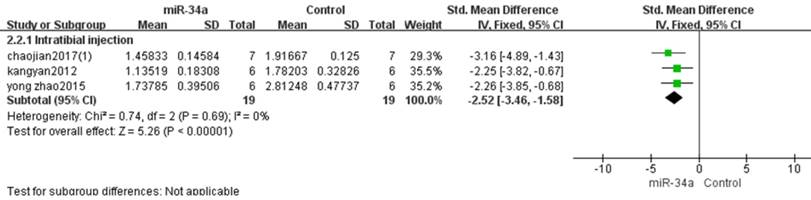
Osteosarcoma xenograft models induced by intratibial injection of osteosarcoma cells were used in three studies [10, 11, 15]. There were 19 mice in the experimental group and 19 mice in the control group. A fixed-effects model was used also due to the low heterogeneity among the included studies, and the tumor weight was significantly decreased after the decreased miRNA-34a was restored. (pooled MD = [-2.52]; 95% confidence interval [CI]: [-3.46, -1.58]; p <0.00001; Figure 6).
4.4.2 Osteosarcoma cell lines used to produce osteosarcoma xenograft models
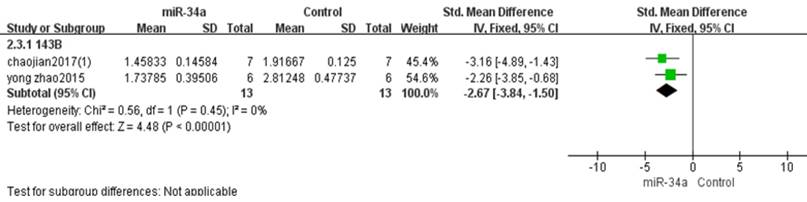
Two studies used 143B to produce osteosarcoma xenograft models [10, 11]. There were 13 mice in the experimental group and 13 mice in the control group. The result of the forest plot with the fixed-effects model suggested that the tumor weight was significantly decreased by restoring the down-regulated miRNA-34a.The pooled MD = [-2.67]; 95% confidence interval [CI]:[-3.84, -1.50]; p < 0.00001 (Figure 7).
Only 1 study used SOSP-9607 cell line to produce osteosarcoma xenograft models, therefore the data could not be pooled for reanalysis. This study confirmed that miR-34a inhibits the development of osteosarcoma in vitro and in vivo”.
All included studies that used tumor weight as the major outcome were pooled for analysis. SD, standard deviation; CI, confidence interval.
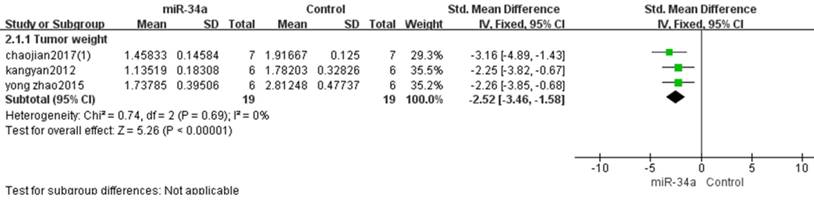
All studies that used tumor weight as the major outcome measure were stratified by injection sites of osteosarcoma cells. SD, standard deviation; CI, confidence interval.
All studies that used tumor weight as the major outcome measure were stratified by osteosarcoma cell lines used to produce osteosarcoma xenograft models. SD, standard deviation; CI, confidence interval.
Discussion
The results of our present meta-analysis demonstrated that tumor volume and tumor weight in the xenograft mouse models were significantly decreased by upregulation of miRNA-34a expression. The overexpression of miRNA-34a inhibited osteosarcoma cell growth both in vitro and in vivo. Therefore, we confirmed that miRNA-34a could act as a tumor suppressor in osteosarcoma.
Osteosarcoma, as the most common primary malignant bone tumor, has high mortality rate and metastasis rate. With the progress of neoadjuvant chemotherapy, the 5-year survival rate in patients without metastasis is less than 70 percent[18], while the survival rate of patients with metastasis or relapse is still lower than 20%[3]. Therefore, it is essential to develop new therapeutic strategies for osteosarcoma.
In recent years, miRNA is a hot topic in the field of osteosarcoma research. Many literatures suggest that miRNAs play important roles in cell proliferation, apoptosis and differentiation. MiRNA-34a was reported low expression in human cancer cells. Cell proliferation was inhibited, cell cycle progression was arrested and cell apoptosis was induced by restoring of the down-regulated miR-34a expression [19, 20]. MiRNA-34a is proven to be the mediator of tumor suppression via transcriptional regulating p53 and NOTCH, as well as epithelial-mesenchymal transition (EMT) and TGF-β signaling pathways[21].These results indicate that miRNAs-34a may be a therapeutic target for osteosarcoma.
It would be surprising if many studies were performed by different researchers in different laboratories with different techniques, all of them ended up by measuring the same fundamental parameters. Furthermore, animal studies are usually small size (with a sample size of about ten in each group). Therefore, the heterogeneity is acceptable, the challenge is to decide on the most fitting method to evaluate heterogeneous studies. When heterogeneity cannot be ignored, one analytical method is to integrate the data into a random-effects model, which hypothesizes that the effects being estimated in diverse studies are not equal, however follow some distribution.
Therefore, we systematically collected all available literatures of miRNA-34a on osteosarcoma in the mouse models and performed a meta-analysis. This is the first meta-analysis to evaluate the potential value of miRNA-34a serving as diagnostic and prognostic bio-marker for osteosarcoma. We carried out a systematic literature search that included both English and Chinese databases to make sure the comprehensiveness of the studies that were assessed. Two researchers reviewed the studies individually, evaluated the methodological quality, and extracted the data to evade the bias. Though this is not a comprehensive list of all therapies that have ever been tried in pre-clinical models of osteosarcoma, but rather, a systematic review and meta-analysis of specific therapy that are being considered for human translation.
Although all included publications and data were collected strictly based on the inclusion/ exclusion criteria in this meta-analysis to reduce the bias and improve the quality of the article, some inevitable factors still existed. As we can see Tables 1 and 2, one studies did not explain gender of mice, no study has described sample-size calculation, allocation concealment, blinded assessment of outcomes and animals excluded from analysis.
In conclusion, an important finding in this meta-analysis is that miRNA-34ahas good anti-osteosarcoma potential thus may have future value as a therapeutic and prognostic biomarker in osteosarcoma. This will provide a powerful evidence for the future development of animal experiments and new therapeutic targets for clinical treatment of osteosarcoma. However, more reliable animal experiments and clinical trials need to be carried out before a miRNA-based treatment could be translated from animal studies to human use.
Acknowledgements
Grant support: This study was supported by grants from 1) National Nature Science Foundation (Nos. 81674006, 81603343, 81330085), 2) the Program for Innovative Research Team in Ministry of Science and Technology (No. 2015RA4002), 3) the Program for Innovative Research Team of Ministry of Education of China (IRT1270).
Author contributions statement
Yanping Yang and Qi Shi designed and coordinated the overall process of this meta-analysis; Yanping Yangand Yongjun Wangedited the manuscript; Wenyi Wang, Shaopu Hu, Hongfeng Ruan and Junli Chang performed literature search and selection; Wenyi Wang and Wenlan Zhi drafted the manuscript; Shaopu Hu and Xiaobo Wang prepared the figures.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang Y, Jia LS, Yuan W, Wu Z, Wang HB, Xu T. et al. Low miR-34a and miR-192 are associated with unfavorable prognosis in patients suffering from osteosarcoma. American journal of translational research. 2015;7(1):111-9 PubMed PMID: 25755833; PubMed Central PMCID: PMC4346528
2. Zhi C, Wu B. Serum miRNA-34a serves as a diagnostic and prognostic bio-marker in osteosarcoma. Int J Clin Exp Pathol. 2016;9(3):3459-64
3. Han K, Zhou Y, Tseng KF, Hu H, Li K, Wang Y. et al. PAK5 overexpression is associated with lung metastasis in osteosarcoma. Oncol Lett. 2018;15(2):2202-10 doi: 10.3892/ol.2017.7545. PubMed PMID: 29434926; PubMed Central PMCID: PMC5777019
4. Chang J, Yao M, Li Y, Zhao D, Hu S, Cui X. et al. MicroRNAs for osteosarcoma in the mouse: a meta-analysis. Oncotarget. 2016;7(51):85650-74 doi: 10.18632/oncotarget.13333. PubMed PMID: 27852052; PubMed Central PMCID: PMC5356766
5. Jamieson NB, Morran DC, Morton JP, Ali A, Dickson EJ, Carter CR. et al. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(2):534-45 doi: 10.1158/1078-0432.CCR-11-0679. PubMed PMID: 22114136
6. Voortman J, Goto A, Mendiboure J, Sohn JJ, Schetter AJ, Saito M. et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer research. 2010;70(21):8288-98 doi: 10.1158/0008-5472.CAN-10-1348. PubMed PMID: 20978195; PubMed Central PMCID: PMC2970724
7. Gallardo E, Navarro A, Vinolas N, Marrades RM, Diaz T, Gel B. et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30(11):1903-9 doi: 10.1093/carcin/bgp219. PubMed PMID: 19736307
8. He M, Gao L, Zhang S, Tao L, Wang J, Yang J. et al. Prognostic significance of miR-34a and its target proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and DLBCL. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014;17(3):431-41 doi: 10.1007/s10120-013-0313-3. PubMed PMID: 24232982
9. Liu W, Zhao Z, Wang Y, Li W, Su Q, Jia Q. et al. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/beta-catenin signaling pathway. Cell death & disease. 2018;9(3):343. doi: 10.1038/s41419-018-0363-x. PubMed PMID: 29497056
10. Zhao Y, Tu MJ, Yu YF, Wang WP, Chen QX, Qiu JX. et al. Combination therapy with bioengineered miR-34a prodrug and doxorubicin synergistically suppresses osteosarcoma growth. Biochemical pharmacology. 2015;98(4):602-13 doi: 10.1016/j.bcp.2015.10.015. PubMed PMID: 26518752; PubMed Central PMCID: PMC4725324
11. Jian C, Tu MJ, Ho PY, Duan Z, Zhang Q, Qiu JX. et al. Co-targeting of DNA, RNA, and protein molecules provides optimal outcomes for treating osteosarcoma and pulmonary metastasis in spontaneous and experimental metastasis mouse models. Oncotarget. 2017;8(19):30742-55 doi: 10.18632/oncotarget.16372. PubMed PMID: 28415566; PubMed Central PMCID: PMC5458164
12. Zhao Y, Tu MJ, Wang WP, Qiu JX, Yu AX, Yu AM. Genetically engineered pre-microRNA-34a prodrug suppresses orthotopic osteosarcoma xenograft tumor growth via the induction of apoptosis and cell cycle arrest. Scientific reports. 2016;6:26611. doi: 10.1038/srep26611. PubMed PMID: 27216562; PubMed Central PMCID: PMC4877571
13. Tiram G, Segal E, Krivitsky A, Shreberk-Hassidim R, Ferber S, Ofek P. et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS nano. 2016;10(2):2028-45 doi: 10.1021/acsnano.5b06189. PubMed PMID: 26815014
14. Wu X, Zhong D, Gao Q, Zhai W, Ding Z, Wu J. MicroRNA-34a inhibits human osteosarcoma proliferation by downregulating ether a go-go 1 expression. International journal of medical sciences. 2013;10(6):676-82 doi: 10.7150/ijms.5528. PubMed PMID: 23569431; PubMed Central PMCID: PMC3619116
15. Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q. et al. MicroRNA-34a Inhibits the Proliferation and Metastasis of Osteosarcoma Cells Both In Vitro and In Vivo. Plos one. 2012;7(3):e33778. doi: 10.1371/journal.pone.0033778.g001
16. Wen J, Zhao YK, Liu Y, Zhao JF. MicroRNA-34a inhibits tumor invasion and metastasis in osteosarcoma partly by effecting C-IAP2 and Bcl-2. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39(6):1010428317705761. doi: 10.1177/1010428317705761. PubMed PMID: 28635396
17. Jian C. Novel Combination Therapy with Doxorubicin, Bioengineered miRNA-34a Prodrug and Sorafenib Suppresses Osteosarcoma and Pulmonary Metastases. 2017.
18. Li S, Cheng D, Zhu B, Yang Q. The Overexpression of CARM1 Promotes Human Osteosarcoma Cell Proliferation through the pGSK3beta/beta-Catenin/cyclinD1 Signaling Pathway. International journal of biological sciences. 2017;13(8):976-84 doi: 10.7150/ijbs.19191. PubMed PMID: 28924379; PubMed Central PMCID: PMC5599903
19. Duan J, Zhou K, Tang X, Duan J, Zhao L. MicroRNA-34a inhibits cell proliferation and induces cell apoptosis of glioma cells via targeting of Bcl-2. Molecular medicine reports. 2016;14(1):432-8 doi: 10.3892/mmr.2016.5255. PubMed PMID: 27176117
20. Wu S, He X, Li M, Shi F, Wu D, Pan M. et al. MiRNA-34a overexpression inhibits multiple myeloma cancer stem cell growth in mice by suppressing TGIF2. American journal of translational research. 2016;8(12):5433-43 PubMed PMID: 28078014; PubMed Central PMCID: PMC5209494
21. Imani S, Zhang X, Hosseinifard H, Fu S, Fu J. The diagnostic role of microRNA-34a in breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(14):23177-87 doi: 10.18632/oncotarget.15520. PubMed PMID: 28423566; PubMed Central PMCID: PMC5410295
Author contact
![]() Corresponding author: YanpingYang, PhD. and MD. Longhua Hospital, Shanghai University of TCM, 725 S. Wanping Road, Shanghai200032, China. Phone: 86-21-6438-5700; Fax: 86-21-6439-8310; E-mail: yanpingyangkscom
Corresponding author: YanpingYang, PhD. and MD. Longhua Hospital, Shanghai University of TCM, 725 S. Wanping Road, Shanghai200032, China. Phone: 86-21-6438-5700; Fax: 86-21-6439-8310; E-mail: yanpingyangkscom

 Global reach, higher impact
Global reach, higher impact