Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(23):4391-4397. doi:10.7150/jca.26437 This issue Cite
Research Paper
Oncolytic Adenovirus Harboring Interleukin-24 Improves Chemotherapy for Advanced Prostate Cancer
1. Department of Urinary Surgery, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221000, China.
2. Radiotherapy Department, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221000, China.
*Equal contributors.
Received 2018-4-2; Accepted 2018-7-29; Published 2018-10-22
Abstract
Purpose: Oncolytic adenoviruses emerge as new agents for cancer therapy. This study aimed to investigate the synergistic anti-tumor activity of oncolytic adenovirus armed with IL-24 (ZD55-IL-24) and docetaxel (DTX) on advanced prostate cancer in vitro and in vivo.
Methods: DU145 prostate cancer cells or nude mice xenografted with DU145 prostate cancer cells were treated by ZD55-IL-24 and DTX alone or in combination.
Results: DTX did not affect ZD55-IL-24 replication and IL-24 expression in DU145 cells. In vitro, the combination of ZD55-IL-24 and DTX showed synergistic inhibitory effects on prostate cancer cell viability and invasion. In vivo, ZD55-IL-24 and DTX synergistically inhibited the growth and activated the apoptosis of DU145 xenografts, accompanied by significantly decreased PARP-1 levels and increased caspase-3 and caspase-8 levels as well as decreased CD31 expression.
Conclusion: We reported the synergistic anti-tumor efficacy of ZD55-IL-24 and DTX on prostate cancer. Our results suggest that chemotherapy combined with oncolytic adenovirus mediated gene therapy is a promising strategy for the treatment of advanced prostate cancer.
Keywords: docetaxel, prostate cancer, IL-24, Oncolytic adenovirus
Introduction
Prostate cancer is the second cause of cancer-related death in the men with high mortality worldwide [1-3]. Common treatments for prostate cancer include prostatectomy, radiotherapy, chemotherapy and androgen deprivation therapy. Radical resection and radical radiotherapy are the main treatment methods for early stage prostate cancer. For advanced prostate cancer, androgen deprivation therapy is the preferred treatment [4]. However, after a median time of about 18-24 months nearly all prostate cancer cases progress into castration-resistant prostate cancer (CRPC), and subsequently progress into metastatic castration-resistant prostate cancer (mCRPC). During this period, hormone deprivation therapy will be ineffective, the patients's prognosis is poor and the survival time is usually less than two years [5]. In the majority of cases, the first-line treatment for CRPC is docetaxel (DTX) or cabazitaxel, without or with radiation therapy, but late-stage prostate cancer becomes resistant to chemotherapy. Therefore, combination treatment strategy is urgently needed.
Interleukin (IL-24), also called melanoma differentiation associated gene-7, could exert inhibiting effect on various cancer cells, such as prostate cancer, kidney cancer, osteosarcoma, melanoma, colon cancer, cervical cancer, malignant glioma, breast cancer, lung cancer without significant side effect on normal cells [6]. The anticancer effect of IL24 is mainly achieved by promoting tumor cell apoptosis, inhibiting tumor growth, angiogenesis and metastasis, and enhancing immune regulation [7-9]. IL-24 also has a special bystander antitumor activity to kill nearby tumor cells [10,11].
Oncolytic adenovirus has gained increasing attention as a novel anti-tumor agent due to many advantages, such as selective replication in malignant cells and the lysis of targeted cells via the induction of primary cell death, the activation of anti-tumor immunity and bystander effects [12,13]. Our previous study showed that inserting IL-24 into E1B-55 gene-deleted oncolytic adenovirus ZD55 had stronger anti-tumor effect than that of ZD55, and ZD55-IL-24 had no toxicity to normal cells [14]. In this study, we evaluated the synergistic anti-tumor effect of oncolytic adenovirus ZD55-IL-24 and chemotherapy agent docetaxel in prostate cancer. We further explored the underlying mechanism to provide a potential strategy for the clinical therapy of prostate cancer.
Materials and Methods
Cell culture and agent
DU145 cell line and HEK393 cell line were provided by Institute of Biochemistry, Chinese Academy of Sciences and were cultured in RPMI-1640 and DMEM medium, respectively, supplemented with 10% fetal bovine serum (Gibco, MA, USA) and 1% penicillin/streptomycin (Gibco), maintained at 5% CO2 and 37℃ in a humidified incubator. Docetaxel (DTX) was obtained from the Jiangsu Hengrui pharmaceutical and stored at 4℃.
Recombinant adenoviruses
ZD55-IL-24 and ZD55-EGFP were provided by Institute of Biochemistry, Chinese Academy of Sciences. Mass preparation of viruses was carried out in HEK293 cells using Adeno-XTM Maxi Purification Kit (Clontech, USA). The virus titers were determined using QuickTiterTM Adenovirus Titer Immunoassay Kit (Cell Biolabs, San Diego, CA, USA).
MTT assay
DU145 cells were seeded in 96-well plates with 3,000 cells per well. Then the cells were treated with different concentration of docetaxel and different titers of ZD55-IL-24. Then every 24 h, 20 uL MTT (Amresco, stock solution 4 mg/mL) was added to each well and incubated at 37°C for 4 h. Then the supernatants were discarded and the remains were dissolved in 100 uL DMSO (Amresco). After shaking for 15 min, the well was measured at wave length 490 nm by Microplate Reader (Thermo scientific, MA, USA).
Transwell assay
Cells were seeded in 6-well plates with 1×106 cells per well. The cells were treated with DTX (1 ng/ml) and/or ZD55-IL-24 (10 MOI). 24 h later, the cells were harvested and seeded into the upper chamber precoated with Matrigel at 1×105 cells per well. 700 ul RPMI-1640 medium supplemented with 10% serum were added to the lower chamber. After 24 h incubation, the cells remained on the upper surface of the filters were wiped by cotton swabs and the cells on the filters were fixed, stained with crystal violet, and the cells in five randomly selected fields were counted under a microscope.
Xenograft tumor model
5-weeks old male BALB/c nude mice were purchased from Vital River Laboratory Animal Technology (Beijing, China). Experimental procedures followed the Guide for the Care and Use of Laboratory Animals. The xenograft model was built by injecting 1×106 DU145 cells subcutaneously in each mouse. Nude mice were divided into 4 groups randomly (n=8): (1) standard control group (Con): xenograft tumor treated with intraperitoneal injection of PBS; (2) adenovirus treatment group: xenograft tumor treated with intratumoral injection of ZD55-IL-24 (1×109 pfu) every three days; (3) drug treatment group: xenograft tumor administrated with intraperitoneal injection of docetaxel (10 mg/kg) once a week; (4) adenovirus+ drug treatment group: xenograft tumor treated with intratumoral injection of ZD55-IL-24 and intraperitoneal injection of docetaxel. The volume of tumors was measured on the 7th day after injection, and then measured every three days, mice were killed on the 28th day. The tumor volume (TV) was calculated as: TV (mm3) = length× width2×0.5.
Western blot analysis
Cells or tumor tissues were lysed and the proteins were extracted and separated by SDS-PAGE. Then the proteins were transferred to PVDF membrane and incubated with antibodies for caspase-3, caspase-8, PARP-1, cleaved PARP-1, IL-24, E1A and GAPDH (Abcam, UK) at 4°C overnight. Membranes were incubated with secondary antibodies for 2 h. Finally, the protein bands were tested by ECL reagents according to the instructions. The gray value of the bands was analyzed with Image-J software.
Hematoxylin-eosin and immunohistochemical staining
Xenograft tumor tissues were dissected and fixed in 10% formalin, then were embedded and cut into 5 μm sections. The samples were deparaffinized, hydrated, stained with hematoxylin-eosin, then mounted using neutral resin. For immunohistochemical staining, endogenous peroxidase was quenched by 3% hydrogen peroxide and the sections were blocked with 2% goat serum at room temperature for 30 min, then incubated with antibodies for caspase-3, caspase-8, PARP-1, and CD31 (Abcam, UK) at 4°C overnight. The staining was detected using DBA kit (ZSGB-Bio, Beijing, China) and observed under a light microscope (Nikon DS-Ri1, Japan), the staining was quantified by Image J software.
Terminal deoxynucleotidyl transferase-mediated digoxigenin-11-dUTP nick end labeling (TUNEL) assay
Apoptotic cells in tumor tissues were quantified using in situ apoptosis detection kit (KeyGenBio, Nanjing, China) following the manufacturer's protocol. The nucleus were counterstained with DAPI. The apoptotic cells were observed under fluorescent microscope and cells in five randomly selected fields were counted.
Statistical analysis
The data are expressed as mean±SD and analyzed by SPSS16.0 software. T-test or one-way ANOVA was used to compare the difference. P<0.05 was considered statistically significant.
Results
Synergistic inhibitory effects of DTX and ZD55-IL-24 on the viability of prostate cancer cells
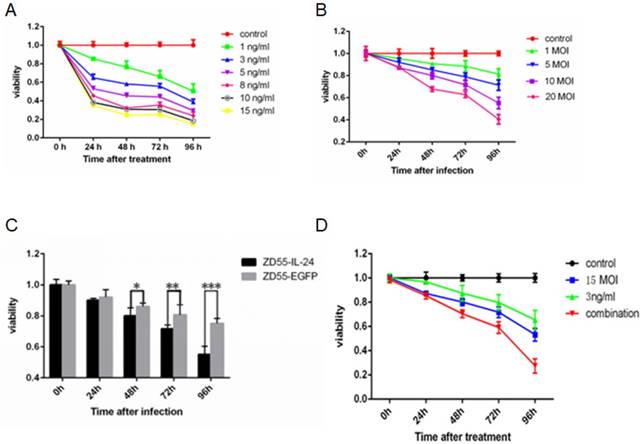
MTT assay showed that DTX inhibited the viability of prostate cancer cells in a time and concentration dependent manner (Fig. 1A). Similarly, ZD55-IL-24 inhibited the viability of prostate cancer cells in a time and concentration dependent manner (Fig. 1B). We chose 1 ng/ml DTX and 10 MOI ZD55-IL-24 for subsequent experiments. ZD55-IL-24 was more potent than ZD55-EGFP in killing prostate cancer cells when the titer was 10 MOI (Fig. 1C). Notably, we found that cell viability was significantly lower in ZD55-IL-24 (10 MOI) plus DTX (1 ng/ml) combination treatment group than in ZD55-IL-24 (10 MOI) or DTX treatment alone group (Fig. 1D).
Synergistic inhibitory effects of DTX and ZD55-IL-24 on the invasion of prostate cancer cells
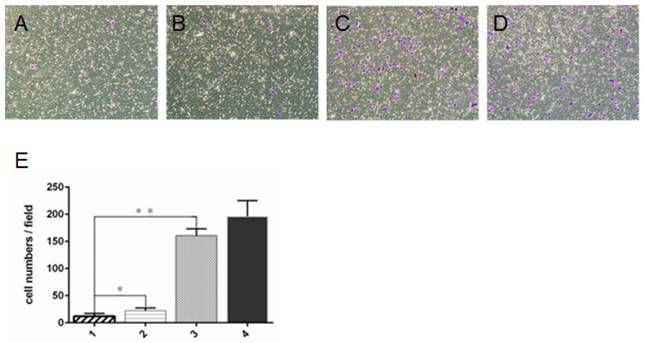
Transwell invasion assay showed that cell invasion was significantly lower in ZD55-IL-24 (10 MOI) plus DTX (1 ng/ml) combination treatment group than in ZD55-IL-24 (10 MOI) or DTX treatment alone group (Fig. 2).
ZD55-IL-24 and DTX exhibited synergistic effects to inhibit DU145 cell viability. (A) DU145 cells were treated with DTX at indicated concentration for up to 4 days. (B) DU145 cells were treated with ZD55-IL-24 at the indicated concentration for up to 4 days. (C) DU145 cells were treated with ZD55-IL-24 or ZD55-EGFP (10 MOI) for up to 4 days. (D) DU145 cells were treated with ZD55-IL-24 (10 MOI) in combination with DTIC (1 ng/ml) for up to 4 days. For comparison, DU145 cells were treated with vehicle (control), ZD55-IL-24 (15 MOI) or DTX (3 ng/ml). Cell viability was detected by MTT assay. *P<0.05, **P<0.01, ***P<0.001.
ZD55-IL-24 and DTX exhibited synergistic effects to inhibit DU145 cell invasion. Transwell assay of the invasion of DU145 cells treated by ZD55-IL-24 (10 MOI) in combination with DTIC (1 ng/ml) (A), ZD55-IL-24 (15 MOI) alone (B), DTX (3 ng/ml) alone (C), or vehicle (D). Amplification 100 x. E. Quantitative analysis of the number of cells that invaded the filter. 1. ZD55-IL-24 plus DTX, 2. ZD55-IL-24, 3. DTX, 4. Vehicle control. *P<0.05, **P<0.01.
Synergistic stimulatory effects of DTX and ZD55-IL-24 on the apoptosis of prostate cancer cells
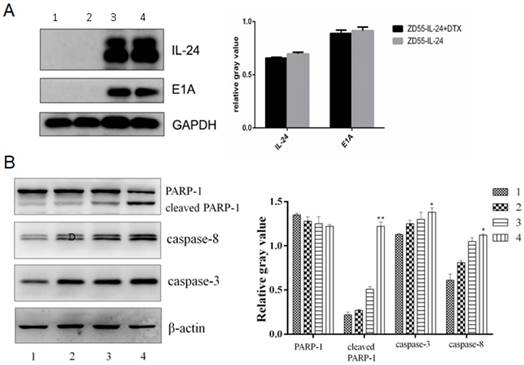
Western blot analysis confirmed that IL-24 protein and E1A protein were expressed efficiently in ZD55-IL-24 plus DTX group and ZD55-IL-24 group, and the expression levels among the two groups had not significant difference (P>0.05), which suggested that DTX had no effect on expression of IL-24 (Fig. 3A). However, protein levels of caspase-8, caspase-3 and cleaved PARP-1 were significantly higher in ZD55-IL-24 (10 MOI) plus DTX (1 ng/ml) combination treatment group than in ZD55-IL-24 (10 MOI) or DTX treatment alone group (Fig. 3B).
ZD55-IL-24 and DTX exhibited synergistic effects to promote DU145 cell apoptosis. A. Western blot analysis of IL-24 and E1A protein levels in DU145 cells in each group. GAPDH was loading control. B. Western blot analysis of caspase-8, caspase-3 and total and cleaved PARP-1 levels in each group. β-actin was loading control. 1. Vehicle control, 2. DTX, 3. ZD55-IL-24, 4. ZD55-IL-24 plus DTX. *P<0.05, **P<0.01 vs. control.
Synergistic inhibitory effects of DTX and ZD55-IL-24 on the growth of prostate cancer xenograft
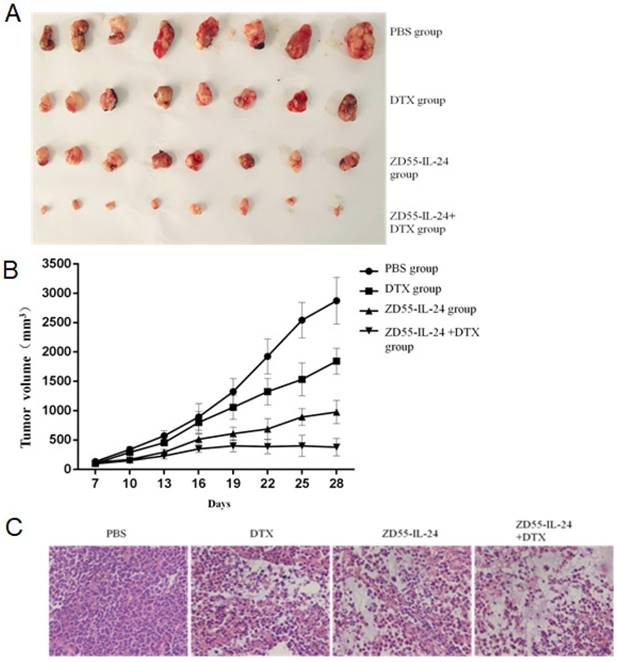
Based on the above in vitro data, we examined the effects of ZD55-IL-24 combined with DTX on prostate tumor growth in vivo. The final tumor size of ZD55-IL-24 plus DTX treatment group was obviously smaller than that of single treatment group (Fig. 4A). Tumor growth curve showed that the growth of xenografted DU145 cells was significantly slower in ZD55-IL-24 plus DTX combination treatment group than in single treatment group (Fig. 4B). Furthermore, histological analysis based on HE staining showed that a mass of tumor cells scattered in nests or clusters with the atypical and enlarged cell nuclei containing prominent nucleoli in combination treatment group (Fig. 4C).
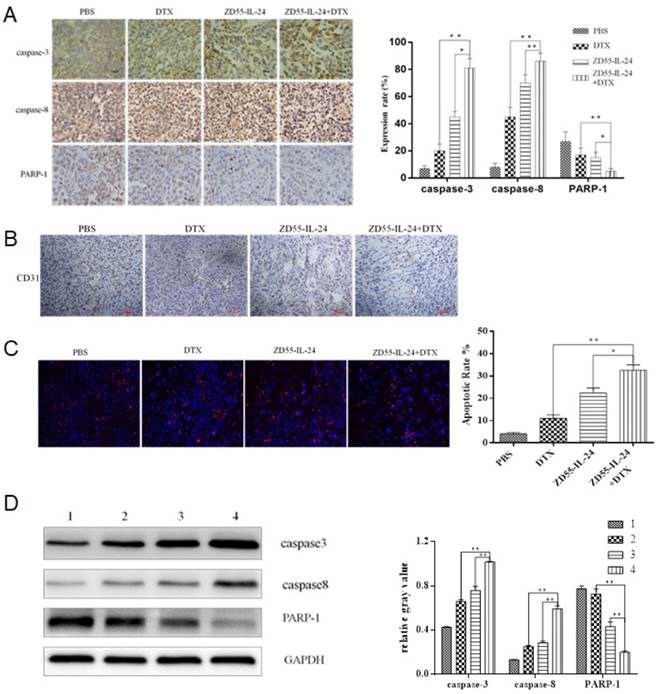
Next we aimed to understand how combination treatment achieved better anti-tumor efficacy. By immunohistochemical staining we found that protein levels of caspase-3 and caspase-8 increased significantly while protein levels of PARP-1 decreased significantly in ZD55-IL-24 plus DTX combination treatment group than in single treatment group (Fig. 5A). Furthermore, CD31 staining was significantly weaker in ZD55- IL-24 plus DTX combination treatment group than in single treatment group (Fig. 5B). These data suggest that the induction of apoptosis and the inhibition of angiogenesis may contribute to the stronger efficacy of combination treatment.
TUNEL assay confirmed that apoptosis rate was significantly higher in ZD55- IL-24 plus DTX combination treatment group than in single treatment group (Fig. 5C). Western blot analysis showed that protein levels of caspase-3 and caspase-8 were significantly higher while protein levels of PARP-1 were significantly lower in ZD55- IL-24 plus DTX combination treatment group than in single treatment group (Fig. 5D).
Discussion
Currently, how to establish high efficiency and low toxicity combination therapy for prostate cancer is a big challenge. In recent years, oncolytic virotherapy has shown promise and has been used in clinical practice [14]. However, currently the administration of oncolytic virus is limited to intratumoral injection, because intravenous administration is easy to produce antibodies against the virus. Therefore, clinical trials of oncolytic viruses are limited to superficial tumors such as head and neck cancer [15]. Due to special anatomical structure of the prostate, viral particle can be accurately injected into prostate cancer lesions guided by ultrasound in phase I clinical trials [16,17]. However, oncolytic virus has limited anti-tumor effect when used alone and the combination with chemotherapy can achieve a better outcome [18-20]. ZD55-IL-24 is an engineered conditionally replicative oncolytic adenovirus which has shown anti-tumor effect on colon cancer, lung cancer, and pancreatic cancer [21,22].
ZD55-IL-24 and DTX exhibited synergistic effects to inhibit the growth of prostate cancer xenograft. A. Prostate tumor xenografts were harvested from each group of mice at the end of treatment. B. Tumor growth curve showing the growth of prostate tumor xenografts in each group of mice. C. H&E staining of xenografts in each group of mice. Amplification 400 x.
ZD55-IL-24 and DTX exhibited synergistic effects to induce the apoptosis of prostate cancer xenograft. A. Immunohistochemical staining of xenografts in each group of mice. Amplification 400 x. B. CD31 staining of xenografts in each group of mice. Amplification 100 x. C. TUNEL assay of xenografts in each group of mice. Amplification 100 x. D. Western blot analysis of caspase-8, caspase-3 and total PARP-1 levels in xenografts in each group of mice. GAPDH was loading control. 1. Vehicle control, 2. DTX, 3. ZD55-IL-24, 4. ZD55-IL-24 plus DTX. *P<0.05, **P<0.01.
In this study we aimed to develop combination therapy with oncolytic adenovirus ZD55-IL-24 and chemotherapy agent docetaxel in prostate cancer. We found that the combination of the two agents showed better inhibitory effects on prostate cancer cell viability and invasion in vitro. Furthermore, DTX had no significant effect on the replication of ZD55-IL-24 and the expression of IL-24. These results suggest the feasibility of the combination therapy strategy. Therefore, we used nude mouse xenograft model and confirm that combination therapy showed much stronger inhibitory effects on the growth of prostate cancer xenograft in vivo than single treatment.
CD31 (platelet endothelial cell adhesion molecule-1, PECAM-1) is expressed in blood vessels and lymphatic endothelial cells, and participates in angiogenesis and tumor metastasis [23]. Thus we examined CD31 expression in prostate cancer xenograft and found that CD31 expression was inhibited at a greater extent in the combined treatment group compared to single treatment group. These results suggested that ZD55-IL-24 plus DTX could inhibit the angiogenesis of prostate cancer.
The induction of apoptosis is an important mechanism of anti-cancer therapy. To elucidate the mechanism by which combination therapy achieved better anti-tumor efficacy, we examined apoptosis and the expression of apoptosis related proteins. The proteins that regulate apoptosis mainly consist of three families: Caspase family, Bcl-2 family and IAPs family [24,25]. Among them, cysteine-aspartic acid protease (caspase) family is a protease system that directly leads to the disintegration of apoptotic cells [26]. Caspase family includes many members such as caspase-3, caspase-8 and caspase-9 which play important role in the execution of apoptosis. Activation of caspase-8 stimulates exogenous apoptotic pathway mediated by death receptor, while activation of caspase-9 stimulates endogenous apoptotic pathway mediated by the mitochondrial. Caspase-3 is a downstream effector protein, and once it is activated a cascade reaction occurs. PARP is the downstream effector protein of caspase. Increased cleavage of caspase-3 has been shown to increase the sensitivity of prostate cancer to DTX [27]. In this study, we found that DTX combined with ZD55-IL-24 significantly decreased PARP-1 levels and increased caspase-3 and caspase-8 levels in vitro and in vivo, compared to single treatment. Furthermore, TUNEL assay showed significantly higher apoptosis rate in tumor tissues treated by DTX combined with ZD55-IL-24 than in tumor tissues treated by DTX or ZD55-IL-24 alone.
In conclusion, this is the first report on the synergistic anti-cancer activity of ZD55-IL-24 plus DTX on advanced prostate cancer. Our results suggest that chemotherapy combined with oncolytic adenovirus mediated gene therapy is a promising strategy for cancer therapy.
Acknowledgements
This study was funded by grants from Jiangsu Province Science Foundation (No. BK2007032), Project of Invigorating Health Care through Science, Technology and Education, and Jiangsu Provincial Medical Youth Talent.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29
2. Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer. 2016;139:2436-46
3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86
4. Santer FR, Erb HH, McNeill RV. Therapy escape mechanisms in the malignant prostate. Semin Cancer Biol. 2015;35:133-44
5. Amaral TM, Macedo D, Fernandes I, Costa L. Castration-resistant prostate cancer: mechanisms, targets, and treatment. Prostate Cancer. 2012;2012:327-53
6. Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH. et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168(12):6041-46
7. Chang S, Yang J, Chen W, Xie Y, Sheng W. Antitumor activity of an adenovirus harboring human IL-24 in colon cancer. Mol Biol Rep. 2011;38(1):395-401
8. Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P. et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a 'magic bullet' for cancer therapy? Expert Opin Biol Ther. 2007;7(5):577-86
9. Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K. et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63(16):5105-13
10. Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK. et al. Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunol Immunother. 2007;56(2):205-15
11. Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM. et al. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11(5):724-33
12. Li Y, Hong J, Oh JE, Yoon AR, Yun CO. Potent antitumor effect of tumor microenvironment-targeted oncolytic adenovirus against desmoplastic pancreatic cancer. Int J Cancer. 2018:142 392-413
13. Lv SQ, Ye ZL, Liu PY, Huang Y, Li LF, Liu H, Zhu HL, Jin HJ, Qian QJ. 11R-P53 and GM-CSF Expressing Oncolytic Adenovirus Target Cancer Stem Cells with Enhanced Synergistic Activity. J Cancer. 2017;8:199-206
14. Liu XY. The Excellent Anti-Tumour Strategy (CTGVT, OV-gene) and the Excellent Anti-Tumor Gene (IL-24). Int J Biomed Sci. 2012;8(2):87-93
15. LaRocca CJ, Han J, Salzwedel AO, Davydova J, Herzberg MC, Gopalakrishnan R. et al. Oncolytic adenoviruses targeted to Human Papilloma Virus-positive head and neck squamous cell carcinomas. Oral Oncol. 2016;56:25-31
16. DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M. et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61(20):7464-72
17. Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J. et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63(21):7497-506
18. Zhong S, Yu D, Wang Y, Qiu S, Wu S, Liu XY. An armed oncolytic adenovirus ZD55-IL-24 combined with ADM or DDP demonstrated enhanced antitumor effect in lung cancer. Acta Oncol. 2010;49(1):91-9
19. Liu Z, Li J, Li J, Huang J, Ke F, Qi Q. et al. Mannan-modified Ad5-PTEN treatment combined with docetaxel improves the therapeutic effect in H22 tumor-bearing mice. Int J Nanomedicine. 2012;7:5039-49
20. Ning X, Sun Z, Wang Y, Zhou J, Chen S, Chen D. et al. Docetaxel plus trans-tracheal injection of adenoviral-mediated p53 versus docetaxel alone in patients with previously treated non-small-cell lung cancer. Cancer Gene Ther. 2011;18(6):444-49
21. He B, Huang XY, Liu XY, Xu B. Cancer targeting gene-viro-therapy for pancreatic cancer using oncolytic adenovirus ZD55-IL-24 in immune-competent mice. Molecular Biology Reports. 2013;40(9):5397-405
22. Zhao LL, Gu JF, Dong AW, Zhang YH, Zhong L, He LF. et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Human Gene Therapy. 2005;16(7):845-58
23. Jacquemier J, Mathoulin-Portier MP, Valtola R, Charafe-Jauffret E, Geneix J, Houvenaeghel G. et al. Birnbaum DPrognosis of breast-carcinoma lymphagenesis evaluated by immunohistochemical investigation of vascular-endothelial-growth-factor receptor 3. Int J Cancer. 2000;89(1):69-73
24. Duckett CS. IAP proteins: sticking it to Smac. Biochem J. 2005;385(1):1-2
25. Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281(2):1080-90
26. Bratton SB, MacFarlane M, Cain K, Cohen GM. Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis. Exp Cell Res. 2000;256(1):27-33
27. Gao Q, Zheng J. microRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73. Biomed Pharmacother. 2017;97:528-34
Author contact
![]() Corresponding author: Department of Urinary Surgery, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221000, China. Email: 100002006069edu.cn
Corresponding author: Department of Urinary Surgery, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221000, China. Email: 100002006069edu.cn

 Global reach, higher impact
Global reach, higher impact