Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(1):35-42. doi:10.7150/jca.26637 This issue Cite
Research Paper
Validation of Prognosis Value of Cumulative Prognostic Scores Based on Serum High-Density Lipoprotein Cholesterol and Albumin Levels in Patients with Colorectal Cancer
1. Department of Laboratory Medicine, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong 510060, China;
2. Department of Laboratory Medicine, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong 510060, China;
3. Guangdong Esophageal Cancer Institute, Guangzhou, Guangdong, China.
* Equal contributors
Received 2018-4-12; Accepted 2018-10-20; Published 2019-1-1
Abstract
Aims: Noninvasive tools for the prognosis of colorectal cancer (CRC) are in urgent need. Lipids and proteins have been studied in CRC several years, thus a prognostic indicator based on preoperative serum high-density lipoprotein cholesterol (HDL-C) and serum albumin (ALB) levels (HA score) in CRC patients and to compare the correlation with survival to that of the Glasgow prognostic score.
Patient and methods: In the present study, the patient characteristics, clinicopathological factors, and the level of pre-treatment serum markers (HDL-C, ALB, CEA and CA19-9) were analyzed retrospectively in 248 patients with CRC.
Results: In HA score, patients with reduced HDL-C and decreased ALB levels were allocated a score of 2, those with only one of these abnormalities were assign as score 1, and those with neither of these abnormalities were allocated a score of 0. The cut-off value of HDL-C and ALB were defined as median. Among these, the distribution of the HA score were 66 patients of score 2 (26.61%), 112 patients of score 1 (45.16%), and 70 patients of score 0(28.23%). The prognostic significance of the HA score was then determined by Univariate and multivariate cox hazards in CRC. Univariate analysis revealed that tumor invasion depth, lymph node metastasis, metastasis, TNM stage, CEA, CA19-9, HA score and GPS had a significant association with the OS and DFS of CRC, furthermore HA score (P<0.001, P<0.001) TNM stage(P<0.001, P<0.001) were retained as the prognostic factors that were associated with OS and DFS according to multivariate analyses.
Conclusions: These results suggest that the overall survival (OS) and disease-free survival (DFS) were shorter in CRC patients with a high level of HA score. Thus, our study has proposed that the evaluation of preoperative serum HA score may be used to predict OS and DFS of CRC.
Keywords: high-density lipoprotein cholesterol, albumin, CRC, prognosis, overall survival
Introduction
Colorectal cancer (CRC) is one of the most common malignancies and the third leading cause of cancer death worldwide[1]. Although advanced diagnostic tools, surgical techniques and therapy are available for patients with CRC, the 5-year survival rate remains low[2, 3]. As treatment plans are becoming more individualized for each patient, it is important to assess disease progression in a timely manner and accurately evaluate the prognosis[4]. Thus, a more effective and simple biomarker to recognize the biological characteristics of CRC needs to be identified in order to guide individualized treatment.
So far, several clinical factors, including clinical stage, and pathological status, serum biomarkers have been identified as independent predictors of survival in patients with CRC[5]. However, clinical stage is depend on physical examination and performance, and is frequently inaccurate. Also, pathological examination caused hurt to the body. Furthermore, many serum biomarkers have been used to predict the survival of CRC. However, the sensitivity and specificity are not sufficient and reliable.
The association between cancer and inflammation is well recognized, and almost all types of cancer are associated with systemic inflammatory response, such as the Glasgow prognostic score (GPS). The GPS is composed of the measurement C-reactive protein (CRP) and albumin(ALB)[6], it has been reported that high score of GPS is associated with decreased survival in patients of lung cancer[7], hepatocellular cancer[8], esophageal cancer[9], gastric cancer[10], colorectal cancer[11], especially the serum CRP level. Moreover, the elevated in serum tumor markers (e.g., carcinoembryonic antigen (CEA) and Carbohydrate antigen 19-9 (CA19-9) have been associated with tumor progression and decreased overall survival(OS). Therefore, more accurate and relevant measurements of patient conditions, including the extent of tumor and nutritional status, are desirable to improve outcomes for patients with CRC, and these markers have been measured through routine noninvasive methods.
Some researchers have reported that abnormal levels of lipids are closely correlated with the cancer, and low high-density lipoprotein cholesterol (HDL-C) was associated with increased risk of CRC. Furthermore, the correlation between low HDL-C and poor prognosis have been reported in various types of cancer, such as CRC and lung cancers. Therefore, we hypothesized that in combination with ALB, which reflects malnutrition and poor survival outcome of cancer patients[12], HDL-C and ALB system may provide a potent predictive scoring system for the CRC patients.
The aim of this retrospective study was to develop a prognostic grouping system as HDL-C/ ALB (HA) score and evaluate the prognostic significance in CRC. However, the relationship between GPS and HA score is still unknown. The further goal of the present analysis was to assess the relationship between GPS and HA score in CRC patients, thus identifying meaningful and new prognostic subsets of the study population.
Methods
Patients
Between January 2007 and July 2009, 248 eligible patients (143 male and106 female; ages 26-85 years) with diagnosed CRC at the Sun Yat-sen University Cancer Center were enrolled into this retrospective study. The demographic details are described in Table 1. All of the patients met the diagnostic criteria for CRC. Exclusion criterias were as follows: (1)patients treating with medication or taking hormone replacement therapy or taking curative resection; (2) patients with concomitant diseases that were associated with increasing serum lipids and proteins levels (i.e., diabetes, hyperlipidemia, or metabolic syndrome); (3) other types of malignancy. On account of their medical records, the tumor differentiation grades were classified according to the World Health Organization criteria. Stage was recorded based on American Joint Committee on Cancer Staging system (AJCC, 2002; Greene). All the patients were received treatment. The clinical information, including demographic data, pathological tumor, node, metastasis stage, smoking status, alcohol consumption and OS data were available for all patients. Smoking and alcohol Tobacco status was classified as follows: patients were divided into two groups: smoking and nonsmoking; Alcohol status was assessed as drinking or not drinking. The overall patient survival, defined as the time from surgery to death or final follow up, whichever came first, was used to assess the prognosis. Disease-free survival (DFS) was calculated from the day of surgery to the day of recurrence or most recent follow-up.
Prior to use of these serum, informed consent was obtained from each of the patients. All of them provided written informed consent. In our institution, patients were generally followed up every 3 months in the first years, every 6 months for the following 2 years, and annually thereafter for patients without evidence of recurrence. The last follow-up was in October 2014, inform consent and survival status was verified again through direct telecommunication with the patient or their family (performed by The Medical Information Unit in our Cancer Center). This study was approved by the Institute Research Ethics Committee of the Sun Yat-Sen University Cancer Center, Guangzhou, China.
The relationship between Clinical characteristics and the HA score (n = 248)
| Variables | N (%) | Score 0 (n=70) | Score 1 (n=112) | Score 2 (66) | P value |
|---|---|---|---|---|---|
| Number of patients | 248(100) | 70(28.23) | 112(45.16) | 66(26.61) | |
| Age(years) | |||||
| <60 | 124(50) | 39(55.71) | 54(48.21) | 31(46.97) | 0.522 |
| ≥60 | 124(50) | 31(44.29) | 58(51.78) | 35(53.03) | |
| Gender | |||||
| Male | 143 (57.66) | 35(50.00) | 65(58.04) | 43(68.25) | 0.201 |
| Female | 105(42.34) | 35(50.00) | 47(41.96) | 23(34.85) | |
| Family history | |||||
| Yes | 35(14.11) | 13(18.57) | 14(12.50) | 8(12.12) | 0.448 |
| No | 213(85.89) | 57(81.43) | 98(87.50) | 58(87.88) | |
| BMI | |||||
| <18.5 | 32(12.90) | 8(11.43) | 14(12.50) | 10(15.15) | 0.941 |
| 18.5-23.9 | 144(58.06) | 43(61.43) | 64(57.14) | 37(56.06) | |
| ≥24 | 69(27.82) | 19(27.14) | 33(29.20) | 17(25.76) | |
| Smoking | |||||
| No | 178(71.77) | 57 (81.43) | 80(71.43) | 41(62.12) | 0.044 |
| Yes | 70(28.23) | 13 (18.57) | 32(28.57) | 25(37.87) | |
| Alcohol status | |||||
| No | 216(87.10) | 65(92.86) | 99(88.39) | 52(78.79) | 0.043 |
| Yes | 32(12.90) | 5(7.14) | 13(11.61) | 14(21.21) | |
| Pathology | |||||
| Adenocarcinoma | 248(100) | 70 | 112 | 66 | |
| pT status | |||||
| pT 1 | 13(5.24) | 3(4.28) | 9(8.03) | 1(1.51) | 0.039 |
| pT 2 | 28(11.29) | 11(15.71) | 13(11.61) | 4(6.06) | |
| pT 3 | 72(28.03) | 24(34.29) | 34(30.36) | 14(21.21) | |
| pT 4 | 135(54.44) | 32(45.71) | 56(50.00) | 47(71.21) | |
| pN status | |||||
| pN 0 | 123(49.60) | 35(50.00) | 58(51.79) | 30(45.45) | 0.922 |
| pN 1 | 64(25.81) | 17(24.29) | 29(25.89) | 18(27.27) | |
| pN 2 | 61(24.60) | 18(25.71) | 25(22.32) | 18(27.27) | |
| pM status | |||||
| pM 0 | 191(77.01) | 61(87.14) | 86(76.79) | 44(66.67) | 0.018 |
| pM 1 | 57(22.98) | 9(12.86) | 26(23.21) | 22(33.33) | |
| Clinical stage | |||||
| Ⅰ | 30(12.10) | 9(12.86) | 17(15.18) | 4(6.06) | 0.084 |
| Ⅱ | 79(31.85) | 23(32.86) | 36(32.14) | 20(30.30) | |
| Ⅲ | 83(33.47) | 29(41.43) | 34(30.36) | 20(30.30) | |
| Ⅳ | 56(22.58) | 9(12.86) | 25(22.32) | 22(33.33) | |
| CEA(ng/mL) | |||||
| ≤5 | 147(59.27) | 42(60.00) | 74(66.07) | 31(46.97) | 0.094 |
| >5 | 93(37.50) | 25(35.71) | 37(33.04) | 31(46.97) | |
| CA19-9 | |||||
| ≤35 | 182(73.39) | 51(72.86) | 91(81.25) | 40(60.61) | 0.017 |
| >35 | 54(21.77) | 14(20.00) | 18(16.07) | 22(33.33) |
Laboratory Measurements
As part of the physical examination, peripheral blood was collected from the patient between 7 and 8 a.m before treatment, clotted at room temperature, centrifuged at 3500 r/min for 8 min. The levels of CEA and CA199 were measured by a Modular Analytics E170 immunoassay unit (Roche Diagnostics, Germany), and serum HDL-C, ALB and CRP were measured using a Hitachi 7600 automatic biochemical analyzer (Hitachi High-Technologies, Japan). GPS was calculated by CRP and albumin as follows: GPS 0, patients with a CRP ≤10 mg/L and albumin ≥35 g/L; GPS 1, patients with only higher CRP or lower albumin; GPS 2, patients in whom CRP was >10 mg/Land albumin concentration <35 g/L.
Statistical analysis
All statistical tests were performed with SPSS 16.0 for Windows software (SPSS, Chicago, IL, USA). As recommended by the manufacturers, the cut-off for CEA and CA199 was 5 ng/mL, 35U/mL. Continuous variables (HDL-C and ALB) were categorized using median values as cut-off points. HA score was calculated by HDL-C and ALB as follows: Score0, HDL-C ≥1.13 mmol/L and ALB ≥41.1 g/L); Score1: HDL-C ≥1.13 mmol/L or ALB ≥41.1 g/L; Score2: HDL-C <1.13 mmol/L and ALB < 41.1 g/L. The patients with a HA score of score 0 or 1 were classified into the low HA group, and those with a GPS of 2 were classified into the high-GPS group. The correlation between HA score, clinical characteristics and GPS was assessed using the Mann-Whitney U test and χ2 tests.
Univariate and multivariate analyses of clinical variables were performed using Cox proportional hazards regression models. Firstly, we used Univariate analyses to analyze all the variables and then we found that factors had a significant association with CRC survival. Secondly, we carried out multivariate analysis by full model to determine whether these factors could be used as an independent prognostic factor for survival. We also eliminated the influence of statistical colinearity, Results of this survey were analysed using the Kaplan-Meier survival curves with the log-rank test and proportional hazard model. P values < 0.05 were regarded as indicating statistically significant differences. All reported P values are two sided.
Results
The correlations between the HA score and clinical characteristics
The relationships between the HA score and clinical characteristics of the patients are summarized in Table 1. A total 248 patients with CRC cancer were eligible for the final analysis. The median age of the patients was 60 years (range, 26-85 years), and 57.66 % of patients were males. All the pathology of the patients was adenocarcinoma. The clinical stage I, II, III and IV were observed in 30 (12.10 %), 79 (31.85%), 83 (33.47 %), and 56 (22.58 %) of the patients, respectively. The median serum HDL-C level was 1.13 mmol/L and the median serum ALB level was 41.1 g/L. Among these, the distribution of the HA score were 70 patients of score 0(28.23%), 112 patients of score 1 (45.16%), and 66 patients of score 2 (26.61%). χ2 test showed that HA score positively correlated with smoking(P=0.044, more common in smoking patients than not), alcohol status(P=0.043, more common in patients with drinking than not), tumor invasion depth(P=0.039, more common in pT4 than the other), metastasis(P=0.019, more common in patients with metastasis than not) and CA19-9(P=0.017, more common in higher CA19-9 than low CA19-9). No significant differences in age, gender, family history, BMI, lymph node metastasis and CEA were identified between the groups. Especially, the higher HA score was significantly observed more frequently among patients in clinical stage II or higher stage.
Associations between HA score and patient survival analysis
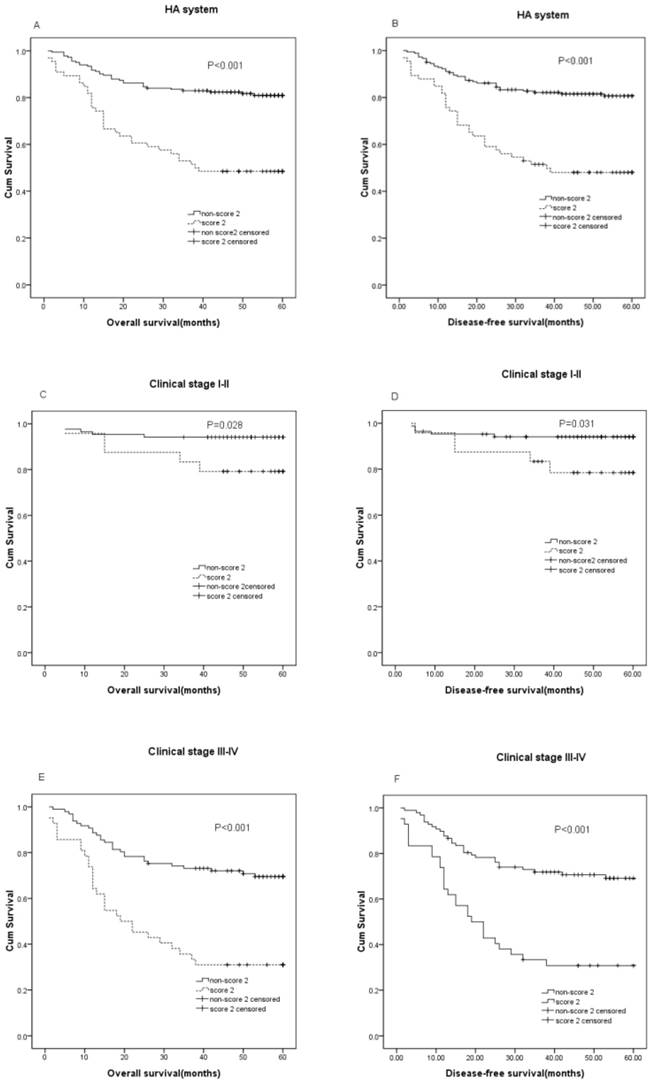
The median follow-up time of the 248 CRC patients was 55 months with 180 alive and 68 cancer-related deaths at the last clinical follow-up. The median OS was 55 (range, 1-60) months and the median DFS was 50 (range, 1-60) months. The patients with a HA score of 2 was classified into the high HA group, and those with GPS of 0 and 1 were classified into the low HA group. In the Kaplan-Meier analysis, the HA score was closely associated with OS and DFS, and a higher HA score in patient with shorter DFS(P<0.001) and OS(P<0.001). For the whole cohort, the OS was 13.24 months shorter in patients HA score score2 (mean, 35.17 months) than those with non-score2 (mean, 48.41months), and the DFS was 12.44 months shorter in patients with score2 (mean, 33.24 months) than those with non- score2 (mean, 45.68 months) (P<0.001). Furthermore, this analysis showed that HA score could distinguish OS when stratified by clinical stage(TNM stage I-II, P=0.032; TNM stage III-IV, P<0.001), and also the HA score in patients was independently associated with DFS(TNM stage I-II, P=0.035; TNM stage III-IV, P<0.001)(Figure 1).
Univariate and multivariate analyses of prognostic factors
With univariate survival analysis, the analysis found that tumor invasion depth (P=0.005), lymph node metastasis (P<0.001), metastasis (P<0.001), TNM stage (P<0.001), CEA (P=0.012), CA19-9 (P=0.001), HA score (P=0.000)and GPS P<0.001) had a significant association with the OS of CRC, also tumor invasion depth (P=0.006), lymph node metastasis (P<0.001), metastasis (P<0.001), TNM stage (P<0.001), CEA (P=0.010), CA19-9 (P<0.001), HA score (P=0.000)and GPS (P<0.001) had effect on DFS (Table 2). All the potentially important factors identified in univariate analysis except tumor invasion depth, lymph node metastasis, and metastasis, were included in the multivariate analysis (Cox proportional hazards model) (Table 2), because of the influence of collinearity. Consequently, the multivariate analysis showed that TNM stage (HR=4.986; 95%CI: 2.395-10.380; P < 0.001), CA19-9 (HR=1.770; 95%CI: 1.006-3.114; P =0.048) and HA score (score 2 and non-score 2) (HR=1.728; 95%CI: 1.322-2.376; P<0.001) were identified as significantly independent predictors of OS of all patients, and TNM stage (HR=5.006; 95%CI: 2.406-10.416; P < 0.001) and HA score(HR=1.724; 95%CI: 1.320-2.251; P < 0.001) were also independent prognostic indicators of DFS(Table 3).
Comparison of HA score with GPS and Other Predictive Factors
After stratification by GPS, there are 54 and 185 patients were classified as score 1 and score 0, and only 9 patients were assigned a score of 2. However, the distribution of patients based on HA score and GPS were not similar between the two groups(P < 0.001). Univariate analysis showed that HA score and GPS were significantly correlated with OS and DFS. Furthermore, in multivariate Cox regression analysis, only HA score was significant independent predictor of OS and DFS(P < 0.001 and P < 0.001, respectively), whereas GPS was not(P =0.052 and P =0.051, respectively).
Clinicopathological factors, HA score, disease-free survival and overall survival: univariate analysis (n =248)
| Disease-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | P* Value | HR | 95%CI | P* Value |
| Age (years) | ||||||
| <60 vs.≥60 | 0.976 | 0.607-1.570 | 0.920 | 0.961 | 0.597-1.546 | 0.869 |
| Gender | ||||||
| Male vs. Female | 1.121 | 0.695-1.810 | 0.639 | 1.122 | 0.695-1.810 | 0.638 |
| Family history | ||||||
| No vs. Yes | 1.033 | 0.528-2.020 | 0.925 | 1.034 | 0.528-2.023 | 0.922 |
| BMI | ||||||
| <18.5 vs. 18.5-23.9 vs. ≥24 | 0.805 | 0.541-1,198 | 0.286 | 0.800 | 0.538-1.189 | 0.270 |
| Smoking | ||||||
| No vs. Yes | 1.132 | 0.677-1.894 | 0.637 | 1.144 | 0.684-1.913 | 0.609 |
| Alcohol status | ||||||
| No vs. Yes | 1.103 | 0.564-2.158 | 0.774 | 1.140 | 0.582-2.230 | 0.703 |
| T classification | ||||||
| T3-4 vs. T1-2 | 7.333 | 1.796-29.942 | 0.006 | 7.625 | 1.867-31.135 | 0.005 |
| N classification | ||||||
| No vs. Yes | 3.636 | 2.074-6.372 | <0.001 | 3.649 | 2.082-6.396 | <0.001 |
| Metastasis | ||||||
| No vs. Yes | 4.580 | 2.839-7.389 | <0.001 | 4.689 | 2.904-7.571 | <0.001 |
| TNM stage | ||||||
| III-IV vs. I-II | 5.460 | 2.789-10.690 | <0.001 | 5.506 | 2.813-10.780 | <0.001 |
| CEA | ||||||
| ≤5 vs. >5 | 1.833 | 1.131-2.972 | 0.014 | 1.866 | 1.151-3.025 | 0.011 |
| CA19-9 | ||||||
| ≤35 vs. >35 | 2.409 | 1.438-4.037 | 0.001 | 2.438 | 1.455-4.085 | 0.001 |
| HA Score | ||||||
| Low HA Score vs. High HA Score | 1.834 | 1.445-2.328 | <0.001 | 1.850 | 1.457-2.348 | <0.001 |
| GPS | ||||||
| Score0 vs. Score1 vs. Score2 | 1.911 | 1.299-2.811 | 0.001 | 1.923 | 1.310-2.821 | 0.001 |
Prognostic significance of serum HA score in CRC. The patients were categorized into a low 'HA score' and a high 'HA score' according to the media value of HDL-C and ALB. The five-year OS and DFS rate were calculated using the Kaplan-Meier method and analyzed with the log-rank test. Preoperative HA score is significantly predictive of DFS and OS, with lower HA score among patients with better DFS and OS in the entire CRC cohort (A: P < 0.001, B: P < 0.001), clinical stage Ⅰ-Ⅱ (C: P =0.028, D: P =0.031), clinical stageⅢ-Ⅳ(E: P < 0.001, F: P < 0.001)
Clinicopathological factors, HA score, disease-free survival and overall survival: multivariate analysis (n =248)
| Characteristics | Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P* Value | HR | 95%CI | P* Value | |
| TNM stage | ||||||
| III-IV vs. I-II | 5.006 | 2.406-10.416 | <0.001 | 4.986 | 2.395-10.380 | <0.001 |
| CEA | ||||||
| ≤5 vs. >5 | 0.942 | 0.547-1.620 | 0.828 | 0.936 | 0.543-1.612 | 0.811 |
| CA19-9 | ||||||
| ≤35 vs. >35 | 1.729 | 0.984-3.040 | 0.057 | 1.770 | 1.006-3.114 | 0.048 |
| GPS | ||||||
| Score0 vs. Score1 vs. Score2 | 1.541 | 0.998-2.380 | 0.051 | 1.539 | 0.997-2.376 | 0.052 |
| HA Score | ||||||
| Low HA Score vs. High HA Score | 1.724 | 1.320-2.251 | <0.001 | 1.728 | 1.322-2.376 | <0.001 |
Discussion
Lipid and protein have been reported in several cancers, such as CRC. To date, associations between HDL-C, ALB, GPS and CRC survival have not been well developed. In this study, the univariate analysis revealed that HA score and GPS were prognostic factors of CRC, and multivariate analysis showed that high HA score score was an independent prognostic predictor of poor CRC OS(HR=1.728; 95%CI: 1.322-2.376; P < 0.001). Furthermore, the HA score significantly associated with clinical stage and survival in patients with CRC, indicating that patients with higher HA score show more progressed disease and poorer prognosis.
The HDL-C and ALB tests are simple, inexpensive tests that are widely used in clinical laboratories to detect the function of nutrition and inflammation. Chi el al. reported that the decreased level of preoperative HDL-C was found to be associated with poor survival in patients with NSCLC. Serum HDL-C level may be a clinical prognosis factor for NSCLC patients[13]. Liu et al. published a study which indicated a statistically significant relationship between metabolic syndrome and colorectal adenomas in men, and that only central obesity, low HDL-C, and high triglycerides were independently associated with colorectal adenomas [14]. Coppola JA et al also found that a direct association between triglyceride plasma levels and an inverse association between plasma HDL-C levels and adenoma risk[15]. The role of HDL-C is reverse cholesterol transport, which is very important to prevent from the cardiovascular diseases. Cholesterol is a structural component of the cell membrane which localized in membrane microdomains that assemble the signal transduction machinery and associate to proteins implicated in key cellular signaling pathways that are closely associated with malignant transformation[16]. But, the function of HDL-C in carcinogenesis is not well understood. It is interesting to consider the reason for the observed association between HDL-C level and incident cancer risk, which may be attributed to its multiple properties, including anti-inflammatory and antioxidant properties. As we all known, cancer is a pro-inflammatory state, in which inflammatory cells actively participate in the occurrence of tumor development, such as tumor cell proliferation, survival, and migration. and the HDL-C may influence some of the pro-inflammatory mediators involved in carcinogenesis. Furthermore, reactive oxygen species may be conducive to the vitality of cancer cells and drive signaling transduction pathways, which lead to activation of redox-sensitive transcription factors and genes involved in cancer cell growth, proliferation, and survival. Su et al [17] demonstrated that HDL-C mimetic peptide significantly reduced proliferation of colon cancer cell in BALB/c mice through the possible mechanism of anti-inflammatory and antioxidant. HDL-C level was negatively correlated with the occurrence of CRC. Decreased HDL-C level can promote the production of inflammatory cytokines IL-6, and inhibit the secretion of anti-inflammatory cytokines such as IL-10, so as to promote the proliferation and differentiation of colorectal cancer cells and inhibit their apoptosis. Meanwhile, low HDL level can promote oxidative stress and insulin resistance and participate in the occurrence of colorectal cancer.
However, ALB has been regarded as a nutritional indicator to measure the nutritional status and liver function of the body. In recent years, the role of albumin in malignant tumors has been paid more and more attention, which is considered to be an important indicator of systemic inflammatory reaction in the course of malignant tumors [5, 18].The synthesis of ALB is suppressed by malnutrition and inflammation[19]. Systemic inflammatory response is part of the tumor. The pro-inflammatory cytokines are released, which could stimulate liver production of CRP and increases the demand for certain amino acids; The cytokines, such as IL-6 and tumor necrosis factor, may modulate the production of ALB by hepatocytes and through increase the permeability of the microvasculature to increased transcapillary passage of ALB; Furthermore, the kuffer cell in liver have influenced by the micrometastatic tumor cells, could produce various cytokines (IL-6 and TNF) thus modulate the synthesis of ALB in hepatocytes[20, 21]. The potential advantage of serum ALB level as a prognostic factor in cancer patients is that it is inexpensive, reproducible and powerful[22]. Therefore, combine with HDL-C and ALB may be a potent prognostic indicator for CRC outcomes. Glasgow prognostic score (GPS), a scoring system based on inflammation(CRP and ALB), was validated as an useful tool in predicting prognosis for various cancers, including gastric cancer[23], lung cancer[24], pancreatic cancer[25], hepatocellular cancer[26], esophageal cancer[27], and cervical cancer[28]. Especially, GPS have been reported to be associated with the prognosis in patients with CRC.
In this study, we reviewed preoperative serum HDL-C and ALB levels. We defined the HDL-C cut-off value as 1.13 mmol/L according to the median, and the ALB cut-off value as 41.1g/L also according to the median. The distribution of patients in HA score is more reasonable than GPS, especially in score 2, there is 66 patients had HA score of score 2 and 9 patients in score 2 of GPS. In fact, there is a correlation between HA score, tumor invasion depth and metastasis, not age and gender. Furthermore, in univariate analysis, increased HA score, GPS and clinical stage, elevated CEA and CA19-9 predicted a higher risk of patients of CRC; But in multivariate analysis, only HA score and clinical stage are significantly linked with cancer survival. Therefore, the HA score is better than GPS as a prognostic indicator in patients with CRC, especially patients with metastasis.
Both HA score and GPS are inflammation factors, systemic inflammation and nutritional deficiencies might be severe in patients with CRC. However, there are many differences between them. Firstly, compared with CRP in GPS, the HDL-C is more specific in CRC. Second, while CRP indicates serum cytokine levels in cancer patients. Whereas HDL-C may work both ways, in the inflammatory state caused by cancer as a depressing force[29] , and the anti-oxidant activity caused by HDL-C states as a inhibiting force for cancer development[30].
We acknowledge the limitations of our retrospective analysis, and we only investigated 248 CRC patients from our institution in 2007-2009. However, our studies suggested an association between HA score and CRC patients. Patients have HA score of score 2 were more likely to have a poor survival, compare patients of non-score 2, and the mechanism of HA score in CRC need to be further study.
Abbreviations
CRC: Colorectal cancer; OS: Overall Survival; DFS: Disease-free Survival; GPS: Glasgow prognostic score; HDL-C: High-density lipoprotein cholesterol; ALB: Albumin; CRP: C-reactive protein; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9.
Acknowledgements
We thank the staff of the biochemical lab of Sun Yat-sen University Cancer Center, who provided various biochemical markers, and all the staff who supported our study.
Funding information
This study was supported by Natural Science Foundation of Guangdong Province, China (grant no. 2018A030310260) and Medical Scientific Research Foundation of Guangdong Province, China (grant no. 2018102516469945).
Authors' contributions
Hao Huang and Lin Zhang carried out the main work and contributed equally. They participated in the design of the study and drafted the manuscript. Du-bo Chen performed the statistical analysis. Pei-song Chen and Min-Liu conceived the study and participated in its design and coordination, Xiao-hong He and Xue-gao Yu helped to draft the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90
2. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G. et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715-722 722-724
3. Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13(5):668-676
4. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903-2909
5. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704-1706
6. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92(10):1834-1836
7. Jiang AG, Chen HL, Lu HY. The relationship between Glasgow Prognostic Score and serum tumor markers in patients with advanced non-small cell lung cancer. Bmc Cancer. 2015;15:386
8. Horino K, Beppu T, Kuroki H, Mima K, Okabe H, Nakahara O, Ikuta Y, Chikamoto A, Ishiko T, Takamori H. et al. Glasgow Prognostic Score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol. 2013;18(5):829-838
9. Da SJ, Mauricio SF, Bering T, Correia MI. The relationship between nutritional status and the Glasgow prognostic score in patients with cancer of the esophagus and stomach. Nutr Cancer. 2013;65(1):25-33
10. Mimatsu K, Oida T, Fukino N, Kano H, Kawasaki A, Kida K, Kuboi Y, Amano S. Glasgow prognostic score is a useful predictive factor of outcome after palliative gastrectomy for stage IV gastric cancer. Anticancer Res. 2014;34(6):3131-3136
11. Dreanic J, Dhooge M, Brezault C, Mir O, Chaussade S, Coriat R. A prognostic indicator of survival in metastatic colorectal cancer patients in the era of molecular-targeted agents: the modified Glasgow Prognostic Score. Oncology-Basel. 2014;86(1):44-45
12. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69
13. Chi PD, Liu W, Chen H, Zhang JP, Lin Y, Zheng X, Liu W, Dai S. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One. 2014;9(3):e91080
14. Liu CS, Hsu HS, Li CI, Jan CI, Li TC, Lin WY, Lin T, Chen YC, Lee CC, Lin CC. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol. 2010;10:51
15. Coppola JA, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Ness RM, Fazio S, Zheng W, Murff HJ. Plasma lipid levels and colorectal adenoma risk. Cancer Causes Control. 2015;26(4):635-643
16. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46-50
17. Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, Navab M, Fogelman AM, Reddy ST, Farias-Eisner R. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11(6):1311-1319
18. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69
19. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4):S118-S125
20. Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35(2):106-110
21. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210-213
22. Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, Shih YL, Chang SJ, Wang JY. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. Bmc Cancer. 2009;9:288
23. Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94(5):637-641
24. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7(4):655-662
25. La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19(9):2917-2923
26. Horino K, Beppu T, Kuroki H, Mima K, Okabe H, Nakahara O, Ikuta Y, Chikamoto A, Ishiko T, Takamori H. et al. Glasgow Prognostic Score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol. 2013;18(5):829-838
27. Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, Yekebas EF, Izbicki JR. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18(4):1130-1138
28. Polterauer S, Grimm C, Seebacher V, Rahhal J, Tempfer C, Reinthaller A, Hefler L. The inflammation-based Glasgow Prognostic Score predicts survival in patients with cervical cancer. Int J Gynecol Cancer. 2010;20(6):1052-1057
29. Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114(1):157-170
30. Robinson JG. Low high-density lipoprotein cholesterol and chronic disease risk marker or causal? J Am Coll Cardiol. 2010;55(25):2855-2857
Author contact
![]() Corresponding authors: Min-Liu and Pei-song Chen, Department of Laboratory Medicine, The First Affiliated Hospital of Sun Yat-sen University, 58 Zhongshaner Road, Guangzhou, Guangdong 510060, China Email: chpssysu.edu.cn
Corresponding authors: Min-Liu and Pei-song Chen, Department of Laboratory Medicine, The First Affiliated Hospital of Sun Yat-sen University, 58 Zhongshaner Road, Guangzhou, Guangdong 510060, China Email: chpssysu.edu.cn

 Global reach, higher impact
Global reach, higher impact