Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(1):156-167. doi:10.7150/jca.28600 This issue Cite
Research Paper
LXRα Promotes the Differentiation of Human Gastric Cancer Cells through Inactivation of Wnt/β-catenin Signaling
1. Department of Gastrointestinal Surgery, Xiangya Hospital of Central South University, Hunan key laboratory of precise diagnosis and treatment of gastrointestinal tumor, Changsha, Hunan, P.R. China;
2. Department of Colorectal and Anus Surgery, Xiangya Hospital of Central South University, Hunan key laboratory of precise diagnosis and treatment of gastrointestinal tumor, Changsha, Hunan, P.R. China.
Received 2018-7-18; Accepted 2018-11-2; Published 2019-1-1
Abstract
LXRα is a subtype of the liver X receptors (LXRs). There is accumulating evidence to support the involvement of LXRα in a variety of malignancies. However, the function and specific mechanism of LXRα in gastric cancer (GC) remain unclear. In this study, the expression of LXRα was significantly lower in poorly differentiated and undifferentiated GC tissues compared with well- and moderately differentiated GC tissues by immunohistochemistry analysis. The activation of LXRα leads to the decreased expression of β-catenin, CD44, and Cyclin D1, whereas the inhibition of LXRα has opposite effect. The same results were obtained in animal experiments. Furthermore, results showed that CD44 and Cyclin D1 expression significantly decreased when Wnt/β-catenin signaling was blocked in LXRα silent GC cells, whereas it was significantly increased when Wnt/β-catenin signaling was activated in LXRα over-expressed GC cells. CD44 and Cyclin D1, downstream targets of Wnt/β-catenin signaling, are specific markers for cell differentiation. Therefore, we conclude that LXRα may promote the differentiation of human GC cells through inactivation of Wnt/β-catenin signaling.
Keywords: liver X receptor alpha, stomach neoplasms, cell differentiation, Wnt beta-catenin signaling pathway, CD44 antigen
Introduction
Gastric cancer (GC) is the fifth most common malignancy in the world. In 2012, approximately one million new cases of stomach cancer were diagnosed (951,000 cases, 6.8% of the total) [1]. It remains the second most deadly cancer despite a decline in incidence and mortality over the last 50 years [2, 3]. Chemotherapy and surgery are the mainstays of treatment; however, novel approaches are urgently required as chemoresistance hinders current treatment strategies [4, 5].
Liver X receptors (LXRs) are involved in cholesterol transport, glucose metabolism, and modulation of the inflammatory response. They are members of the nuclear receptor family and subdivided into LXRα (also known as NR1H3) and LXRβ (also known as NR1H2). Cholesterol derivatives, including oxysterols and 24(S), 25-epoxycholesterol, and synthetic agonists, such as T0901317 and GW3965, activate both LXRs [6]. LXRα is expressed in active metabolic sites, such as in the liver, intestine, kidney, skin, adrenal glands, adipose tissue, and macrophages. LXRβ is found throughout the body [7]. Recent studies indicate that LXRα is associated with many types of cancer [8].
In terms of cellular differentiation, the poor differentiation of tumor cells is often associated with a worse prognosis, particularly in leukemia, thyroid cancer, and colon cancer. In addition, the differentiation of tumor cells is associated with epithelial- mesenchymal transition (EMT) and drug resistance [9, 10]. These relationships are less well understood in GC.
In this study, we first focus our attention on the expression of LXRα in malignant gastric tissues and tumor adjacent mucosas to determine whether LXRα might serve as a diagnostic marker for GC. Second, we investigate the relationship between the expression of LXRα and patient characteristics including age, gender, lymph node metastasis, invasion depth, and the TNM stage of GC. Third, we determine the possible mechanism by which LXRα regulates the differentiation of GC cells and the role of the Wnt/β-catenin signaling pathway.
Materials and Methods
Materials
Anti-LXRα (ab41902), anti-β-catenin (ab32572), anti-CD44 (ab51037), and anti- Cyclin D1 (ab134175) antibodies were supplied by Abcam (Abcam, USA). GAPDH (10949-1-AP) and β-tubulin (10094-1-AP) antibodies were supplied by Proteintech Group (Proteintech, USA). Dimethyl sulfoxide and GW3965 were purchased from Sigma-Aldrich (St Louis, MO, USA). XAV939 (S1180) and Wnt agonist 1 (S8178) were purchased from Selleck (Selleck, USA).
Subjects and samples
In total, 124 patients, 93 males and 31 females, aged between 29 years and 83 years (median age 58 years) were enrolled from November 2015 to December 2016. Each patient had a unique hospital number. All patients were treated with surgery in Xiangya Hospital, affiliated with Central South University, Hunan, China. Neither chemotherapy nor irradiation was performed prior to tumor resection. This study was approved by the Medical Ethics Committee of the Xiangya Hospital of Centre South University (approval number 201503158). Written informed consent from the donor was obtained for the use of samples in this research. Samples of cancer tissue, and tumor adjacent mucosa (2 cm distance from the cancer) were collected from each patient during the operation. Next, the samples were fixed in 10% formaldehyde solution and subsequently embedded in paraffin wax. All these samples were diagnosed as adenocarcinoma after pathologic examination. Differentiation was graded as follows: well and moderate (n=19, 15.3%), and poor and undifferentiated (n=105, 84.7%).
Immunohistochemistry
Serial cross sections, 4 μm thick, were collected and stained immunohistochemically as per manufacturer's instructions for the Histostain®-Plus kits (Zymed, Carlsbad, USA). The samples were deparaffinized, rehydrated, and incubated with fresh 0.3% hydrogen peroxide in methanol for 10 min at 37 °C. Sections were autoclaved for antigen retrieval in citrate buffer at 100 °C for 2 min and incubated with rabbit LXRα polyclonal antibody (ab41902, dilution 1:1000, Abcam, USA) at 4 °C overnight. Sections were washed with PBS and incubated with biotinylated anti-rabbit IgG as a second antibody for 15 min at 37 °C and then with streptavidin-conjugated horseradish peroxidase for 15 min at 37 °C (Zymed, Carlsbad, USA). An immune reaction was demonstrated with DAB. The sections were counterstained with hematoxylin, dehydrated, and mounted. PBS substituted for primary antibody in negative controls, with no evident detectable staining. LXRα -positive oral cancer was a positive control in this study.
Evaluation of LXRα immunohistochemical staining
The average integral optical density (sumIOD/ area) measurement helped determine the positive- staining density [11]. The imaging system was a Leica CCD camera DFC420 connected to a Leica DMIRE2 microscope (Leica Microsystems Imaging Solutions, Ltd., Cambridge, UK), and high-power magnification (×400) was used to photograph the representative fields. The software was Leica Qwin Plus v3. In each image, the sumIOD/area was measured and counted using Image-Pro Plus v6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA).
RNA interference
shRNA duplexes targeting LXRα were synthesized as follows: NR1H3-RNAi(29985-1): 5′-TTCCTCAAGGATTTCAGTT-3′, NR1H3-RNAi(29983-1): 5-GACTGATGTTCCCACGGAT-3′, and NR1H3-RNAi(2998 6-1): 5′-GAAGAAACTGAAGCGGCAA-3′. shRNA duplexes containing non-specific sequences were used as a negative control: 5′-TTCTCCGAACGTGTCACGT -3′.
Cell culture
The human gastric adenocarcinoma cell lines AGS and SGC7901 were obtained from Central South University (Changsha, China). The cells were cultured in PRMI 1640 (HyClone, Waltham, MA, USA) supplemented with 10% fetal bovine serum (HyClone) in a humidified atmosphere of 37 °C at 5% CO2. We exposed the cells to GW3965 (a LXRα agonist), XAV939 (a β-catenin inhibitor), and Wnt agonist 1 (a Wnt/β-catenin signaling agonist).
Lentivirus transfection
GeneChem Biomedical Co., Ltd. (Shanghai, China), created the LXRα coding sequence and LXRα shRNA using a recombinant gene delivery system. A total of 5×105 AGS or SGC7901 cells were seeded in each well of a six-well plate. When the cells reached 80%-90% confluence on the day of transfection, the overexpression or inhibition of LXRα or a blank vector recombinant lentivirus gene delivery system was transfected into the cells. The six groups of cells included cells treated with 10 μmol/L GW3965 for 48 h (“LXR GW”), untransfected (“blank”) cells, cells transfected with LXRα overexpression vector (“LXRα”), cells transfected with empty GFP vector (“GFP”), cells transfected with LXRα-shRNA vector (“LXRα Sh”), and cells transfected with scrambled shRNA vector (“Sh Ctrl”). Later in the study, “Sh Ctrl” and “LXRα Sh” cells were treated with either 10 μmol/L XAV939 (a Wnt/β-catenin signaling inhibitor) or dimethyl sulfoxide for 24 h. “LXRα” cells were treated with 10 μmol/L Wnt agonist 1 (a Wnt/ β-catenin signaling activator S8178, Selleck, USA) or dimethyl sulfoxide for 24 h in order to assay the influence of Wnt/β-catenin signaling LXRα function.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. First-strand cDNA was synthesized by using the PrimeScript™ RT reagent Kit (TaKaRa) according to the manufacturer's instructions. After the RT reaction, 1 μl of the complementary DNA was used for subsequent qRT-PCR (SYBR ® Premix Ex Taq, TaKaRa) following the manufacturer's protocol. The primers for qRT-PCR are listed in Table 1. Relative quantification analysis was conducted according to the 2 -ΔΔCt method. Each sample was analyzed in triplicate, and all experiments were carried out three times independently. The relative levels of target genes' mRNA were expressed as the ratio of target to GAPDH and calculated from the standard curve as directed. All reported results are the average ratios of more than three different independent experiments.
Flow cytometry analysis
Four groups of GC cells AGS (“LXRα Sh”, “Sh Ctrl”, “GFP” and “LXRα”) at each phase of the cell cycle were measured by the discriminative DNA content stained with propidium iodide (PI). Cells were fixed with chilled 70% ethanol and kept in a -20°C container. After 12 h, cells were washed with cold PBS, and then the RNA were removed by the incubation of the cells with 2 mg/mL RNase A at 37°C for 30 min. To stain cellular DNA, cells were incubated with PI (50 µL/mL solution) at room temperature for 1 h in the dark. DNA content was recorded by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and then analyzed by CellQuest software v3.3 (Becton Dickinson, Franklin Lakes, NJ, USA).
The sequences of gene primers
| Gene | Primer | Sequence (5'—3') |
|---|---|---|
| LXRα | Forward | AGA ACA GAT CCG CCT GAA GA |
| Reverse | CCT CTC GAT CAT GCC CAG TT | |
| CCND1 | Forward | AAC TAC CTG GAC CGC TTC CT |
| Reverse | CCA CTT GAG CTT GTT CAC CA | |
| β-catenin | Forward | GCC AAG TGG GTG GTA TAG AGG |
| Reverse | GGG ATG GTG GGT GTA AGA GC | |
| CD44 | Forward | TGA CAA CGC AGC AGA GTA ATT C |
| Reverse | TTC CAC CTG TGA CAT CAT TCC T | |
| GAPDH | Forward | TGG GTG TGA ACC ATG AGA AGT |
| Reverse | TGA GTC CTT CCA CGA TAC CAA |
Western blot analysis
Whole-cell extracts or fresh tumor tissue homogenates of mice were prepared with 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 1% sodium dodecyl sulfate (SDS) supplemented with a protease inhibitor (Sigma-Aldrich). Fifty micrograms of proteins was used in each well. Protein was electrophoretically separated on a 10% SDS PAGE gel and then transferred to a PVDF membrane. Blots were incubated in appropriate primary antibody [anti- LXRα (ab41902; Abcam; 1:1000), anti-beta-catenin antibody (ab32572; Abcam; 1:5,000), anti-CD44 antibody (ab51037; Abcam; 1:5,000), anti-Cyclin D1 antibody (ab14175; Abcam; 1:10,000), GAPDH (10949-1-AP; Proteintech; 1:10,000), and β-tubulin (10094-1-AP; Proteintech; 1:1,000)]. They were developed using enhanced chemiluminescence (GE Biosciences). Using NIH Image J software, optical densities of the bands were analyzed. For figure panels, contrasts were adjusted linearly for ease of viewing the bands.
In vivo tumorigenesis
Four-week-old male nude athymic BALB/c nu/nu mice were used to examine tumorigenicity. To evaluate the role of LXRα in tumor formation, two groups of GC cells AGS (“GFP” and “LXRα”) were propagated and inoculated subcutaneously into the flanks of nude mice (1×107 cells in 0.1 mL volume). Tumor size was measured every five days. Tumor volumes were determined according to the following formula: A×B2 /2, where A is the largest diameter and B is the diameter perpendicular to A. Tumors were measured periodically, and mice were sacrificed before tumors reached 1 cm3 or ulcerated. After 24 days, the mice were killed and tumor mass was weighed. An individual animal has one subcutaneous tumor. The maximum diameter of a single subcutaneous tumor was 2cm.The tumor tissues of the mice were homogenized. Total RNA and protein were extracted from the homogenate of tissue. The relative expression of RNA and protein of LXRα, β-catenin, CD44, and Cyclin D1 in tissues was measured by qRT-PCR and Western blot analysis, respectively. The experiments were performed using five mice per group. All animal experiments were performed in accordance with recommendations in the National Research Council Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Animal Care and Use Medical Ethics Committee of Xiangya Hospital of Central South University.
Statistical analysis
All statistical analyses were performed with SPSS software package 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Because of skewed distribution, the data of the sumIOD/area were presented as median (minimum, maximum). The other quantitative data were presented as mean±SD. For tissue array immunohistochemistry analysis, Mann-Whitney U test was used to assay the association between LXRα expression and clinicopathological variables in GC tissres and tumor adjacent mucosas. For qRT-PCR and Western blot analysis, Student's t-test was used to assay differential expression in different cell groups. P-values less than 0.05 were considered significant.
Results
Expression of LXRα in GC and tumor adjacent mucosa
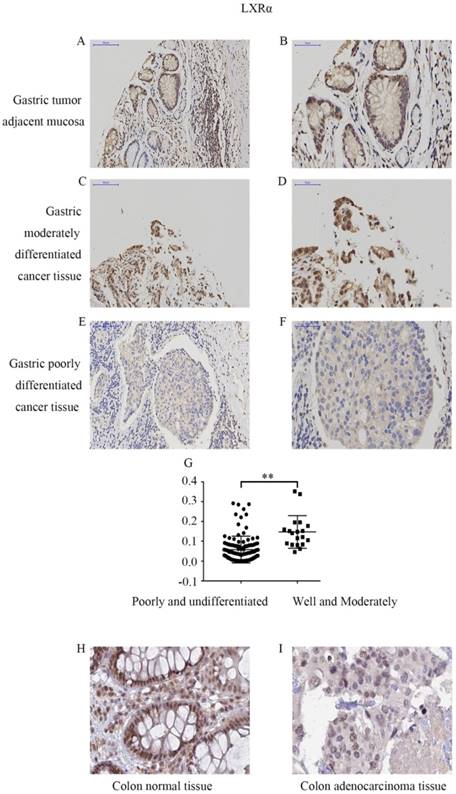
We first determined the LXRα expression levels in cancer tissues and tumor adjacent mucosas of GC patients by immunohistochemistry analysis (Figure 1A-F). In 124 cases of gastric carcinoma tissue and its adjacent mucosa, the relative expression of LXRα was expressed by the average integral optical density (sumIOD/area), with a median (minimum, maximum). The relative expression of LXRα in the gastric tumor adjacent mucosa group was 0.1483 (0.0314, 0.2627), and that in the GC group was 0.0478 (0.0003, 0.3523). Mann-Whitney U test indicated that the expression of LXRα was significantly lower in GC tissue than in tumor adjacent mucosas (P<0.001) (Table 2).
LXRα expression level is associated with differentiation of GC
The LXRα expression levels in different differentiation of GC tissue were determined by immunohistochemistry analysis (Figure 1C-G). The relative expression of LXRα was expressed by the average integral optical density (sumIOD/area), with a median (minimum, maximum). The relative expression of LXRα in the well- and moderately differentiated group was 0.1210 (0.0464, 0.3523), and that in the poorly and undifferentiated group was 0.0393 (0.0003, 0.2873). Mann-Whitney U test indicated that the expression of LXRα was significantly lower in poorly and undifferentiated GC tissue and comparatively higher in the well- and moderately differentiated GC tissues (P<0.001). Clinicopathological analysis revealed that the expression of LXRα protein was significantly negatively correlated with the degree of tumor differentiation and was not related to the patients' age, gender, lymph node metastasis, invasion depth, and TNM stage of cancer (P>0.05) (Table 3).
The sumIOD/area of LXRα in gastric cancer and adjacent normal gastric mucosas
| Clinicopathological Factors | n | LXRα (sumIOD/area) | P-value |
|---|---|---|---|
| Adjacent normal mucosas | 124 | 0.1483(0.0314-0.2627) | 0.000 |
| Cancer tissue | 124 | 0.0478(0.0003-0.3523) |
Correlation between LXRα, differentiation of gastric cancer cells and clinicopathologic features
| Clinicopathological Factors | n | LXRα (sumIOD/area) | P-value |
|---|---|---|---|
| Age(years) | |||
| ≤65 | 94 | 0.0462(0.0003-0.3523) | 0.646 |
| >65 | 30 | 0.6542(0.0005-0.3383) | |
| Gender | |||
| Male | 93 | 0.0492(0.0003-0.3523) | 0.363 |
| Female | 31 | 0.0462(0.0006-0.3383) | |
| Histologic type | |||
| Well and Moderately | 19 | 0.1210(0.0464-0.3523) | 0.000 |
| Poorly and undifferentiated | 105 | 0.0393(0.0003-0.2873) | |
| pTNM stage | 0.448 | ||
| I-II | 39 | 0.0594(0.0006-0.3383) | |
| III-IV | 85 | 0.0393(0.0003-0.3523) | |
| Lymph node metastasis | 0.216 | ||
| Positive | 86 | 0.0567(0.0005-0.3523) | |
| Negative | 38 | 0.0462(0.0003-0.2623) | |
| Invasion depth | 0.231 | ||
| T1-T2 | 43 | 0.0594(0.0006-0.3383) | |
| T3-T4 | 81 | 0.0393(0.0003-0.3523) |
Efficiency of LXRα inhibition and overexpression in GC cells transfected with lentivirus vector
After 72 h of transfection, we first compared the number of cells observed under the fluorescence microscope to the number of cells observed under normal light. In all transfection groups, the number of cells with green fluorescence was 80% greater than the number of cells seen under normal light sources. Then, we used qRT-PCR to detect the inhibition rate of the “LXRα Sh” vector and the overexpression rate of “LXRα” vector. The results showed that the average rates of inhibition efficiency were 85.6% in the “LXRα Sh” vector of AGS cells, and 77.6% in the “LXRα Sh” vector of SGC7901 cells, compared with the “blank” vector. Similarly, the average rates of inhibition efficiency of protein were 70.8% in AGS cells, and 77.1% in SGC7901 cells. The average rate of overexpression efficiency was 26.5 times higher in the “LXRα” vector of AGS cells than in the “blank” vector, and 33.2 times higher in the “LXRα” vector of SGC7901 cells than in the “blank” vector (Figure 2A-E). The above results show that all the transfection cell groups met the experimental requirements.
Expression levels of LXRα in different differentiation of gastric cancer and adjacent normal gastric mucosas by immunohistochemistry. (A) Expression of LXRα in well differentiated gastric cancer tissues,the image is at a magnification of ×200. (B)Expression of LXRα in well differentiated gastric cancer tissues,the image is at a magnification of ×400. (C) Expression of LXRα in poorly differentiated gastric cancer tissues, the image is at a magnification of ×200. (D) Expression of LXRα in poorly differentiated gastric cancer tissues, the image is at a magnification of ×400. (E) Expression of LXRα in adjacent normal gastric mucosas, the image is at a magnification of ×200. (F) Expression of LXRα in adjacent normal gastric mucosas, the image is at a magnification of ×400. (G) The expression of LXRα was significantly lower in poorly and undifferentiated GC tissue and comparatively higher in the well- and moderately differentiated GC tissues (P<0.01).(H) Expression of LXRα in colon normal tissue, the image is at a magnification of ×400, downloaded from THE HUMAN PROTEIN ATLAS website. (I) Expression of LXRα in colon adenocarcinoma tissue, the image is at a magnification of ×400, downloaded from THE HUMAN PROTEIN ATLAS website.
Relative expression level of LXRα mRNA in SGC7901 and AGS cells after LXRα inhibition and overexpression by quantitative real-time PCR. (A)In SGC7901 cell lines, the relative expression of LXRα of “LXRα Sh” group is 0.223667± 0.056048 vs “Sh Ctrl” group is 1. (B) In AGS cell lines, the relative expression of LXRα of 'LXRα Sh' group is 0.143667± 0.050013 vs “Sh Ctrl”group is 1. (C) In SGC7901 cell lines, the relative expression of LXRα of “LXRα” group is 33.1855± 3.01001 vs “GFP” group is 1. (D) In AGS cell lines, the relative expression of LXRα of “LXRα” group is 26.5073± 3.01638 and “GFP” group is 1. (E) Relative expression level of LXRα protein in SGC7901 and AGS cells after LXRα inhibition by Western blot analysis. In SGC7901 cell lines, the relative expression of LXRα protein of “LXRα Sh” group is 0.2973±0.0214 vs “Sh Ctrl” group is 1.312±0.1557,and in AGS cell lines, the relative expression of LXRα of 'LXRα Sh' group is 0.2913±0.03891 vs “Sh Ctrl”group is 0.9905± 0.1556. (F-K) Overexpression of LXRα increased the relative ratio of cell populations accumulating at the G0/G1 phase. The GC cells AGS with LXRα was knocked down or overexpressed were analyzed by flow-cytometry. The values represent the mean ± SD (n≥3). *P < 0.05, **P < 0.01.
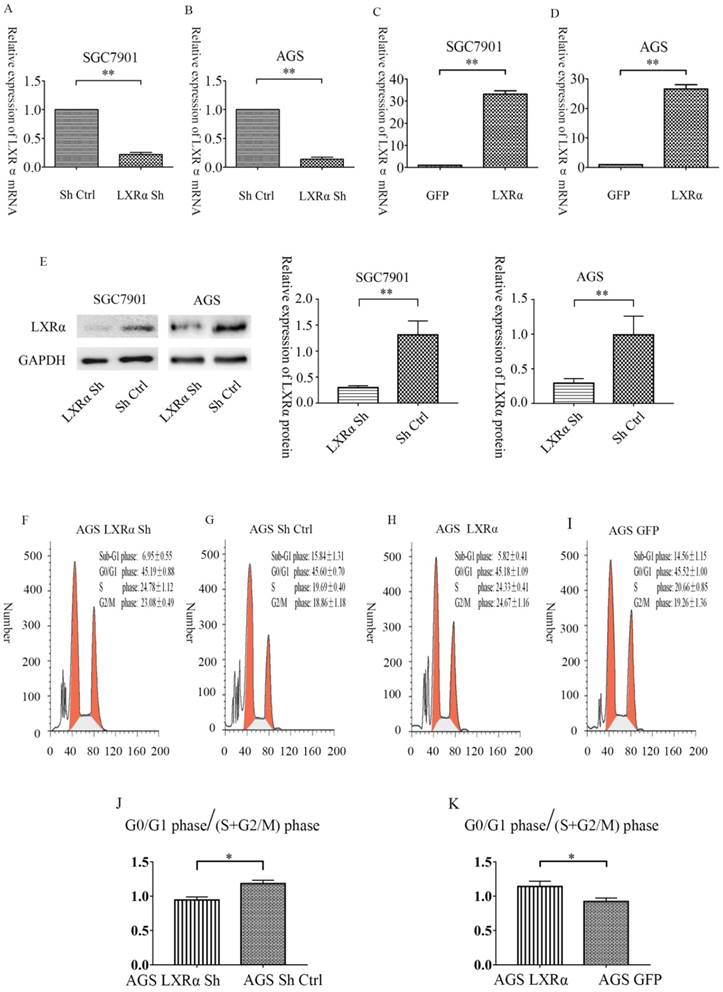
Overexpression of LXRα leads to cell cycle arrest at the G0/G1 phase in GC cells AGS
We further analyzed the effect of LXRα on cell cycle progression; the ratio of cell populations at each cell cycle phase was analyzed. The “LXRα Sh” cell population at the G0/G1 phase was 45.19±0.88%, S phase was 24.78±1.12% and G2/M phase was 23.08± 0.49%; the “Sh Ctrl” cell population at the G0/G1 phase was 45.60±0.70%, S phase was 19.69±0.40% and G2/M phase was 18.86±1.18%; the “LXRα” cell population at the G0/G1 phase was 45.52±1.00%, S phase was 20.66±0.85% and G2/M phase was 19.26±1.36%; the “GFP” cell population at the G0/G1 phase was 45.18±1.09%, S phase was 24.33±0.41% and G2/M phase was 24.67±1.16% (Figure 2F-K). The proportion of G0/G1 phase /(S+G2/M) phase increased after LXR overexpression in AGS cells. The result indicated that overexpression of LXRα leads to cell cycle arrest at the G0/G1 phase in GC cells AGS.
LXRα promotes the differentiation of GC cells and regulates the Wnt/β-catenin pathway in GC cells
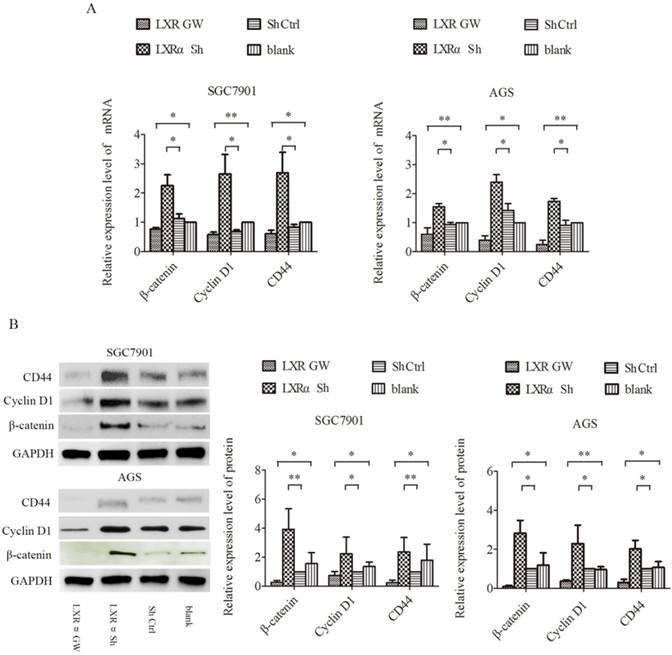
Wnt/β-catenin signaling is well known to play an important role in the process of cell differentiation. In this signaling, β-catenin is a key factor. CD44 and Cyclin D1, downstream targets of Wnt/β-catenin signaling, are specific markers for cell differentiation. We treated AGS and SGC7901 cells with GW3965 for 48 h and LXRα-short hairpin RNA (shRNA) vector, respectively, and then determined their expression levels by qRT-PCR and Western blot analysis. Compared with the “LXRα Sh” and “blank” groups, the mRNA and protein levels of CD44, Cyclin D1 and β-catenin in “Sh Ctrl” and “LXR GW” cells decreased significantly, respectively (Figure 3A and B). The results suggest that LXRα is a driving force for differentiation in GC cells and may be involved in inhibiting the Wnt/β-catenin pathway in GC cells.
Regulation of CD44,Cyclin D1 and β-catenin by LXRα in gastric cancer cell lines. (A) Relative expression of CD44,Cyclin D1 and β-catenin mRNA in “LXR GW”, “LXRα Sh”, “Sh Ctrl” and “blank” groups cells were evaluated by quantitative real-time PCR. GAPDH was used as an internal control. (B)Relative expression of CD44,Cyclin D1 and β-catenin proteins in “LXR GW”, “LXRα Sh”, “Sh Ctrl” and “blank” groups cells were assessed by Western blot analysis. GAPDH was used as an internal control. The values represent the mean ± SD(n≥3). *P < 0.05, **P < 0.01.
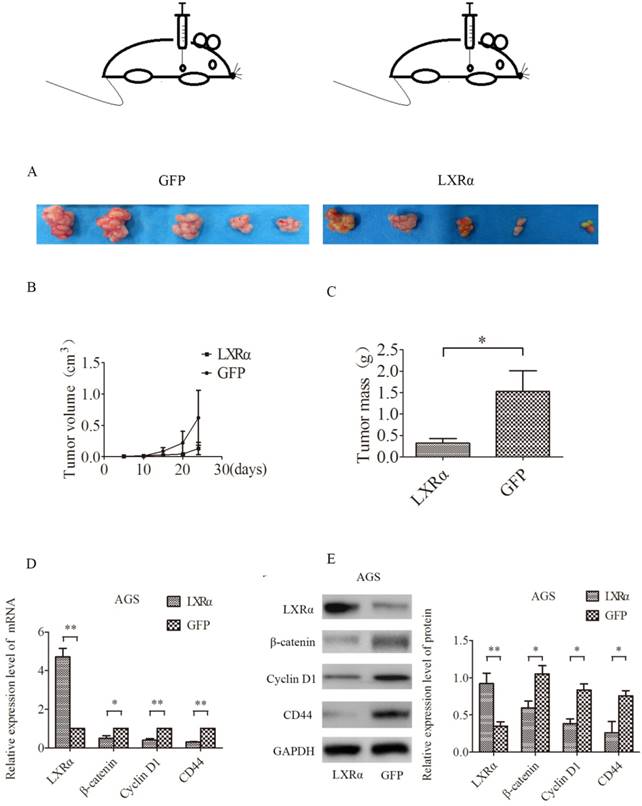
We next tested whether LXRα could have a role in tumorigenesis in vivo by using a nude mouse xenograft model (Figure 4A). Nude mice transplanted with AGS cells developed solid tumors in 24 days. Tumor volume and weight were decreased when LXRα was stably overexpressed in AGS cells (“LXRα”) as compared with the control group (“GFP”). However, there was no statistically significant difference in tumor volume (Figure 4B), whereas the difference in tumor quality was statistically significant (Figure 4C). The relative expression of mRNA and protein of LXRα, β-catenin, CD44 and Cyclin D1 in tumor tissues was measured by qRT-PCR and Western blot analysis, respectively. We found that LXRα was upregulated in (“LXRα”) tumors, whereas β-catenin, CD44, and Cyclin D1 were downregulated compared with the control group (“GFP”) (Figures 4D, E).
Nude mouse xenograft experiment. (A) AGS cells (“LXRα” and “GFP”) were injected subcutaneously into nude mice. (B) Tumor volume was measured every 5 days. (C) After 24 days, the mice were killed and tumors in individual mice were weighed. Each group had five mice. LXRα promotes the differentiation of GC cells in vivo. (D) Relative expression level of β-catenin, CD44 and Cyclin D1 mRNA in different groups of tumor of mice were evaluated by quantitative real-time PCR. GAPDH was used as an internal control. (E)Relative expression level of β-catenin, CD44 and Cyclin D1proteins in different groups of tumor of mice were assessed by Western blot analysis. GAPDH was used as an internal control. The values represent the mean ± SD(n≥3). *P < 0.05, **P < 0.01.
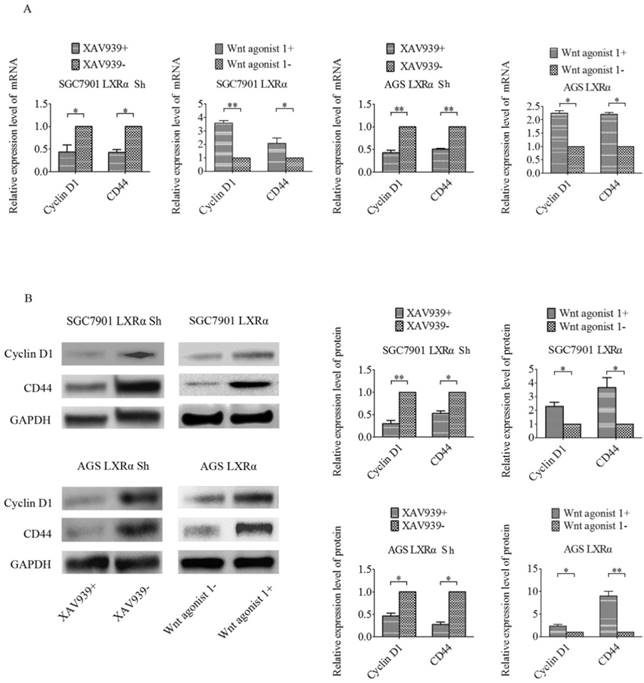
In previous experiments, we found that β-catenin showed higher expression in GC tissues with respect to tumor adjacent mucosa and that nuclear expression of β-catenin was related to the differentiation of tumor cells. To further explore whether LXRα promotes differentiation through the Wnt/β-catenin pathway, we used either the Wnt/β-catenin signaling-specific inhibitor XAV939 or Wnt/β-catenin activator Wnt agonist 1 to control the Wnt/β-catenin signaling in GC cell lines (SGC7901 and AGS). If LXRα can inhibit Wnt/β-catenin to regulate GC cell differentiation, the inhibition is supposed to be Wnt/β-catenin agonist. In the same way, when LXRα is inhibited, the activation of the Wnt/β-catenin pathway by its inhibitor should be blocked. According to this idea, we added XAV939 to the “LXRα Sh” cells, and the “LXRα Sh” cells without XAV939 were treated as the control group. Wnt agonist 1 was added to the “LXRα” cells, and the “LXRα” cells without Wnt agonist 1 were treated as the control group. We found that the expression of CD44 and Cyclin D1 was lower in XAV939 positive “LXRα Sh” cells than in XAV939-negative cells and higher in Wnt agonist 1 positive “LXRα” cells than in Wnt agonist 1-negative cells (Figure 5). The above results suggest that LXRα promotes differentiation of GC through the Wnt/β-catenin pathway.
LXRα promotes differentiation of GC through the Wnt/β-catenin pathway. (A) The relative mRNA levels of differentiation markers (CD44, Cyclin D1) in different groups of “LXRα” and “LXRα Sh” cells(with different treatment: either 10 μmol/L XAV939 or 10 μmol/L Wnt agonist 1 for 24 h) were assayed by quantitative real-time PCR. GAPDH was used as an internal control. (B) The relative protein levels of CD44 and Cyclin D1 in different groups of “LXRα” and “LXRα Sh” cells (with different treatment: either 10 μmol/L XAV939 or 10 μmol/L Wnt agonist 1 for 24 h) were assessed by Western blot analysis. GAPDH was used as an internal control. The values represent the mean ± SD (n≥3). *P < 0.05, **P < 0.01.
Discussion
In this study, the expression of LXRα was determined using immunohistochemistry analysis. We found a significantly lower LXRα expression in poorly and undifferentiated GC tissue and a comparatively higher expression in well- and moderately differentiated GC tissues. LXRα expression levels were downregulated and negatively correlated with differentiation markers in human GC specimens. Inhibition of LXRα resulted in the upregulation of the differentiation molecular markers CD44 and Cyclin D1, which are well served as the Wnt/β-catenin targets, whereas activating LXRα using GW3965 showed the opposite effect. Furthermore, inhibition of the Wnt/β-catenin pathway by XAV939 negated the effect of LXRα inhibition, whereas activation of the Wnt/β-catenin pathway by Wnt agonist 1 impaired the effect of LXRα overexpression on differentiation of GC cells.
LXRα is a subtype of LXRs. It inhibits the proliferation of various cancers, such as colorectal cancer [12], lung cancer [13], hepatocellular carcinoma [14], oral cancer [15], breast cancer [16], brain cancer [17], and thyroid cancer [18]. However, there are few studies on the expression of LXRα between cancer tissue and normal tissue. A previous study reported a 93% positive rate of LXRα mRNA expression in 15 normal breast tissue compared with 73% in 15 breast cancer tissues [19]. Although LXRα mRNA was not quantified in normal breast tissues and breast cancer tissues in this study, the expression of LXRα mRNA in breast cancer showed a decreasing tendency compared with normal breast tissue. Another study on oral squamous cell carcinoma reported that LXRα protein is overexpressed in 12 oral cancer tissues compared with normal tissue [15].
We found that the expression of LXRα protein in 124 cases of GC was lower than that of tumor adjacent mucosa. This conclusion is consistent with the findings for breast cancer, and contrary to those for oral cancer. Interestingly, in our cases, the expression of LXRα protein in some well-differentiated GC tissue was higher than that in tumor adjacent mucosa. This may explain why our findings contradict those of oral cancer research. After all, in oral cancer research, there is no analysis of factors, such as differentiation of cells, and there are fewer cases. This also suggests that there may be other unknown factors involved in regulating the expression of LXRα in GC. The higher expression of LXRα in tumor adjacent mucosa than in GC tissue suggests that LXRα may have an inhibitory effect on GC. Considering that LXRα is involved in cholesterol transport and glucose metabolism, we suspect that it may be possible to inhibit the tumor by affecting the metabolism of energy substances.
The results of subsequent analysis show that the expression of LXRα protein is associated with differentiation of GC tissue. Different degrees of GC cell differentiation show different heterogeneity. Although the differentiation of GC cells is not directly involved in TNM staging, the differentiation of tumor cells plays an important role in the treatment of GC. Some researchers believe that patients with well-differentiated GC are more likely to use different chemotherapy regimens for preoperative chemotherapy than those with poor differentiation [20]. Surgeons, however, have differing views on whether early GC is suitable for endoscopic resection in poorly differentiated GC [21]. Patients with peritoneal recurrence, advanced T or N stage, and low differentiation are more common than those without peritoneal recurrence; moreover, patients with peritoneal recurrence show worse overall survival compared with those without peritoneal recurrence [22]. In addition, studies suggest that the presence of tumor stem cells is one of the causes of tumor drug resistance [10]. However, drug resistance in GC is an important reason for the unsatisfactory effect of chemotherapy. Therefore, the relationship of LXRα with GC differentiation will help determine its significance as a new target for GC treatment. However, the mechanism of LXRα affecting the differentiation of GC cells is unknown.
The Wnt/β-catenin signaling pathway is one of the important pathways for regulating cell differentiation [23]. This signaling pathway has abnormal activation in various tumor tissues [24], and its key factor β-catenin protein is higher in GC tissues than in normal gastric mucosal tissue [25]. CD44 and Cyclin D1 are the target genes of the Wnt/β-catenin signaling pathway [26, 27], which is involved in the regulation of cell differentiation [26, 28]. A high expression of CD44 and Cyclin D1 is also found in some tumor cells or tissues including GC [29-32].
We found that the expression of β-catenin, CD44 and Cyclin D1 in LXRα-activated GC cells, both at the mRNA and protein levels, is significantly lower than that of negative control GC cells in vitro. This indicates that LXRα can inhibit the Wnt/β-catenin signaling pathway and further inhibit CD44 and Cyclin D1 in GC cells. It should be noted that, as an agonist of LXRs, GW3965 can activate LXRα and LXRβ simultaneously. Therefore, in our experiment, the inhibitory effect of LXRα GW cells on the Wnt pathway and differentiation in GC may not only be generated by LXRα. Aiming at this problem, in the animal experiment, we chose the LXRα overexpression cells. The in vivo results agree with in vitro results. To some extent, it can eliminate LXRβ interference on the experimental results.In addition, although previous studies did not find a relationship between LXRβ and differentiation of GC cells, there may be collaboration between LXRα and LXRβ. Further studies are needed to confirm this. After LXRα inhibition, the Wnt/β-catenin signaling pathway is activated, and CD44 and Cyclin D1 is overexpressed, even higher than the level of parental GC cells. Therefore, Wnt/β-catenin signaling is probably one of the ways wherein LXRα regulates the differentiation of GC cells.
We further demonstrate that expression changes in differentiation markers (CD44 and Cyclin D1) induced by LXRα overexpression or inhibition, can be reversed by XAV939 or Wnt agonist 1, respectively. The results reveal that LXRα potentially inhibits differentiation via Wnt/β-catenin signaling in GC cells. It is known that Wnt/β-catenin signaling has multiple functions in cancer progression. β-catenin and Cyclin D1 are proven to be associated with not only differentiation but also EMT in cancer [25, 33]. Whether LXRα can inhibit invasion or the EMT ability of GC cells is still unknown. Further experiments are needed to confirm this.
Conclusion
In summary, we found that LXRα functions as a differentiation promoter by inhibiting the Wnt/ β-catenin pathway. LXRα is comparatively reduced in several cancers, including GC. Therefore, LXRα is an excellent candidate for the development of a targeted GC therapy that will block metastasis and induce cytotoxicity in tumor while sparing normal cells. However, the mechanism of LXRα in regulating WNT signaling is not clear. Further study of LXRα in phosphorylated and methylated regulation is necessary. In addition, many studies currently use non-specific LXRs agonists such as GW3965 to treat tumors. Specific LXRα agonist may be more helpful for the treatment of cancer.
Abbreviations
DAB: diaminobenzidine; EMT: epithelial-mesenchymal transition; GC: gastric cancer; LXRα: liver X receptor α; LXRβ: liver X receptor β; LXRs: liver X receptors; PCR: polymerase chain reaction; PBS: phosphate buffered solution; PI: propidium iodide; qRT-PCR: quantitative real-time polymerase chain reaction; SDS: sodium dodecyl sulfate; TNM: tumor-node-metastasis.
Acknowledgements
This work was supported by Nature Scientific Foundation of China (81573012). We thank Professor Xueqing Feng (Xiangya Hospital of Central South University) for her technical help.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jacques Ferlay, Isabelle Soerjomataram, Rajesh Dikshit. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015Mar1;136(5):E359-86
2. Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014Aug;40(3):250-60
3. Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World Journal of Gastrointestinal Oncology. 2012Jul15;4(7):156-69
4. Holohan C, Van Schaeybroeck S, Longley DB. et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013Oct;13(10):714-26
5. Andrea Z Lai, Sean Cory, Hong Zhao. et al. Dynamic Reprogramming of Signaling Upon Met Inhibition Reveals a Mechanism of Drug Resistance in Gastric Cancer. Sci Signal. 2014Apr22;7(322):ra38
6. NicoMitro PuiyingA.Mak, LeoVargas et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007Jan11;445(7124):219-23
7. Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Bio. 2012Mar14;13(4):213-24
8. Lin C, Gustafsson J. Targeting liver X receptors in cancer therapeutics. Nat Rev Cancer. 2015Apr;15(4):216-24
9. Li L, Li W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Therapeut. 2015Jun;150:33-46
10. Catherine A. O'Brien, Kornelia Polyak,et al. Stanger.Looking Back: Cancer Stem Cells. Cell Stem Cell. 2017Jun1;20(6):754
11. Ning Tan, Qinyi Liu, Xiaojia Liu. et al. Low expression of B-cell-associated protein 31 in human primary hepatocellular carcinoma correlates with poor prognosis. Histopathology. 2016Jan;68(2):221-9
12. Lo Sasso G, Bovenga F, Murzilli S. et al. Liver X Receptors Inhibit Proliferation of Human Colorectal Cancer Cells and Growth of Intestinal Tumors in Mice. Gastroenterology. 2013Jun;144(7):1497-507
13. Yu-bing Dai, Yi-fei Miao, Wan-fu Wu. et al. Ablation of Liver X receptors α and β leads to spontaneous peripheral squamous cell lung cancer in mice. Proceedings of the National Academy of Sciences. 2016Jul5;113(27):7614-9
14. Na TY, Shin YK, Roh KJ. et al. Liver X Receptor Mediates Hepatitis B Virus X Protein-Induced Lipogenesis in Hepatitis B Virus-Associated Hepatocellular Carcinoma. Hepatology. 2009Apr;49(4):1122-31
15. Tetsuharu Kaneko, Chihiro Kanno, Naoki Ichikawa-Tomikawa. et al. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget. 2015Oct20;6(32):33345-57
16. Nelson ER, Wardell SE, Jasper JS. et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science. 2013Nov29;342(6162):1094-8
17. Villa GR, Hulce JJ, Zanca C. et al. An LXR-Cholesterol Axis Creates a Metabolic Co-Dependency for Brain Cancers. Cancer Cell. 2016Nov14;30(5):683-693
18. Mond M, Alexiadis M, Eriksson N. et al. Nuclear receptor expression in human differentiated thyroid tumors. Thyroid. 2014Jun;24(6):1000-11
19. Vigushin DM, Dong Y, Inman L. et al. The Nuclear Oxysterol Receptor LXRα Is Expressed in the Normal Human Breast and in Breast Cancer. Med Oncol. 2004;21(2):123-31
20. Sun Li-Bo, Zhao Guo-Jie, Ding Da-Yong, et al.Comparison between better, poorly differentiated locally advanced gastric cancer in preoperative chemotherapy. a retrospective, comparative study at a single tertiary care institute. Would Joumal Of Surgical Oncology. 2014Sep8;12:280
21. Kim J. Important considerations when contemplating endoscopic resection of undifferentiated-type early gastric cancer. World J Gastroentero. 2016Jan21;22(3):1172-8
22. Fan Wu, Chunmei Shi, Riping Wu. et al. Peritoneal recurrence in gastric cancer following curative resection can be predicted by postoperative but not preoperative biomarkers: a single-institution study of 320 cases. Oncotarget. 2017May8;8(44):78120-78132
23. Moon RT, Kohn AD, Ferrari GVD. et al. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004Sep;5(9):691-701
24. Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014Jul;13(7):513-32
25. J Huang, D Xiao, G Li. et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/b-catenin pathway in gastric cancer cells. Oncogene. 2014May;22(33):2737-2747
26. Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011Apr;11(4):254-67
27. Katoh M, Katoh M. WNT Signaling Pathway and Stem Cell Signaling Network. Clin Cancer Res. 2007Jul15;13(14):4042-5
28. Fu M, Wang C, Li Z. et al. Minireview: Cyclin D1: Normal and Abnormal Functions. Endocrinology. 2004Dec;145(12):5439-47
29. Casimiro MC, Velasco-Velázquez M, Aguirre-Alvarado C. et al. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Inv Drug. 2014Mar;23(3):295-304
30. Yoku Hayakawa, Yoshihiro Hirata, Hayato Nakagawa. et al. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proceedings of the National Academy of Sciences. 2011Jan11;108(2):780-785
31. Naor D, Wallach-Dayan SB, Zahalka MA. et al. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008Aug;18(4):260-7
32. Musgrove EA, Caldon CE, Barraclough J. et al. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011Jul7;11(8):558-72
33. Hou F, Yuan W, Huang J. et al. Overexpression of EphA2 correlates with epithelial-mesenchymal transition-related proteins in gastric cancer and their prognostic importance for postoperative patients. Med Oncol. 2012Dec;29(4):2691-700
Author contact
![]() Corresponding author: Zhikang Chen, Department of Colorectal and Anus Surgery, Xiangya Hospital of Central South University, Xiangya road No.87, Kaifu district, Changsha, Hunan province, P.R.China. Tel:+86 139 7311 4538. Email: 430445edu.cn Postcode:410008
Corresponding author: Zhikang Chen, Department of Colorectal and Anus Surgery, Xiangya Hospital of Central South University, Xiangya road No.87, Kaifu district, Changsha, Hunan province, P.R.China. Tel:+86 139 7311 4538. Email: 430445edu.cn Postcode:410008

 Global reach, higher impact
Global reach, higher impact