Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(5):1154-1161. doi:10.7150/jca.29052 This issue Cite
Research Paper
PD-L1 expression patterns in tumour cells and their association with CD8+ tumour infiltrating lymphocytes in clear cell renal cell carcinoma
1. Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China
2. Department of Biotherapy, Sun Yat-Sen University Cancer Center, Guangzhou 510060, People's Republic of China
3. Department of Pathology, Sun Yat-Sen University Cancer Center, Guangzhou 510060, People's Republic of China
Received 2018-8-7; Accepted 2019-1-4; Published 2019-1-29
Abstract
Purpose: To evaluate the tumour cell PD-L1 (TC-PD-L1) expression patterns in the local microenvironment of clear cell renal cell carcinoma (ccRCC).
Materials and Methods: 30 fresh primary ccRCC tissues were used to detect the association between TC-PD-L1 and CD8+TILs at mRNA level. The in vitro incubation experiment was used to confirm the association between extrinsic TC-PD-L1 expression and IFNγ. A cohort of 135 ccRCC patients treated between January 2009 and August 2013 was included for survival analysis.
Results: Our results confirmed that ccRCC cell lines were capable of expressing PD-L1. The incubation experiment in vitro demonstrated the positive correlation of TC-PD-L1 expression with interferon-gamma (IFNγ). Additionally, survival analysis was investigated in 135 ccRCC patients and found no independent correlation of TC-PD-L1 expression in multivariate analysis, whereas more distinct prognostic differences were detected when TC-PD-L1-positive ccRCC were sub-classified as with or without CD8+ T cell infiltration.
Conclusion: The intrinsic and extrinsic expression patterns are both detected in ccRCC. High positive rate of TC-PD-L1 correlated closely to the strong infiltration of CD8+ TILs. TC-PD-L1-positive ccRCC patients with abundant CD8+ TILs infiltration confer the high risk of death and disease relapse.
Keywords: PD-L1, local microenvironment, intrinsic expression, extrinsic expression, clear cell renal cell carcinoma
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common histological subtype of renal cell carcinoma (RCC), accounting for most of RCC-related deaths [1]. Although surgery can be curative at the early-stage, deaths due to kidney cancer have not declined primarily because of recurrence and metastasis [2]. Traditional treatment modalities, such as chemotherapy and radiotherapy, do not significantly improve the response rates or survival in metastatic disease [3]. For metastatic cases, cytoreductive nephrectomy with immunotherapy has shown significant increase in long-term survival [4]. In the 20th century, Fyfe et al. demonstrated a 15% objective response rate in RCC patients who treated with high-dose IL-2, this lead the US Food and Drug Administration (FDA) to approve the use of IL-2[5]. However, the treatment-related mortality rate of up to 4% [5] ratifies the urge for newer, novel and improved therapeutics for ccRCC patients.
Presently, immune evasion was added to the list of hallmarks required for tumour formation and metastasis [6]. Several specific immune features in the microenvironment suggest that an ongoing tumour-directed immune response is limited by the interaction between PD-1 and PD-L1 [7]. Currently, several unique clinical trials have shown that the responses to PD-1/PD-L1 checkpoint inhibitions are more frequently observed among ccRCC patients with positively expressed tumour cell PD-L1 (TC-PD-L1) [8-12]. However, these trails had varying percentage of positivity, ranging from 15% to 66%. In addition, Callea M et al. detected the heterogeneity of TC-PD-L1 expression between primary and metastatic sites [11]. However, ccRCC is characterized by intratumoural heterogeneity [13]. Therefore, the expression patterns of TC-PD-L1 in the local microenvironment of ccRCC cannot be ignored.
As such, in this study we systematically estimated the expression patterns of TC-PD-L1 in the local tumour microenvironment of ccRCC and observed a positive association between TC-PD-L1 and CD8+ TILs. The intrinsic and extrinsic expression patterns are both detected in ccRCC. Moreover, CD8+ TILs were associated with significantly poor survival in TC-PD-L1-positive ccRCC patients, which thereby underscore an urge for improved treatment options in these patients.
Materials and Methods
Cell lines
We obtained five cell lines (ACHN, Caki-1, A498, 769-P, and 786-O) and immortalized proximal tubular cell line HK2 from the American Type Culture Collection. The cells were cultured in the RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Life Technologies) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere containing 5% CO2. For the IFNγ stimulation experiments, cells were seeded at 100,000 cells/well in six-well plates then incubated in the presence (20 or 40 ng/ml) or in the absence of IFNγ (Proteintech).
Tissue specimens and patient information
Retrospective assessment of a cohort of 135 primary ccRCC patients treated with radical or conservative surgery with or without a tyrosine kinase inhibitor (TKI)-based targeted therapy (sunitinib/sorafenib), between January 2009 and August 2013 at the Urology Department at Sun Yat-sen University Cancer Center (Guangzhou, China) was performed. Tumour grading and staging were classified according to the 7th AJCC Staging System edition. Fresh primary tissue samples were retrieved from the Pathology Department at the Medical College in Nanchang University (Nanchang, China). Overall survival (OS) was calculated from the date of diagnosis to the date of death. Disease-free survival (DFS) was calculated from the date of diagnosis to the date of first recurrence or distant metastasis (irrespective of the site) till the date of death (irrespective of the cause) or last follow-up visit.
Immunohistochemistry and evaluation
Briefly, 5-μm-thick paraffin sections were deparaffinized with xylene and rehydrated in the water. The sections were submerged into EDTA (1 mmol/L, pH = 9.0) and microwaved for antigenic retrieval. 3% hydrogen peroxide was used to quench endogenous peroxidase activity. The addition of goat serum was used to block nonspecific binding. The slides were selectively incubated with either a rabbit anti-human PD-L1 monoclonal antibody (mAb) (1:100; #13684, Cell Signaling Technology) or a rabbit anti-CD8 polyclonal antibody (1:100; Golden Bridge Biotech, Beijing, China) overnight at 4°C, washed 5 times with PBS and incubated with HRP-conjugated secondary antibodies (EnvisionTM Detection Kit, GK500705, Gene Tech) for 30 min. 3,3'-Diaminobenzidine (DAB) was used to develop positive identification signals, after which they were counterstained with hematoxylin. Lastly, the sections were dehydrated and evaluated.
Scoring evaluations were performed by two expert pathologists (Min Li and Mu-Yan Cai) blinded from the patients' outcomes. The percentage of PD-L1-positive cells occupying the tumour was scored as follows: 0, < 5% of the area; or 1, ≥ 5% of cells of the area. Cases demonstrating ≥ 5% tumour cell expression were considered positive to maintain consistency with previous literatures [14-16]. Meanwhile, we counted the number of CD8+ TILs in five random fields at 200× magnification and for which the average value was obtained [17]. The median of CD8+ TILs counts was identified as the optimal cut-off value.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from tumour samples using the TRIzol reagent (Sigma-Aldrich). Complementary DNA (cDNA) was synthesized using a GoScriptTM Reverse Transcription System (Promega), and qPCR was conducted using GoScript qPCR Master Mix (Promega). The forward and reverse primer sequences for PD-L1 were 5'-CCTACTGGCATTTGCTGAACGCAT-3' and 5'-ACCATAGCTGATCATGCAGCGGTA-3', respectively; for CD8+ were 5'-ATGGCCTTACCAGTGACCG-3' and 5'-AGGTTCCAGGTCCGATCCAG-3', respectively; for IFNγ were 5'-TCGGTAACTGACTTGAATGTCCA-3' and 5'-TCGCTTCCCTGTTTTAGCTGC-3', respectively; and for GAPDH were 5'-TTCTTTTGCGTCGCCAGCCGA-3' and 5'-GTGACCAGGCGCCCAATACGA-3', respectively. The relative expression was calculated as follows: 2-ΔCt, where ΔCt is Ct(gene) - Ct(GAPDH).
Western blot analysis
30 μg of proteins underwent electrophoresis in 10% SDS-PAGE, and then transferred on the polyvinylidene fluoride membranes (Immobilon P; Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk for 60 min and incubated with rabbit anti-PD-L1 (1:1,000, Cell Signaling Technology) or rabbit monoclonal anti-GAPDH (1:5000, Cell Signaling Technology) overnight at 4°C. Afterwards, HRP-conjugated secondary antibody (1:10000, sc-2004, Santa Cruz Biotechnology) was incubated with membranes, and the bands were visualized using a ChemiDoc Touch (Bio-Rad). The intensity of the bands was measured and normalized to GAPDH by using ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All statistical computations were performed using SPSS 19.0. Spearman's rank correlation was used to analyse the CD8+ TILs and TC-PD-L1 expression levels as continuous variables. The chi-squared test was used to determine the association between either TC-PD-L1 expression or the CD8+ TIL count with the patients' clinicopathological features. The correlations among the mRNA expression levels of PD-L1, CD8+ and IFNγ were analysed using Spearman's rank correlation. Kaplan-Meier method was used to depict the survival curves. The log-rank test was conducted to compare the difference. Cox proportional hazards model was performed for univariate analyses and multivariate analysis. A P value less than 0.05 (two-sided) were used to denote statistically significant.
Results
Clinicopathological features of the patients
The average age was 52 years (range 14-78 years), and there were twice as many male patients (n = 91) than female patients (n = 44) in the cohort. Among these patients, 83 (61.5%) patients were diagnosed with clinical stage I, 24 (17.8%) with stage II, 12 (8.9%) with stage III, and 16 (11.8%) with stage IV disease. Over a median follow-up time of 60 months, 27 (20.0%) patients relapsed, and 23 (17.0%) died. The clinicopathological characteristics of the 135 included ccRCC patients are presented in Table 1.
Elevated TC-PD-L1 expression associates with higher CD8+ TILs infiltration and advanced clinicopathological features in ccRCC
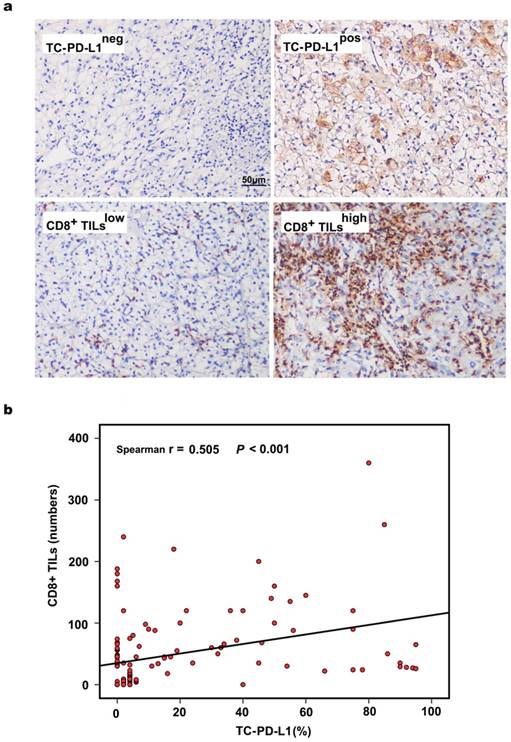
Representative images of TC-PD-L1 expression and CD8+ T lymphocytes from each classification are depicted in Figure 1a. PD-L1 expression was predominantly localized to the cell membrane, and 51 (37.8%) ccRCC patients in our cohort demonstrated positive PD-L1 staining. We further evaluated the association of PD-L1 expression and the presence of CD8+ TILs and observed that PD-L1 expression in tumour cells was significantly correlated with CD8+ T lymphocyte infiltration (r = 0.505, P < 0.001, Figure 1b). Similar results were obtained when TC-PD-L1 expression and CD8+ TILs were analysed as categorical variables (P < 0.001, Table 1). Meanwhile, TC-PD-L1 expression was shown to be associated with clinical stage (P = 0.027) and tumour size (P = 0.004); however, no significant association was observed between TC-PD-L1 expression and the patients' gender, age, tumour location, Fuhrman classification, smoking history, blood creatinine (Cr) or blood urea nitrogen (BUN) (Table 1).
Association between tumour cell PD-L1 expression and the clinicopathological features of clear cell renal cell carcinoma
| Feature | PD-L1 expression | ||||
|---|---|---|---|---|---|
| No. of patients | Negative | Positive | P-value | ||
| Gender | |||||
| Female | 44(32.6%) | 22(50.0%) | 22(50.0%) | 0.058 | |
| Male | 91(67.4%) | 62(68.1%) | 29(31.9%) | ||
| Age (years) | |||||
| < 50 | 59(43.7%) | 40(67.8%) | 19(32.2%) | 0.284 | |
| ≥50 | 76(56.3%) | 44(57.9%) | 32(42.1%) | ||
| Tumour size | |||||
| <5cm | 62(45.9%) | 47(75.8%) | 15(24.2%) | 0.004 | |
| ≥5cm | 73(54.1%) | 37(50.7%) | 36(49.3%) | ||
| Tumour location | |||||
| Left | 81(60.0%) | 51(63.0%) | 30(37.0%) | 0.858 | |
| Right | 54(40.0%) | 33(61.1%) | 21(38.9%) | ||
| Clinical Stage | |||||
| I-II | 107(79.3%) | 72(67.3%) | 35(32.7%) | 0.027 | |
| III-IV | 28(20.7%) | 12(42.9%) | 16(57.1%) | ||
| Fuhrman classification | |||||
| I-II | 110(81.5%) | 71(64.5%) | 39(35.5%) | 0.261 | |
| III-IV | 25(18.5%) | 13(52.0%) | 12(48.0%) | ||
| Smoking history | |||||
| No | 87(64.4%) | 51(58.6%) | 36(41.4%) | 0.271 | |
| Yes | 48(35.6%) | 33(68.8%) | 15(31.3%) | ||
| Cr | |||||
| Normal | 116(85.9%) | 69(59.5%) | 47(40.5%) | 0.130 | |
| High | 19(14.1%) | 15(78.9%) | 4(21.1%) | ||
| BUN | |||||
| Normal | 120(88.9%) | 73(60.8%) | 47(39.2%) | 0.409 | |
| High | 15(11.1%) | 11(73.3%) | 4(26.7%) | ||
| Vital status | |||||
| Alive | 112(83.0%) | 75(67.0%) | 37(33.0%) | 0.018 | |
| Dead | 23(17.0%) | 9(39.1%) | 14(60.9%) | ||
| CD8+TILs | |||||
| Low | 67(49.6%) | 55(82.1%) | 12(17.9%) | <0.001 | |
| High | 68(50.4%) | 29(42.6%) | 39(57.4%) | ||
Cr, creatinine; BUN, urea nitrogen.
Evaluation of PD-L1 expression, CD8+ TILs and their associations in 135 ccRCC tissues. (a) Representative images of TC-PD-L1 expression and CD8+ TILs, ×200 magnification. (b) The significantly positive correlation between the positive rate of TC-PD-L1 and the number of CD8+ TILs as continuous variables.
Intrinsic and extrinsic PD-L1 expression patterns in ccRCC
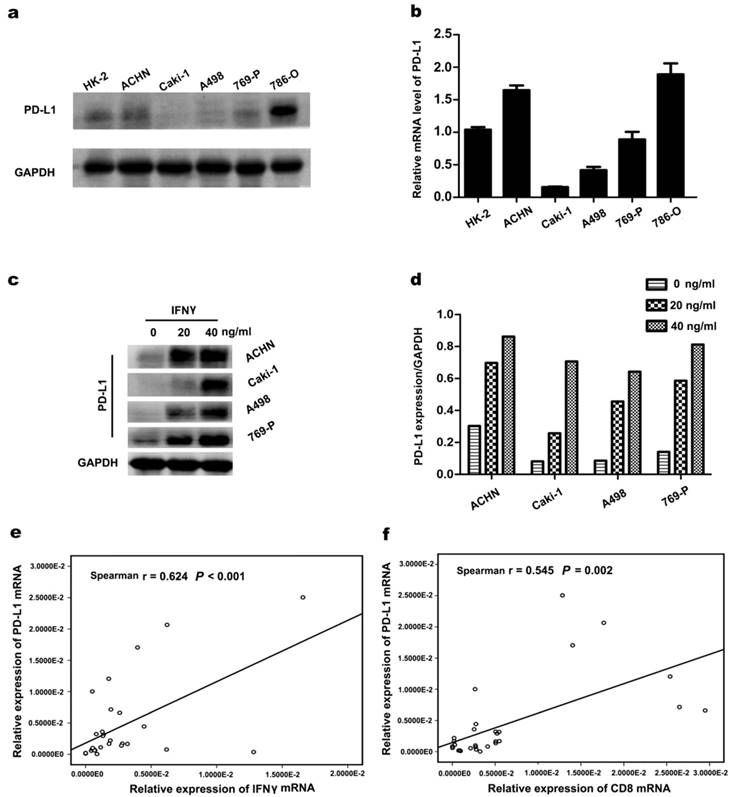
PD-L1 protein and mRNA expression were detected in ccRCC cell lines (ACHN, Caki-1, A498, 769-P, and 786-O) using western blotting and RT-PCR. As illustrated in Figure 2a and 2b, TC-PD-L1 expression was relatively higher in cell line ACHN, 769-P and 786-O compared with Caki-1 and A498. The expression of TC-PD-L1 in ccRCC cell line was further confirmed at the mRNA level. To investigate whether PD-L1 expression in ccRCC cancer cells could be stimulated by IFNγ, ccRCC cell lines were incubated with several concentrations of IFNγ (0, 20 and 40 ng/mL). After 48 hours of IFNγ administration, PD-L1 expression was significantly elevated (Figure 2c and d). To further elucidate the underlying mechanisms responsible for the association between elevated TC-PD-L1 and CD8+ TIL infiltration at mRNA level, a cohort of 30 primary clear cell renal carcinoma specimens was used. Similar results demonstrating significant positive correlation between PD-L1 to either IFNγ or CD8 was observed (r = 0.624, P < 0.001 and r = 0.545, P = 0.002; Figure 2e and 2f, respectively).
Association of PD-L1 expression and CD8+ infiltration levels with prognosis
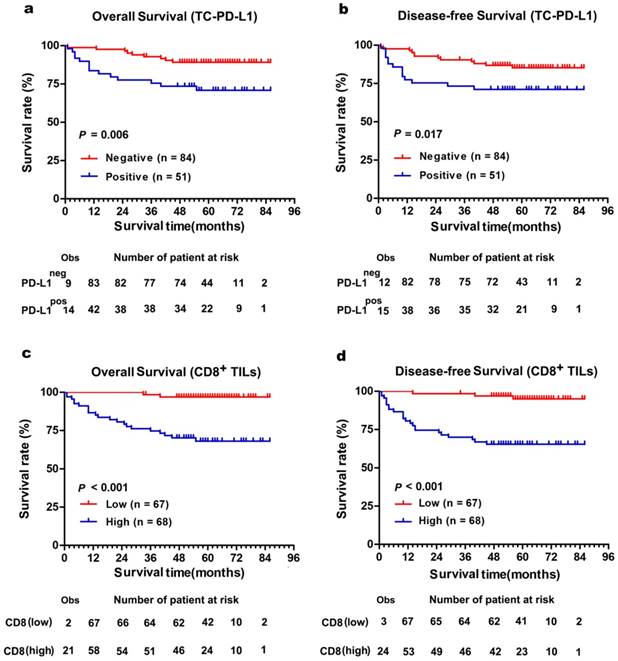
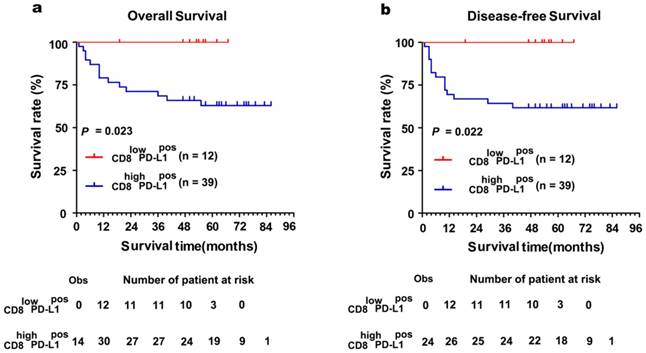
The Kaplan-Meier curves showed that patients with positively expressed TC-PD-L1 had worse OS and DFS (P = 0.006 and 0.017, Figure 3a and 3b, respectively). Moreover, our results demonstrated patients with abundant CD8+ TILs infiltration experienced poorer OS and DFS (both P < 0.001, Figure 3c and 3d, respectively). Intriguingly, subgroup analysis detected more distinct differences in survival when TC-PD-L1-positive ccRCC patients were sub-classified as with or without CD8+ T cell infiltration (OS and DFS, P = 0.023 and P = 0.022, respectively, Figure 4). Univariate analysis revealed higher risks of death (HR, 2.519; P = 0.001) and disease relapse (HR, 2.285; P = 0.001) in patients with high levels of CD8+ TILs (Table 2). Multivariate Cox regression analysis identified that CD8+ TILs and clinical stage as independent prognostic factors for a worse OS (P = 0.038 and P < 0.001, respectively) and DFS (P = 0.011 and P < 0.001, respectively; Table 2), while TC-PD-L1 expression was not identified as an independent prognostic factor.
Correlation between PD-L1 expression and IFNγ or CD8+ in ccRCC. (a) PD-L1 protein in ccRCC cell lines was detected by western blotting. (b) PD-L1 mRNA in ccRCC cell lines. (c) PD-L1 protein was detected after incubated with several concentrations of IFNγ (0, 20 and 40 ng/mL) for 48 h. GAPDH was used as an internal loading control. (d)The target protein levels were normalized to the corresponding GAPDH protein levels. (e) The significant positive correlation between the mRNA of PD-L1 and IFNγ as well as (f) PD-L1 and CD8+ in 30 ccRCC tumour tissues.
Comparison of survival according to PD-L1 expression in tumour cell and the number of CD8+ TILs. (a) Overall survival and (b) disease-free survival were significantly enhanced in TC-PD-L1neg patients compared with TC-PD-L1pos patients. (c) Overall survival and (d) disease-free survival were significantly poor in patients with high CD8+ TILs infiltration compared with low CD8+ TILs infiltration. The small vertical tick marks of “Obs” represented the observed number of events patients. The “number of patients at risk” represented the number of patient possible happened events in the follow-up time.
Subgroup survival analysis based on the CD8+ TILs in TC-PD-L1-positive ccRCC patients. (a)Kaplan-Meier curves for overall survival and (b) disease-free survival in TC-PD-L1-positive patients with abundant or absence of CD8+ TILs infiltration. "Obs" means the observed number of events patients. The "number of patients at risk" represented the number of patient possible happened events in the follow-up time.
Univariate and multivariate Cox regression analysis of the association of various clinicopathological features with overall survival and disease-free survival in patients with clear cell renal cell carcinoma
| Feature | Overall survival | Disease-free survival | |||
|---|---|---|---|---|---|
| Regression coefficient (95%CI) | P | Regression coefficient (95%CI) | P | ||
| I | |||||
| Gender (F vs M) | -0.528(0.259-1.346) | 0.210 | -0.428(0.302-1.405) | 0.275 | |
| Age, years (<50 vs ≥50) | 0.656(0.792-4.684) | 0.148 | 0.723(0.902-4.708) | 0.086 | |
| Tumour size (<5cm vs ≥5cm) | 1.257(1.304-9.472) | 0.013 | 1.022(1.175-6.578) | 0.020 | |
| Tumour location (Left vs Right) | 0.703(0.796-5.125) | 0.139 | 0.745(0.890-4.981) | 0.090 | |
| Clinical Stage (I,II vs III,Iv) | 3.808(13.210-153.575) | <0.001 | 3.222(9.927-63.303) | <0.001 | |
| Fuhrman classification (I,II vs III,Iv) | 1.617(2.215-11.449) | <0.001 | 1.665(2.476-11.273) | <0.001 | |
| Smoking history (No vs Yes) | 0.161(0.509-2.715) | 0.706 | 0.054(0.483-2.305) | 0.893 | |
| Cr (Normal vs High) | -1.363(0.035-1.899) | 0.183 | -0.755(0.111-1.984) | 0.304 | |
| BUN (Normal vs High) | -1.053(0.047-2.588) | 0.303 | -0.432(0.154-2.741) | 0.557 | |
| TC-PD-L1 (Negative vs Positive) | 1.103(1.304-6.970) | 0.010 | 0.893(1.142-5.222) | 0.021 | |
| CD8+ TILs (Low vs High) | 2.519(2.909-53.000) | 0.001 | 2.285(2.956-32.679) | 0.001 | |
| II | |||||
| Tumor size (<5cm vs ≥5cm) | 0.709(0.706-5.855) | 0.189 | 0.266(0.469-3.626) | 0.610 | |
| Clinical Stage (I,II vs III,Iv) | 3.282(7.504-94.491) | <0.001 | 2.774(5.996-42.825) | <0.001 | |
| Fuhrman classification (I,II vs III,Iv) | 1.368(0.523-3.575) | 0.523 | 0.539(0.669-4.393) | 0.262 | |
| TC-PD-L1 (Negative vs Positive) | 0.303(0.517-3.542) | 0.537 | 0.050(0.456-2.422) | 0.907 | |
| CD8+ TILs (Low vs High) | 1.693(1.099-26.897) | 0.038 | 1.719 (1.494-20.842) | 0.011 | |
Cr, creatinine; BUN, urea nitrogen.
Discussion
Numerous reports have detected PD-L1 expression in primary RCC, whereas highly variable percentages of the positive tumour area were reported ranging from 15% to 66% [8-10, 16, 18-20]. In ours, the detection rate was 37.8%, similar to previous reports with positivity rates of 32% [11] and 37.2% [12] in ccRCC, indicating the heterogeneity PD-L1 expression among the subtypes of RCC cannot be ignored. Meanwhile, our study observed that patients with higher TC-PD-L1 expression have increased CD8+ immune cell infiltration. The ccRCC cell line treated with increasing concentrations of recombinant IFNγ led to an increase in PD-L1 expression. These findings underscore the mechanism that CD8+ TILs recruited into the local environment release IFNγ thus driving PD-L1 expression and reflect the presence of an adaptive immune resistance in ccRCC patients. Further, the absence of the CD8+ TILs infiltration in high expressing TC-PD-L1 was identified as being 23% (12/51), which demonstrated the existence of intrinsic immune resistance in ccRCC patients. According to the previous criteria [21, 22], 23% PD-L1 positive expression ccRCC patients detected in present study may fail to respond to the treatments of PD-1/PD-L1 inhibitions for the lack of pre-existing T cell infiltrates, combination therapy designed to bring the T cells into tumours would be considered. Additionally, RCC patients with higher TC-PD-L1 have been demonstrated to be related to the increased tumour aggressiveness such as a higher TNM stage and larger tumour size [12, 23-25]. Our study also reported significantly difference of TC-PD-L1 expression in disease stage and tumour size at presentation indicating that checkpoint inhibitions may have activity in patients with late stage disease or a high tumour burden.
Immunohistochemical studies have concluded that CD8+ TILs exert antitumour activities, which indicates that CD8+ TILs must serve as a positive\prognostic factor. In contrast, Osamu Nakano et al. found that patients with more abundant CD8+ TILs infiltration experienced poorer survival and suggested the immune cell reactions are more pronounced as the biological malignancy progresses for the increased antigenicity of tumour cell [26]. The present study detected similar negative association of high CD8+ T cell infiltration with survival. Further analysis revealed that TC-PD-L1-positive ccRCC patients with high concentration of CD8+ TILs infiltration were associated with a poor prognosis, underscoring a need for improved treatment options in these patients. This seemingly paradoxical observation supports the new idea that the adaptive resistance is active in the tumour microenvironment [14, 27, 28]. In that scenario, TC-PD-L1 can interact with its receptor thus inducing CD8+ TILs anergy or exhaustion[29] . Moreover, previous study observed the expression of genes that were associated with immunosuppression (PD-L1, IDO1, and Tregs) was associated with the presence of CD8+ TILs and INFγ [30]. Likely due to the highly suppressive process in the local microenvironment, the poor prognosis was detected in the subgroup analysis. However, for the limited number of patients, further prospective research is needed.
To the best of our knowledge, the present study is the first to comprehensively and specifically investigate the PD-L1 expression patterns in the primary microenvironment. However, inevitable selection bias may exist in this retrospective study.
Conclusion
The CD8+ TILs were elucidated to influence the expression of TC-PD-L1 in ccRCC patients and associated with significant poor survival in TC-PD-L1-positive ccRCC patients.
Abbreviations
AJCC: American Joint Committee on Cancer; BUN: blood urea nitrogen; ccRCC: clear cell renal cell carcinoma; Cr: blood creatinine; DFS: disease-free survival; FDA: Food and Drug Administration; TC: Tumour cell; TKI: tyrosine kinase inhibitors.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation for Young Scholar of China (81402560) and the National Natural Science Foundation of China (NO.81572865).
Ethics Committee Approval and Patient Consent
For the use of human's clinical data, prior patients' consents and approval from Sun Yat-sen University Cancer Center Institutional Review Board were obtained. All patients included in this study provided verbal informed consent.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
2. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-4
3. Harris DT. Hormonal therapy and chemotherapy of renal-cell carcinoma. Semin Oncol. 1983;10:422-30
4. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071-6
5. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688-96
6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
7. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-7
8. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54
9. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR. et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33:1430-7
10. Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS. et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084-91
11. Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP. et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015;3:1158-64
12. Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS. et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174-9
13. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-92
14. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL. et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37
15. Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s-15s
16. Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381-5
17. Xie QK, Zhao YJ, Pan T, Lyu N, Mu LW, Li SL. et al. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology. 2016;5:e1181252
18. Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R. et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015;21:1071-7
19. Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC. et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757-61
20. Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y. et al. PD-L1 Expression in Clear Cell Renal Cell Carcinoma: An Analysis of Nephrectomy and Sites of Metastases. J Cancer. 2014;5:166-72
21. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139-45
22. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72
23. Leite KR, Reis ST, Junior JP, Zerati M, Gomes Dde O, Camara-Lopes LH. et al. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol. 2015;10:189
24. Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH. et al. Clinicopathologic Analysis of PD-L1 and PD-L2 Expression in Renal Cell Carcinoma: Association with Oncogenic Proteins Status. Ann Surg Oncol. 2016;23:694-702
25. Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC. et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749-56
26. Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M. et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132-6
27. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT. et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116
28. Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS. et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21:3969-76
29. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704
30. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800
Author contact
![]() Corresponding author: Prof. Jian-Chuan Xia, Department of Biotherapy, Sun Yat-Sen University Cancer Center, 651 Dong-feng Road East, Guangzhou 510060, People's Republic of China. (E-mail: xiajchsysu.edu.cn)
Corresponding author: Prof. Jian-Chuan Xia, Department of Biotherapy, Sun Yat-Sen University Cancer Center, 651 Dong-feng Road East, Guangzhou 510060, People's Republic of China. (E-mail: xiajchsysu.edu.cn)

 Global reach, higher impact
Global reach, higher impact