Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(6):1409-1416. doi:10.7150/jca.28659 This issue Cite
Research Paper
Nimotuzumab Plus Paclitaxel and Cisplatin as a 1st-Line Treatment for Esophageal Cancer: Long Term Follow-up of a Phase II Study
1. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), The VIP-II Gastrointestinal Cancer Division of Medical Department, Peking University Cancer Hospital & Institute, Beijing 100142, China.
2. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of GI Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China.
* These authors contributed equally.
Received 2018-7-20; Accepted 2019-1-13; Published 2019-2-23
Abstract
The effect of anti-epidermal growth factor receptor targeted treatment in esophageal squamous cell carcinoma (ESCC) is still unclear. We conducted a prospective phase II study of paclitaxel, cisplatin, and nimotuzumab (TPN) as a first-line treatment for unresectable or metastatic ESCC and the objective response rate was 51.8%. Here, we report the long-term follow-up results of the initial trial. Fifty-nine patients were enrolled from Mar 2011 to Apr 2013 and were treated with the TPN regimen. Palliative sequential radiotherapy was given if all tumor lesions were confined to 1-2 radiation fields. Fifty-six patients were eligible for evaluation. After a median follow-up of 32.2months, the median progression-free survival (PFS) and the overall survival (OS) time were 18.1±4.2 months (95% CI: 9.8-26.4) and 26.2±10.0 months (95% CI: 6.6-45.8), respectively, in 29 patients with unresectable local-regional disease, while they were 6.6±0.4 months (95% CI: 5.8-7.5) and 11.5±3.7 months (95% CI: 4.2-18.8), respectively, in 27 patients with metastatic disease. Patients who were male, those with multiple station lymph node metastases, those with visceral metastasis, those who did not response to TPN treatment, and those who did not receive radiotherapy, had a worse OS. In 6 patients with multiple station lymph node metastasis and in 3 patients with recurrent disease and oligo-metastasis (local lymph nodes), TPN with sequential radiation resulted in a mean OS of 17.67±9.50 months and a mean OS of over 40 months, respectively. In conclusion, TPN is effective as a first-line treatment for patients with unresectable and metastatic ESCC. In addition, TPN treatment with sequential radiation might improve survival in patients with limited or oligo lymph node metastases.
Keywords: cisplatin, epidermal growth factor receptor, esophageal squamous cell carcinoma, nimotuzumab, paclitaxel
Introduction
Esophageal cancer is the eighth-most common cancer worldwide and the sixth-most common cause of cancer-related death [1]. In Asia and especially in China, the majority of esophageal cancer cases are squamous cell carcinoma (SCC), which accounts for approximately 90% of all cases [2]. Epidermal growth factor receptor (EGFR) expression is observed in 30-50% of esophageal cancer patients and is associated with a poor prognosis [3, 4]. Previous studies have confirmed that anti-EGFR treatment such as cetuximab can decrease EGFR pathway signaling via the reduction in the phosphorylation of EGFR and AKT in esophageal cancer cell lines [5]. Moreover, some phase 1/2 clinical trials suggested that the addition of cetuximab to standard chemotherapy and/or radiotherapy for esophageal cancer is well tolerated and might increase treatment efficacy [6-8]. Thereafter, a phase 2/3 multicenter randomized trial, SCOPE1, reported that the addition of cetuximab to chemoradiotherapy (capecitabine and cisplatin) led to more frequent toxicities without a benefit of overall survival (OS): 22.1 months vs 25.4 months, p=0.035) [9]. Another phase 3 clinical trial, the REAL3 study, compared chemotherapy (epirubicin, oxaliplatin, and capecitabine) with or without another anti-EGFR agent panitumumab and found that the combination was associated with worse OS (8.8 months vs 11.3 months; p=0.01) [10].
However, both the SCOPE 1 and REAL 3 studies enrolled patients with adenocarcinoma (AC) and patients with SCC. These two subtypes have completely different biological characteristics and prognoses. Thus, the role of anti-EGFR treatment should be studied separately in AC and SCC of the esophagus.
In esophageal squamous cell carcinoma (ESCC), another humanized anti-EGFR monoclonal antibody, nimotuzumab, has been studied in several clinical trials and the results were promising. In a phase 1 study, 19 patients with local advanced esophageal cancer received chemotherapy (cisplatin and 5-FU) and nimotuzumab (400mg in the first week followed by 200mg weekly). Treatment was well tolerated, and the objective response rate (ORR) was 42.1% [11]. In a phase 2 clinical trial, 63 patients received cisplatin, 5-fluorouracil, and radiotherapy, either alone or combined with six weekly infusions of nimotuzumab at a dose of 200 mg. The ORR was 47.8% in the nimotuzumab group and 15.4% in the control group [12]. In another phase 2 study, nimotuzumab was combined with radiotherapy to treat local advanced esophageal cancer in 52 patients. The median OS was 14 months and the 3 year survival rate was 26.2% [13].
Based on the promising results of those studies in ESCC, we conducted a prospective, single-armed, phase 2 study of paclitaxel, cisplatin, and nimotuzumab (TPN) as a first-line treatment in unresectable local-regional or metastatic ESCC. Enrollment began in March 2011 and was completed in April 2013. In all, 59 patients were enrolled, and of those, 56 were eligible for evaluation. The safety and ORR were reported previously, but the median OS was not assessed as part of the first report. The purpose of the current study is to report the long-term follow-up results and to analyze the impact of clinical characteristics on the survival of patients.
Materials and Methods
Patient Eligibility
This was a prospective phase 2 clinical trial (NCT01336049) that was conducted at the Peking University Cancer Hospital. The ethics committee of the Peking University Cancer Hospital approved this trial, and written informed consent was provided voluntarily by subjects before enrollment. All patients had histologically confirmed ESCC with unresectable local-regional or metastatic disease. Local-regional disease was defined as all tumor lesion(s) limited to one radiotherapeutic field and without visceral metastases. Metastatic disease was defined as tumor lesion(s) that exceeded one radiotherapeutic field in local-regional disease or with visceral metastasis or with recurrent disease. The study methods and patient eligibility were described in detail in our previous publication [14]. Briefly, patients who were between 18 and 75 years of age and those of both genders were eligible. Patients should have a Karnofsky performance status >=80 and a life expectancy of >=3 months. Normal bone marrow, renal, and liver function were required. Patients who received prior palliative chemotherapy and radiotherapy were excluded except for who received radiation and adjuvant chemotherapy for nontargeted lesions in an interval of at least 6 months. In addition, those who received adjuvant chemotherapy should not have a prior history of paclitaxel use, and the total dose of cisplatin should have been less than 300mg/m2. Measurable disease according to the RECIST 1.0 criteria was required.
Study Treatment
Cisplatin (Qilu Pharmaceutical Co., Ltd, Shandong, China) at 30 mg/m2 on day 1 and day 2 and paclitaxel (Beijing Xiehe Pharmaceutical Co., Ltd, Beijing, China) at 175 mg/m2 on day 1 were given every 21 days for at least 2 cycles. Nimotuzumab (Biotech Pharmaceutical Co., Ltd, Beijing, China) at a dose of 200 mg was given weekly. The CT scans were performed before treatment, every 2 cycles after treatment, and every 3 months during the follow-up. Sequential radiotherapy was allowed after TPN treatment on the condition that all tumor lesions were in one or two radiotherapeutic fields. It was recommended that nimotuzumab also be administered during radiotherapy. Palliative radiotherapy was an option to control symptoms in patients with metastatic disease after completion of TPN treatment.
Statistical Methods
All descriptive statistics are presented as the mean ± standard error. PFS and OS curves were calculated using the Kaplan-Meier method, and the differences in survival curves were assessed using the log-rank test. Multivariable Cox models were used to estimate adjusted hazard ratios (HRs). SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses, and a p value of <=0.05 was considered statistically significant.
Results
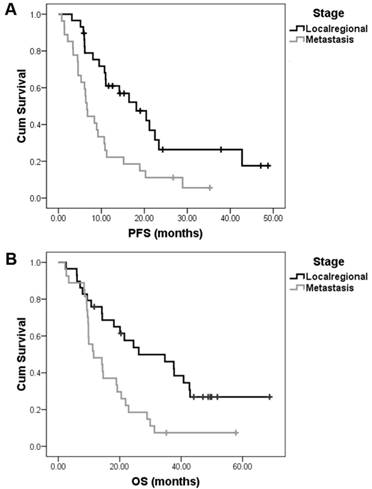
The trial completed enrollment in April 2013. In all, 59 patients were enrolled and 56 were eligible for survival analysis. The patient characteristics are summarized in Table 1. In all, 191 treatment cycles with a median of 4 cycles (range, 1.0-6.0 cycles) per patient were administered. The primary end point was the ORR of 51.8% (28/56) and had previously been reported together with the safety data [14]. After a median follow-up of 32.2 months (range, 9.2-68.8 months), we reported the final survival data. The median PFS and OS for the whole group were 10.8±1.1 months (95% CI: 8.7-12.9) and 19.2±4.2 months (95% CI: 10.9-27.5), respectively. Twenty-nine patients had unresectable local-regional disease and 27 patients had metastatic disease. The median PFS and OS were 18.1±4.2 months (95% CI: 9.8-26.4) and 26.2±10.0 months (95% CI: 6.6-45.8), respectively, in patients with local-regional disease. However, in patients with metastatic disease, the PFS and OS were 6.6±0.4 months (95% CI: 5.8-7.5) and 11.5±3.7 months (95% CI: 4.2-18.8), respectively (Fig. 1).
We firstly analyzed the impact of lymph node metastasis patterns on survival. According to the Japanese Classification of Esophageal Cancer (tenth edition by the Japan Esophageal Society) [15], the N stage was classified based on the involved lymph node station of the primary tumor. Out of 56 patients, 49 could be classified by the sites of metastatic lymph nodes (7 patients were excluded due to an uncertain location of metastatic lymph nodes). Our results showed that the median PFS in 9 patients with single lymph node station metastasis was longer than that in 40 patients with multilymph node station metastasis, but this difference was not statistically significant (20.4±5.6 vs 8.4±1.7 months, p=0.17). However, the median OS in patients with single lymph node station metastasis was much longer than that in patients with multilymph node station metastasis (40.8±4.1 months vs 14.4±5.2 months, p=0.026).
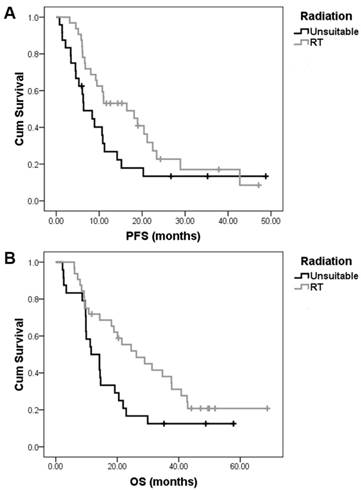
In the present study, patients in whom all tumor lesions were confined to one or two radiotherapeutic field may have received palliative sequential radical radiotherapy to control symptoms after TPN treatment. In all, 23 patients with local-regional disease received radical radiation treatment (RT). Six patients with metastatic disease of multiple lymph node station metastasis received palliative RT. In addition, 3 patients with oligo-metastases (limited local lymph node metastases) after esophagectomy also received palliative RT (Table 2). Compared with those who did not received RT, patients who received RT exhibited longer median PFS and OS (PFS16.4±5.7 vs 6.4±1.8 months, p=0.064; OS 26.2±6.5 vs 11.5±2.6 months, p=0.036) (Fig. 2).
As shown in table 2, noticeably, 6 patients with metastatic disease who received palliative RT after TPN treatment had a mean PFS of 12.66±8.53 months and a mean OS of 17.67±9.50 months. In 3 patients with oligo-metastases, the responses to TPN treatment were PR, SD, and PD for each of the patients. Those patients received palliative RT and all are still alive at the time of this writing; each patient had a survival time of 49.9, 40.8 and 68.8 months. This implied that ESCC patients with oligo-metastases (limited local lymph nodes metastasis) have relatively better prognosis and that TPN treatment with radiation might lead to longer survival.
Patient characteristics and survival after TPN treatment
| N | Median PFS | p Value | Median OS | p Value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 46 | 8.9±2.6 | 0.028 | 14.3±4.2 | 0.045 |
| Female | 10 | 23.4±7.8 | 28.9±6.7 | ||
| Age | |||||
| <60 yrs | 24 | 6.8±1.5 | 0.028 | 9.8±2.8 | 0.34 |
| >= 60yrs | 32 | 11.0±3.5 | 21.5±2.5 | ||
| Primary tumor location | |||||
| Cervical and upper thoracic esophagus | 5 | 22.5±1.5 | 0.22 | 42.5±4.2 | 0.074 |
| Middle thoracic esophagus | 20 | 9.5±3.0 | 14.6±4.8 | ||
| Lower thoracic esophagus | 28 | 9.2±1.6 | 14.3±4.9 | ||
| Unknown | 3 | / | / | ||
| Differentiation | |||||
| Well | 1 | 0.46 | 0.21 | ||
| Median | 25 | 10.8±3.9 | 21.9±7.2 | ||
| Poor | 29 | 10.7±1.6 | 14.2±2.5 | ||
| Unknown | 1 | / | / | ||
| Lymph node station involved* | |||||
| Single | 9 | 20.4±5.6 | 0.17 | 40.8±4.1 | 0.026 |
| Multiple | 40 | 8.4±1.7 | 14.4±5.2 | ||
| Unclassified | 7 | / | / | ||
| Visceral metastasis | |||||
| No | 38 | 14.2±4.8 | 0.003 | 24.5±5.8 | 0.004 |
| Yes | 18 | 5.3±1.7 | 11.2±1.7 | ||
| Stage | |||||
| Local-regional | 29 | 18.1±4.2 | 0.003 | 26.2±10.0 | 0.003 |
| Metastatic disease | 27 | 6.6±0.4 | 11.5±3.7 | ||
| TPN treatment response | |||||
| CR and PR | 30 | 15.2±4.7 | 0.003 | 21.5±2.5 | 0.018 |
| SD and PD | 26 | 8.4±2.5 | 10.7±2.9 | ||
| Radiation treatment | |||||
| No | 24 | 6.4±1.8 | 0.064 | 11.5±2.6 | 0.036 |
| Yes | 32 | 16.4±5.7 | 26.2±6.5 | ||
* According to the Japanese classification of esophageal cancer [15]. PFS, progression free survival; OS, overall survival; TPN, nimotuzumab with paclitaxel and cisplatin treatment; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The median PFS and OS of the combination of nimotuzumab, paclitaxel, and cisplatin (TPN) as a 1st line treatment in local-regional and metastatic esophageal squamous cell carcinoma. After a median follow-up of 32.2 months, TPN treatment resulted in a median PFS of 18.1±4.2 months in 29 patients with local-regional disease and 6.6±0.4 months in 27 patients with metastatic disease (A). The median OS time of these patients were 26.2±10.0 months and 11.5±3.7 months, respectively (B).
In the univariate analysis, the location of the primary tumor and cell differentiation had no relation to survival. However, compared with female patients, male patients exhibited worse median PFS (8.9±2.6 vs 23.4±7.8 months, p=0.028) and a worse median OS (14.3±4.2 vs 28.9±6.7 months, p=0.045). Patients younger than 60 years of age also exhibited a worse median PFS (6.8±1.5 months vs 11.0±3.5 months, p=0.028). However, the median OS was similar in the two different age arms (9.8±2.8 months vs 21.5±2.5 months, p=0.34). In both the local-regional and metastatic disease groups, patients with CR and PR had a longer median OS than those with SD and PD (local-regional disease: 37.6±11.1 vs 20.1±15.5 months; metastatic disease: 18.9±3.3 vs 9.3±0.2 months, p=0.018).
We also analyzed the impact of the patient characteristics on survival using a multivariable Cox model that included gender, age, primary tumor location, nodal status, tumor grade, radiotherapy, and response to TPN treatment as the covariates and where the patients were stratified by staging of local-regional disease and metastatic disease. As shown in Table 3, in the multivariable Cox regression analyses, the gender of the patients showed an independent association with PFS (female vs male: HR=0.28, 95% CI: 0.10-0.80, p=0.02). In addition, TPN treatment showed an independent association with OS (SD and PD vs PR: HR=2.32, 95% CI: 1.06-5.05, P =0.03).
Characteristic of ESCC patients who received palliative or palliative radiotherapy
| Patient No. | Gender | Age | Primary Tumor | Differentiation | Metastatic Lymph Node Sites | Esophagectomy | Response to Chemo | Radiation | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | Middle Thorax | Poor | CE, SV, PE, PC | N | PR | Palliative | 6.63 | 31.3 |
| 2 | M | 62 | Lower Thorax | Poor | PE, CA ,PA | N | SD | Palliative | 9.2 | 9.2 |
| 3 | M | 51 | Middle Thorax | Poor | CE, SV, PE, PS, CA | N | SD | Palliative | 6.77 | 9.3 |
| 4 | F | 71 | Middle Thorax | Poor | SV, PE, PS, CA | N | PR | Palliative | 28.9 | 28.9 |
| 5 | M | 66 | Middle Thorax | Poor | PS, CA, PA | N | SD | Palliative | 5.5 | 8.4 |
| 6 | M | 70 | Lower Thorax | Moderate | PE, HI, CA, PA | N | PR | Palliative | 18.9 | 18.9 |
| 7 | M | 66 | Lower Thorax | Poor | SV, PE | Y | PR | Palliative | 21.2 | 49.9 |
| 8 | M | 62 | Middle Thorax | Unknown | PE | Y | PD | Palliative | 3.1 | 40.8 |
| 9 | F | 58 | Middle Thorax | Moderate | PE | Y | SD | Palliative | 37.8 | 68.8 |
M, male; F, female; CE, cervical; SV, supraclavicular; PE, paraesophageal; PC, paracardial; CA, celiac artery; PA, paraaortic; PS, parastomach; HI, hilar; ESCC, esophageal squamous cell carcinoma; N, no; Y, yes; PFS, progression free survival; OS, overall survival; PR, partial response; SD, stable disease; PD, progression.
Impact of patient characteristics on survival: multivariable analysis
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
p Value | HR | 95.0% CI | p Value | HR | 95.0% CI | |||
| Lower | Upper | Lower | Upper | |||||
| Gender (Female) | 0.02 | 0.28 | 0.10 | 0.80 | 0.49 | 0.69 | 0.24 | 1.98 |
| Age (<60yrs) | 0.17 | 0.62 | 0.31 | 1.23 | 0.20 | 0.61 | 0.28 | 1.30 |
| Lymph node station (Single) | 0.48 | 0.67 | 0.22 | 2.06 | 0.43 | 1.58 | 0.51 | 4.94 |
| Visceral metastasis | 0.35 | 1.86 | 0.51 | 6.77 | 0.66 | 1.35 | 0.36 | 5.06 |
| Without radiation | 0.48 | 1.59 | 0.44 | 5.68 | 0.87 | 1.12 | 0.28 | 4.40 |
| TPN treatment (SD and PD vs PR) | / | / | / | / | 0.03 | 2.32 | 1.06 | 5.05 |
PFS, progression free survival; OS, overall survival; TPN, nimotuzumab with paclitaxel and cisplatin treatment; PR, partial response; SD, stable disease; PD, progressive disease; HR, hazard ratio.
The impact of sequential radiotherapy after nimotuzumab, paclitaxel, and cisplatin (TPN) combination treatment on the survival of esophageal squamous cell carcinoma patients. In all, 23 patients with local-regional ESCC received sequential radical radiation treatment (RT) after TPN treatment. Six patients with metastatic disease and 3 patients with recurrent disease but with oligo-metastases (limited local lymph node metastases) after esophagectomy also received palliative RT after TPN treatment. Compared with those who did not received RT, patients who received sequential RT exhibited longer median PFS (PFS16.4±5.7 vs 6.4±1.8 months, p=0.064) (A) and OS (26.2±6.5 vs 11.5±2.6 months, p=0.036) (B).
Discussion
Surgical resection is currently the standard treatment for patients with local-regional esophageal cancer. However, most patients are diagnosed with unresectable or metastatic disease and at an advanced stage. Fluorouracil, cisplatin, and taxane form the backbone of treatment for recurrent or metastatic ESCC, and as a first-line treatment, the ORR is 25-35%; moreover, the median OS of these patients is 7-9 months [16-18]. Thus, more effective therapies are critically needed to improve the outcome of esophageal cancer.
The overexpression of EGFR in esophageal cancer has been reported to be as high as 30-80% of tumors, and this correlates with increased invasion, poorly differentiated histology, and worse prognosis [19, 20]. In preclinical studies, cetuximab inhibited cellular proliferation and enhanced the activities of cytotoxic agents in esophageal cancer cell lines [21, 22]. Moreover, the success of anti-EGFR targeted treatment in non-small cell lung cancer [23], colorectal cancer [24], and squamous-cell head and neck cancer [25] provides the rationale for the use of anti-EGFR targeted treatment in esophageal cancer.
Although some phase 1 and 2 studies have suggested the safety and efficacy of cetuximab in esophageal cancer [6-8], two large-scale multicenter randomized clinical trials, SCOPE1 and REAL3, showed negative results [9, 10]. It should be noted that the SCOPE1 study did not separate SCC from AC, and more than 20% of patients were diagnosed with AC [9]. In the REAL3 study, almost all patients (99%, 545/553) were pathologically diagnosed with AC [10]. SCC and AC are substantially different in their underlying etiology and tumorigenesis. Therefore, the findings of these clinical trials on esophageal squamous cell carcinoma should be reviewed.
Recently, another anti-EGFR agent, nimotuzumab, had been widely used to treat cancers in the clinic. Nimotuzumab is a recombinant humanized monoclonal IgG1 antibody against human EGFR that has shown clinical efficacy in head and neck cancer [26], non-small cell lung cancer [27], and glioma [28] as a combination therapy with radiotherapy or chemotherapy.
In ESCC, several phase 1 and phase 2 studies showed that nimotuzumab with chemoradiotherapy or radiotherapy led to either a higher response rate or improved survival [12, 13, 29, 30]. Radiotherapy or chemoradiotherapy is recommended as a curable and standard treatment for esophageal cancer patients with local-regional disease. However, for patients with late-stage disease including unresectable or metastatic disease, first-line chemotherapy is the recommended treatment. Radiation was palliative and given to control the symptoms including esophageal obstruction caused by the primary tumor.
A few studies have investigated the combination of nimotuzumab and chemotherapy. Liang et al treated 19 ESCC patients with nimotuzumab, 5-FU, and cisplatin. In 16 evaluable patients, the ORR and DCR were 42.1% and 68.4%, respectively, but survival data were not reported [11]. Xu S et al treated 205 cancer patients with nimotuzumab at different dosages along with standard chemotherapy. However, only 21 ESCC patients were enrolled and the authors did not separately analyze the efficacy and safety of this population [31]. Han X et al treated 21 ESCC patients with late-stage disease using nimotuzumab with paclitaxel-, fluorouracil-, or gemcitabine-based chemotherapy. The ORR and DCR were 38.1% and 81%, respectively. The mean PFS was 7 months and the 18-month OS rate was 10% [32]. Compared with the studies described above, the present study was a prospective phase 2 clinical trial. This study enrolled more patients (59 patients and 56 evaluable) with late-stage ESCC. The treatment design was uniform (nimotuzumab, paclitaxel, and cisplatin). The ORR was 51.8% and was reported previously with the safety data [14]. After a follow-up of 32.2 months, we analyzed the survival data and the impact of patient characteristics on survival. The median PFS and OS of the whole group were 10.8±1.1 months (95% CI: 8.7-12.9) and 19.2±4.2 months (95% CI: 10.9-27.5), respectively. One of our previous phase 2 studies showed that, in 39 ESCC patients with unresectable and/or recurrent and/or metastatic disease, chemotherapy consisting of paclitaxel and cisplatin (TP) resulted in a similar ORR of 48.6%. However the median TTP and OS were only 7.0 months (95% CI, 4.83-9.16 months) and 13.0 months (95% CI, 10.5-15.4 months), respectively [33]. Thus, the addition of nimotuzumab to the TP regimen in the present study resulted in better PFS and OS compared with chemotherapy alone. Recently, in a retrospective study, Saumell Y et al [34] analyzed the efficacy of nimotuzumab in 93 patients with locally advanced or metastatic ESCC. Although the treatment design was inconsistent, an increase in the median OS in patients treated with nimotuzumab (11.9 vs 6.5 months) and an increase of the 1-year survival rate (54.0% vs 21.9%) were observed. Based on these promising results, a randomized, placebo-controlled, double-blind phase 3 study (TPN versus TP chemotherapy alone) was performed to verify the efficacy of TPN combination treatment for ESCC. Furthermore, we previously analyzed the EGFR expression by immunohistochemistry in ESCC patients who were treated with nimotuzumab including off-label use. The expression of EGFR failed to predict the response of patients to nimotuzumab [35]. Other biomarkers should be investigated in future studies.
Previous studies have suggested that lymph node metastasis is a strong prognostic predictor. Takeno et al. analyzed the metastatic pattern of lymph nodes in 126 ESCC patients. Their results showed that multiple-station metastasis was a significant negative prognostic parameter compared with single-station metastasis (p=0.0035)[36]. Peng et al. analyzed 1,351 patients with ESCC who underwent radical-intent surgical resection. They found that survival could easily be distinguished according to the number of metastatic lymph node stations: patients with a single station involved, 2-3 stations involved, and >=4 stations involved (p=0.001) [37]. For inoperable patients, the status of lymph node metastasis is normally evaluated by CT scan and the sensitivity is approximately only 50% [38]. Therefore, we used the classification and the anatomical lymphatic spread represented by the Japanese classification [15] instead of the number of involved metastatic lymph nodes in the 7th edition of the AJCC staging system. Our results showed that the median OS in patients with single lymph node station metastasis was longer than that of patients with multilymph node station metastasis (40.8±4.1 months vs 14.4±5.2 months, p=0.026). However, the multivariate analysis failed to demonstrate a relationship between lymph node station metastasis and OS. Therefore, more studies should be performed to explore the impact of the lymph node metastatic pattern on survival in ESCC.
In this study, the univariate analysis also showed that gender was associated with prognosis. Female patients had a relatively better PFS and OS. However, the multivariate analysis only confirmed that females had a favorable PFS but not a favorable OS. Epidemiological studies have reported that compared with males, females have a relatively low incidence of esophageal cancer [1]. In addition, hormone therapy users have a lower risk of ESCC compared with those who have never used hormone therapy [39, 40]. Some investigations also suggested that women with ESCC have a favorable prognosis. Su et al. analyzed the prognosis of 674 ESCC patients who underwent surgical resection and found that the 5-year survival rate for females was much higher than that for males (46.8% vs 36.7%, p=0.003) [41]. Morita et al. analyzed 1,000 consecutive ESCC patients who underwent esophagectomy and found that female gender was an independent favorable prognostic factor (hazard ratio=0.74) [42]. A retrospective analysis, which included more samples, was then performed to explore the role of gender in patients with unresectable ESCC who received TPN treatment.
Radiotherapy is an important treatment for unresectable or metastatic esophageal cancer. Definitive concurrent chemoradiotherapy (cCRT) is the recommended standard treatment for patients with advanced local-regional ESCC [43, 44]. Some studies have shown that sequential chemoradiotherapy (sCRT) is a treatment with similar efficacy but less toxicity compared with cCRT [45, 46]. In the present study, patients who received sCRT had a better OS compared with those without RT. Even in the 6 patients with multiple-station lymph node metastasis, palliative sCRT also resulted in a better PFS and OS. Therefore, sCRT might be an effective treatment for ESCC patients with unresectable local-regional disease.
Though metastases are generally widely disseminated, a special metastatic pattern, oligo-metastases, has been recognized and has received increased attention. Oligo-metastases have a distinct natural history and a better prognosis compared with wide metastatic disease in a number of cancers such as prostate, breast, lung and colorectal cancers [47, 48]. For ESCC, some ESCC patients have only local- regional lymph node metastasis [49, 50]. Radiotherapy and/or chemotherapy has been demonstrated to be a palliative treatment for local-regional recurrent esophageal carcinoma with a median OS of approximately 12.0 months [51-53]. Noticeably, in the present study, TPN and palliative sCRT in 3 patients with oligo-metastases (limited local lymph node metastasis) led to much better survival. The responses to TPN treatment in each patient were PR, SD, and PD. All patients are still alive at the time of this writing, and have each survived 49.9, 40.8 and 68.8 months. Thus, a potential for a cure still exists for those ESCC patients with recurrent disease but with oligo-metastases of the lymph nodes. Further studies should be conducted to identify the optimal treatment pattern for ESCC patients with oligo-metastasis after esophagectomy. TPN and sequential radiation might be a feasible option for this subpopulation of ESCC patients.
Abbreviations
AC: adenocarcinoma; cCRT: concurrent chemoradiotherapy; EGFR: epidermal growth factor receptor; ESCC: esophageal squamous cell carcinoma; HR: hazard ratio; OS: overall survival; ORR: objective response rate; PD: progressive disease; PFS: progression-free survival; PR: partial response; RT: radiation treatment; SCC: squamous cell carcinoma; sCRT: sequential chemoradiotherapy; SD: stable disease; TP: paclitaxel and cisplatin; TPN: paclitaxel, cisplatin and nimotuzumab.
Acknowledgements
We thank all the staff at the Department of GI Oncology, Peking University Cancer Hospital & Institute, for their help in the administration, data collection, and follow-up of the patients.
Funding
This study was partially funded by the “Capital's Funds for Health Improvement and Research” project, Beijing/China (Dr. Xiaodong Zhang/grant number 2016-2-2152).
Authors' Contributions
The conception and design of the work: Dr. Xiaodong Zhang and Dr. Lin Shen. Collection and analysis of data: Dr. Xiaodong Zhang, Dr. Jun Jia, Dr. Ming Lu, Dr. Xicheng Wang, Dr. Jian Li, Dr. Jie Li, Dr. Yan Li, Dr. Xiaotian Zhang, Dr. Jun Zhou, Dr. Zhihao Lu, Dr. Jifang Gong, Dr. Jing Yu, Dr. Zhiwei Sun, Dr. Ying Yang, Dr. Chuanling Liu and Dr. Yanjie Xiao. Interpretation of data: Dr. Xiaodong Zhang, Dr. Ming Lu and Dr. Jun Jia. Final approval of the version to be published: Dr. Xiaodong Zhang and Dr. Lin Shen. Drafting and revising the work: Dr. Jun Jia, Dr. Ming Lu and Dr. Lin Shen.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The ethics committee of the Beijing Cancer Hospital (Peking University Cancer Hospital & Institute) approved this trial. This phase 2 clinical trial was registered in the ClinicalTrials.gov database; the registration number is NCT01336049. Written informed consent for publication of the datasets was obtained from all individual participants at the point of recruitment to the clinical trial.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
2. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-7
3. Gibault L, Metges JP, Conan-Charlet V, Lozac'h P, Robaszkiewicz M, Bessaguet C. et al. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer. 2005;93:107-15
4. Yu WW, Guo YM, Zhu M, Cai XW, Zhu ZF, Zhao WX. et al. Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepato-gastroenterology. 2011;58:426-31
5. Bettstetter M, Berezowska S, Keller G, Walch A, Feuchtinger A, Slotta-Huspenina J. et al. Epidermal growth factor receptor, phosphatidylinositol-3-kinase catalytic subunit/PTEN, and KRAS/NRAS/BRAF in primary resected esophageal adenocarcinomas: loss of PTEN is associated with worse clinical outcome. Human pathology. 2013;44:829-36
6. Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P. et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20:1667-73
7. Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P. et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06). J Clin Oncol. 2011;29:626-31
8. Lledo G, Huguet F, Chibaudel B, Di Fiore F, Mineur L, Galais MP. et al. Chemoradiotherapy with FOLFOX plus cetuximab in locally advanced oesophageal cancer: The GERCOR phase II trial ERaFOX. Eur J Cancer. 2016;56:115-21
9. Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J. et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. The lancet oncology. 2013;14:627-37
10. Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. The lancet oncology. 2013;14:481-9
11. Ling Y, Chen J, Tao M, Chu X, Zhang X. A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis. 2012;4:58-62
12. Ramos-Suzarte M, Lorenzo-Luaces P, Lazo NG, Perez ML, Soriano JL, Gonzalez CE. et al. Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther. 2012;13:600-5
13. Liang J, E M, Wu G, Zhao L, Li X, Xiu X. et al. Nimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a Phase II clinical trial. OncoTargets and therapy. 2013;6:1589-96
14. Lu M, Wang X, Shen L, Jia J, Gong J, Li J. et al. Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: A single centre prospective phase II trial. Cancer Sci. 2016;107:486-90
15. Soc JE. Japanese Classification of Esophageal Cancer, tenth edition: parts II and III Japan Esophageal Society. Esophagus-Tokyo. 2009;6:71-94
16. Homs MY, v d Gaast A, Siersema PD, Steyerberg EW, Kuipers EJ. Chemotherapy for metastatic carcinoma of the esophagus and gastro-esophageal junction. The Cochrane database of systematic reviews. 2006 CD004063
17. Shim HJ, Cho SH, Hwang JE, Bae WK, Song SY, Cho SB. et al. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. American journal of clinical oncology. 2010;33:624-8
18. Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi K, Nishimura H, Tanigawa N. Evaluation of combined docetaxel and nedaplatin chemotherapy for recurrent esophageal cancer compared with conventional chemotherapy using cisplatin and 5-fluorouracil: a retrospective study. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2008;21:496-501
19. Sunpaweravong P, Suwiwat S, Sunpaweravong S, Puttawibul P, Mitarnun W. Correlation of epidermal growth factor receptor mutation, immunohistochemistry, and fluorescence in situ hybridization in esophageal squamous cell carcinoma. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2009;92:1136-42
20. Zhang W, Zhu H, Liu X, Wang Q, Zhang X, He J. et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;98:513-9
21. Kwon J, Yoon HJ, Kim JH, Lee TS, Song IH, Lee HW. et al. Cetuximab inhibits cisplatin-induced activation of EGFR signaling in esophageal squamous cell carcinoma. Oncol Rep. 2014;32:1188-92
22. Sato F, Kubota Y, Natsuizaka M, Maehara O, Hatanaka Y, Marukawa K. et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther. 2015;16:933-40
23. Hirsch FR, Janne PA, Eberhardt WE, Cappuzzo F, Thatcher N, Pirker R. et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol. 2013;8:373-84
24. Hubbard JM, Grothey A. Colorectal cancer in 2014: progress in defining first-line and maintenance therapies. Nature reviews Clinical oncology. 2015;12:73-4
25. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-27
26. Reddy BK, Lokesh V, Vidyasagar MS, Shenoy K, Babu KG, Shenoy A. et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral oncology. 2014;50:498-505
27. Babu KG, Prabhash K, Vaid AK, Sirohi B, Diwakar RB, Rao R. et al. Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non-small-cell lung cancer: a multicenter, randomized, open-label Phase II study. OncoTargets and therapy. 2014;7:1051-60
28. Solomon MT, Selva JC, Figueredo J, Vaquer J, Toledo C, Quintanal N. et al. Radiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: results from a randomized, double blind trial. BMC Cancer. 2013;13:299
29. Kato K, Ura T, Koizumi W, Iwasa S, Katada C, Azuma M. et al. Nimotuzumab combined with concurrent chemoradiotherapy in Japanese patients with esophageal cancer: A phase I study. Cancer Sci. 2018;109:785-93
30. de Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, de Araujo Lima Franca B. et al. A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer. 2018;88:21-30
31. Xu S, Ramos-Suzarte M, Bai X, Xu B. Treatment outcome of nimotuzumab plus chemotherapy in advanced cancer patients: a single institute experience. Oncotarget. 2016;7:33391-407
32. Han X, Lu N, Pan Y, Xu J. Nimotuzumab Combined with Chemotherapy is a Promising Treatment for Locally Advanced and Metastatic Esophageal Cancer. Med Sci Monit. 2017;23:412-8
33. Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. American journal of clinical oncology. 2008;31:29-33
34. Saumell Y, Sanchez L, Gonzalez S, Ortiz R, Medina E, Galan Y. et al. Overall Survival of Patients with Locally Advanced or Metastatic Esophageal Squamous Cell Carcinoma Treated with Nimotuzumab in the Real World. Advances in therapy. 2017;34:2638-47
35. Jia J, Cui Y, Lu M, Wang X, Li J, Li J. et al. The relation of EGFR expression by immunohistochemical staining and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2016;18:592-8
36. Takeno S, Yamashita SI, Yamamoto S, Takahashi Y, Moroga T, Kawahara K. et al. Number of metastasis-positive lymph node stations is a simple and reliable prognostic factor following surgery in patients with esophageal cancer. Experimental and therapeutic medicine. 2012;4:1087-91
37. Peng J, Wang WP, Dong T, Cai J, Ni PZ, Chen LQ. Refining the Nodal Staging for Esophageal Squamous Cell Carcinoma Based on Lymph Node Stations. Ann Thorac Surg. 2016;101:280-6
38. van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547-57
39. Bodelon C, Anderson GL, Rossing MA, Chlebowski RT, Ochs-Balcom HM, Vaughan TL. Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer prevention research. 2011;4:840-50
40. Wang BJ, Zhang B, Yan SS, Li ZC, Jiang T, Hua CJ. et al. Hormonal and reproductive factors and risk of esophageal cancer in women: a meta-analysis. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2016;29:448-54
41. Su XD, Zhang X, Xie HJ, Lin P, Zhang L, Rong T. Younger women have a better prognosis among patients with esophageal squamous cell carcinoma after esophagectomy. J Thorac Dis. 2016;8:872-9
42. Morita M, Yoshida R, Ikeda K, Egashira A, Oki E, Sadanaga N. et al. Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery. 2008;143:499-508
43. Versteijne E, van Laarhoven HW, van Hooft JE, van Os RM, Geijsen ED, van Berge Henegouwen MI. et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2015;28:453-9
44. Lloyd S, Chang BW. Current strategies in chemoradiation for esophageal cancer. Journal of gastrointestinal oncology. 2014;5:156-65
45. Gupta A, Roy S, Majumdar A, Hazra A, Mallik C. A randomized study to compare sequential chemoradiotherapy with concurrent chemoradiotherapy for unresectable locally advanced esophageal cancer. Indian journal of medical and paediatric oncology: official journal of Indian Society of Medical & Paediatric Oncology. 2014;35:54-9
46. Bhandari V, Gupta KL, Taran R. A comparison of results by sequential and concurrent chemo radiotherapy in locally advanced carcinoma esophagus. Indian journal of cancer. 2013;50:341-4
47. Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Japanese journal of clinical oncology. 2010;40:107-11
48. Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491-524
49. Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World journal of surgery. 2007;31:1107-14
50. Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-11
51. Baxi SH, Burmeister B, Harvey JA, Smithers M, Thomas J. Salvage definitive chemo-radiotherapy for locally recurrent oesophageal carcinoma after primary surgery: retrospective review. Journal of medical imaging and radiation oncology. 2008;52:583-7
52. Shioyama Y, Nakamura K, Ohga S, Nomoto S, Sasaki T, Yamaguchi T. et al. Radiation therapy for recurrent esophageal cancer after surgery: clinical results and prognostic factors. Japanese journal of clinical oncology. 2007;37:918-23
53. Zhang J, Peng F, Li N, Liu Y, Xu Y, Zhou L. et al. Salvage concurrent radio-chemotherapy for post-operative local recurrence of squamous-cell esophageal cancer. Radiation oncology. 2012;7:93
Author contact
![]() Corresponding authors: Prof. Xiaodong Zhang and Prof. Lin Shen, No. 52 Fucheng Road, Haidian District, Peking University Cancer Hospital & Institute, Beijing 100142, China. Tel & Fax: +86-10-88196961; Email: xdz0829com and lin100com.cn
Corresponding authors: Prof. Xiaodong Zhang and Prof. Lin Shen, No. 52 Fucheng Road, Haidian District, Peking University Cancer Hospital & Institute, Beijing 100142, China. Tel & Fax: +86-10-88196961; Email: xdz0829com and lin100com.cn

 Global reach, higher impact
Global reach, higher impact