3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(9):2102-2108. doi:10.7150/jca.28377 This issue Cite
Research Paper
Impact of Age on Risk of Lymph Node Positivity in Patients with Colon Cancer
1. Therapeutics Research Centre, The University of Queensland Diamantina Institute, The University of Queensland, Brisbane, Australia
2. Department of Biliary-Pancreatic Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
3. Department of General Surgery, Changzheng Hospital, Second Military Medical University, Shanghai, China
4. Institute of Health and Biomedical Innovation and School of Biomedical Sciences, Queensland University of Technology, Brisbane, Australia
5. School of Pharmacy and Medical Science, University of South Australia, Adelaide, Australia
*These authors contributed equally to this work.
Received 2018-7-8; Accepted 2019-1-22; Published 2019-5-12
Abstract
Background: Lymph node (LN) positivity is a prognostic indicator in patients with colon cancer regardless of age, and age is an important parameter that impacts therapeutic recommendations. But little is known about the impact of age on LN positivity in patients with colon cancer.
Methods: We analyzed 257,334 patients with colon cancer diagnosed from SEER database. Logistic regression was used to examine the association of age and LN positivity. Poisson regression was used to evaluate whether age was associated with the number of positive LNs.
Results: LN positivity was inversely associated with age (P < .001 for each T stage). Age was predictive of LN positivity after adjustment for number of LNs examined and other covariates (P < .001 for each T stage). Adjusted odds ratios (ORs) for LN positivity for age 20 to 39 vs 80+ were 3.06 for stage T1 (95 % CI, 2.09 to 4.48), 2.46 for stage T2 (95 % CI, 2.00 to 3.02), 1.77 for stage T3 (95 % CI, 1.62 to 1.93), and 1.68 for stage T4 (1.51 to 1.86). Young age was a significant predictor of an increased number of positive LNs (P < .005 for each T stage).
Conclusion: Young age at diagnosis is associated with an increased risk of LN positivity. LN examination and resection could aid younger patients more with detection and removal of metastasis. Guidelines that define postdetection interventions may be needed to limit the overtreatment of older patients, who may be vulnerable to unnecessary tests and treatments.
Keywords: age, lymph node, positivity, colon cancer, prognosis
Introduction
Colon cancer is one of the most common malignancies in the United States. In 2017, an estimated 95,520 new cases were diagnosed [1]. Colon cancer is primarily a disease of the elderly, with the median age at diagnosis in men of 68 and 72 in women [2]. However, it has recently been reported that the incidence of colon cancer in young adults is increasing faster than the old [3, 4]. The incidence rates of colon cancer increased by 1.0% to 2.4% annually since the mid-1980s in adults age 20 to 39 years and by 0.5% to 1.3% since the mid-1990s in adults age 40 to 54 years [3].
The evaluation of lymph nodes (LNs) is critical in staging and treatment recommendations for patients with colon cancer. The LN resection and examination could be beneficial in many cases because it can detect and remove cancers metastasis. On the other hand, overtreatment with this procedure could be harmful, because it may lead to unnecessary biopsy, surgery, and interventions, all of which have associated health risks, especially for the older patients. Although many studies have demonstrated the prognostic significance of LN examined and LN metastases in patients with colon cancer, the impact of age on LN positivity has not been well defined. Several studies revealed that the same surgical procedure on a younger patient will typically result in more LNs being examined [5, 6]. Each additional year of patient age was associated with retrieval of 0.1 fewer LNs [6]. The percentages of patients with positive LNs is also reported to be higher in the young patients compared with the old [7-9]. However, all these previous studies have failed to show a clear and robust association of age and LN positivity, independent of clinical confounders such as T stage and histological type. In this study, we performed descriptive statistics using the SEER database to investigate the impact of age on LN examined, proportion of patients with positive LNs and number of positive LNs for colon cancer patients with all stages and each T stage.
Material and Methods
Data and Study Design
We used the SEER database from 17 population- based cancer registries, currently covering approximately 28% of the US population. Briefly, information collected includes demographics (race, sex, age at diagnosis, year of diagnosis), tumor characteristics (histological type, extension of primary tumor invasion, number of LN examined and positive for metastatic disease), and tumor location. The Ethics Board of Changzheng Hospital, the Second Military Medical determined that this study did not directly involve human participants and, therefore, did not require consent.
Patients
We identified all patients with colon cancer diagnosed between 1988 to 2013. Primary site of colon (C18.0, C18.2 - C18.9) was used. Pathologically staged patients with nonmetastatic adenocarcinoma or signet ring cell carcinoma over the age of 19 years were included. Patients with at least one LN examined were included. All patients included had standard colon cancer operations, based on the SEER coded description of surgical procedures. Local excision and local destruction procedures were excluded because of the lack of expectation of obtaining LNs. Patients with unknown T stage, surgery type, or number of positive LNs were excluded. To eliminate the effect of preoperative radiation on LN harvest and positivity, patients who received radiotherapy prior to surgery were excluded.
Statistical Analysis
Variables
The primary study outcome was LN positivity. Covariates included age, number of LN examined, tumor location, year of diagnosis, tumor grade, sex, and race. In this study, age was included as a categorical variable using 10-year intervals, except for age 20 to 39 years, because of the smaller percentage of patients (1.8 %).
Impact of Age
Differences in patient and tumor characteristics for patients with different age groups were determined using Chi-square tests. Trends in the median number of LN examined by age were estimated using quantile regression models. Trends in LN positivity with age were evaluated with Cochran Armitage trend tests and further stratified by number of LN examined (< 12 and ≥ 12). All analyses were stratified by T stage. In univariate logistic regression analyses with LN positivity as the outcome, the number of LN examined was most predictive of LN positivity as a log-transformed variable, and therefore was utilized in such a way in multivariable analyses. Logistic regression multivariable analyses were performed for each T stage, with LN positivity as the outcome and sex, race (white, black, other), year of diagnosis (1988 to 2003 and 2004 to 2013), age (10 or 20-year intervals), number of LN examined (log transformed), tumor location (4 categories), and grade (I, II, III, IV, or unknown) as covariates. The interaction of age with each covariate was assessed using the covariate-adjusted model. Results are reported as odds ratios with 95% confidence intervals using age ≥ 80 years as the reference category.
Poisson regression
We further assessed whether age was associated with the number of positive LNs in patients with at least one positive LN using Poisson regression. Results of the Poisson regression analyses are presented as rate ratios, using age ≥ 80 years as the reference category. This provides an adjusted estimate of the ratio of the mean number of positive LNs in a specified age group relative to the age ≥ 80 years age group. Evidence of overdispersion was found in initial analysis when examining the mean and variance of the number of positive LNs. To adjust for this, confidence intervals with robust standard errors were estimated, which relaxes the assumption that the variance is equal to the mean. The interaction of age with each covariate was assessed using the covariate-adjusted model. The same variables in the logistic regression models were used for covariate adjustment. We explored this with graphical analysis of the number of LN examined as a categorical variable that indicated that the age association differed in the groups with number of LN examined < 12 vs those with ≥ 12. In the covariate-adjusted multivariable model, we accounted for the interaction by including a categorical variable for age group-LN examined group. We accounted for this interaction in the covariate-adjusted model by including a categorical variable for age-tumor location, using age ≥ 80 years as the reference group. Analyses were done using SAS statistical analysis software, version 9.4. All statistical tests were two-sided, with a 5% type I error.
Results
Table 1 lists the characteristics of the patient population. We identified 257,334 patients who met the eligibility criteria of this study. Most patients were between age 70 and 79 years (30.6 %, n = 78,638). Only 1.8 % of patients were under age 40 years (n = 4,580), with 5.3 % between age 40 and 49 years (n = 13,695) and 13.1 % between age 50 and 59 years (n = 33,725). The greatest proportion of patients were Grade II (68.3 %) and T3 (47.0 %). Most patients had right-sided colon tumors (50.2 %, n = 129,078), with 37.7 % of left-sided (n = 96, 962) and 10.6 % of transverse colon tumors (n = 27,367).
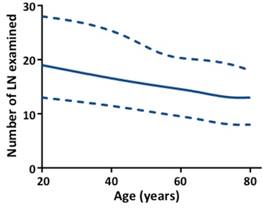
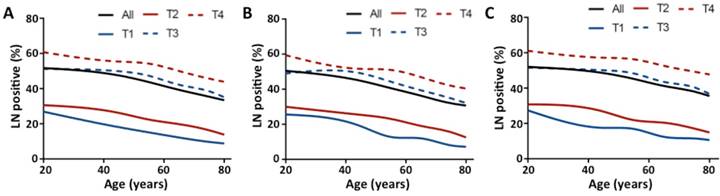
Patients in stage T3 had the highest median number of LN examined (n = 15), and the median number of LN examined was 12, 13, and 12 for stages T1, T2, and T4, respectively (Table S1, see online supporting information). The median number of LN examined decreased as age increased for all stages (Fig 1, P < .0001) and each T stage (Table S1, P < .0001, see online supporting information). For LN positivity, the proportion of patients with at least one positive LN increased by T stage (11.5 %, 17.5 %, 40.4 %, and 48.0 % for stage T1, T2, T3, and T4, respectively). This proportion decreased as age increased for all stages and each T stage (Fig 2A, P < .0001). Then we analyzed the proportion of patients with at least one positive LN by age within each stage, stratified by number of LN examined (< 12 and ≥ 12). As shown in Figs 2B and C, the inverse association between age and LN positivity remained statistically significant for all stages and each T stage (P < .0001). In univariate analyses, younger patients had significantly higher rates of LN positivity for all stages and each T stage (Table 2, P < .0001). In multivariable analyses, age remained a significant predictor of LN positivity for all stages and each T stage (Table 2, P < .0001). Younger patients with early-stage colon cancer were more likely to have LN positivity, with adjusted ORs for age 20 to 39 vs 80+ of 3.06 for stage T1 (95 % CI, 2.09 to 4.48), 2.46 for stage T2 (95 % CI, 2.00 to 3.02), 1.77 for stage T3 (95 % CI, 1.62 to 1.93), and 1.68 for stage T4 (1.51 to 1.86). Other significant predictors of LN positivity in all five models included race, grade, histological type, tumor location, and number of LN examined (Table S2, P < .0001, see online supporting information).
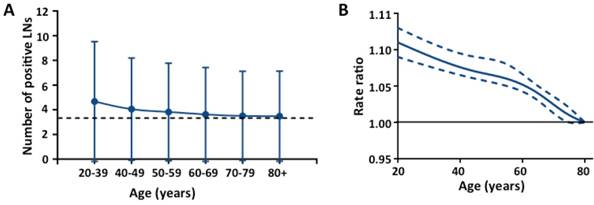
We further assessed the impact of age on the number of positive LNs in patients with at least one positive LN. Fig 3A shows the mean number of positive LNs by age group across all stages. The mean number of positive LNs in the younger age groups were significantly higher than the older age groups for all stages (Fig 3A) and each T stage (Table S3, see online supporting information). In multivariable analyses, the mean number of positive LNs differed by age for all stages after adjusting for covariates (Fig 3B, P < .001) and each T stages (Table S4, P < .001 for T1, T3 and T4; P < .005 for T2, see online supporting information). We then assessed the interaction of age with each covariate in the covariate-adjusted model. For T3, there was a statistically significant interaction between age and tumor location (Table S4b, P < .0001, see online supporting information). For T4, there were statistically significant interactions between age and number of LN examined, and between age and tumor location (Table S4b and S4c, P < .0001, see online supporting information).
Number of LN examined by age for all stages. The upper- and lower-most curves (dashed lines) represent the 25th and 75th number of LN examined and the middle solid line represents the median number of LN examined. Trend was estimated using quantile regression model for all stages (P < .0001).
Discussion
Known clinical and pathologic factors that predict LN positivity in patients with colon cancer include histological type, T stage, and number of LN examined [10-12]. In this large study, we demonstrated that age is another important factor. There is an increased risk of LN positivity in younger patients with colon cancer for all stages and each T stage. In an analysis of 141,411 patients with colon cancer from SEER database, Wang et al. [8] reported that the mean number of positive LNs was higher for patients younger than 40 years old at diagnosis. Similar to our findings, Khan et al. [9] recently analyzed data from 208,077 adult patients with colon cancer from the SEER database, and found that older patients had a significantly lower proportion of node-positive cancers when adequately staged (P < .0001). However, young age has only been reported to increase risk for LN positivity in early-stage rectal cancer (T2-T3) [13], while these studies did not control important factors such as T stage and histological type. Our analysis represents the largest cohort to date of patients with colon cancer (257,334 patients); this afforded us the ability to perform multivariable adjustment for many important factors such as T stage, histological type, number of LN examined and tumor location. Our results suggest that younger patients with colon cancer may have an increased predisposition for LN metastasis for each T stage, excluding the possibilities of easy identification of LN tissue in younger patients during surgery or enriched early stage patients in older population because of cancer screening [13].
More aggressive tumor biology of younger patients could be one possible explanation for our findings. In this study, we found younger patients have more mucinous adenocarcinoma (14.0 % for age 20 to 39 and 11.3 % for age 80+) and signet ring cell carcinoma (3.1 % for age 20 to 39 and 1.2 % for age 80+). Another study also reported that there are more adenocarcinomas with mucinous and signet ring features in young patients with colorectal cancer [14]. These two histological types of colon cancer have more adverse biological features such as invasion and metastasis [15]. Until now, our knowledge regarding the detailed molecular features of sporadic young-onset colon cancer is limited. Recent studies on three major genetic pathways including chromosomal instability [16], microstatellite instability and the cytosine-phosphate-guanine island methylator phenotype pathway [17] in the development of colon cancer support the notion that it may behave more aggressively in younger patients than in older patients, and fit with our findings.
Patient and tumor characteristics by age at diagnosis
| Age at diagnosis, y | |||||||
|---|---|---|---|---|---|---|---|
| 20-39 | 40-49 | 50-59 | 60-69 | 70-79 | 80+ | ||
| Characteristic | No. (%) | Percent (within age group) | |||||
| Age at diagnosis, y | |||||||
| 20-39 | 4,580 (1.8) | ||||||
| 40-49 | 1,3695 (5.3) | ||||||
| 50-59 | 33,725 (13.1) | ||||||
| 60-69 | 56,041 (21.8) | ||||||
| 70-79 | 78,638 (30.6) | ||||||
| 80+ | 70,655 (27.5) | ||||||
| Sex | |||||||
| Male | 134,367 (52.2) | 47.7 | 48.7 | 46.5 | 46.5 | 51.2 | 61.6 |
| Female | 122,967 (47.8) | 52.3 | 51.3 | 53.5 | 53.5 | 48.8 | 38.4 |
| Year of diagnosis | |||||||
| 1988 to 2003 | 125,922 (48.9) | 44.8 | 43.1 | 41.9 | 48.4 | 53.7 | 48.8 |
| 2004 to 2013 | 131,412 (51.1) | 55.2 | 56.9 | 58.1 | 51.6 | 46.3 | 51.2 |
| Race | |||||||
| White | 211,894 (82.3) | 71.9 | 73.0 | 75.2 | 79.5 | 84.2 | 88.4 |
| Black | 25,662 (10.0) | 14.4 | 15.8 | 15.1 | 12.1 | 8.7 | 5.8 |
| Other | 19,778 (7.7) | 13.7 | 11.2 | 9.7 | 8.4 | 7.1 | 5.8 |
| Grade | |||||||
| I | 19,228 (7.5) | 6.1 | 7.0 | 7.7 | 7.9 | 7.6 | 7.0 |
| II | 178,313 (69.3) | 64.6 | 70.4 | 71.5 | 70.7 | 69.3 | 67.2 |
| III | 49,332 (19.2) | 24.1 | 18.5 | 16.7 | 17.5 | 19.0 | 21.7 |
| IV | 3,582 (1.4) | 2.3 | 1.3 | 1.2 | 1.2 | 1.4 | 1.6 |
| Unknown | 6,879 (2.7) | 2.9 | 2.8 | 2.8 | 2.8 | 2.7 | 2.4 |
| Histological type | |||||||
| Adenocarcinoma | 227,111 (88.3) | 82.9 | 87.3 | 89.4 | 89.2 | 88.2 | 87.5 |
| Mucinous adenocarcinoma | 27,303 (10.6) | 14.0 | 11.2 | 9.6 | 9.8 | 10.7 | 11.3 |
| Signet ring cell carcinoma | 2,920 (1.1) | 3.1 | 1.5 | 1.0 | 1.0 | 1.1 | 1.2 |
| LNE | |||||||
| < 12 | 100,749 (39.2) | 19.2 | 27.2 | 32.6 | 38.5 | 42.3 | 42.9 |
| ≥ 12 | 156,585 (60.8) | 80.8 | 72.8 | 67.4 | 61.5 | 57.7 | 57.1 |
| T Stage | |||||||
| T1 | 14,029 (5.5) | 3.4 | 4.0 | 6.3 | 6.2 | 5.9 | 4.3 |
| T2 | 40,168 (15.6) | 10.3 | 12.5 | 14.7 | 15.8 | 16.7 | 15.6 |
| T3 | 120,924 (47.0) | 50.2 | 50.3 | 49.0 | 46.4 | 44.7 | 48.2 |
| T4 | 82,213 (31.9) | 36.1 | 33.2 | 30.0 | 31.6 | 32.6 | 31.9 |
| Tumor location | |||||||
| Right | 129,078 (50.2) | 39.1 | 38.0 | 40.1 | 46.7 | 52.5 | 58.2 |
| Left | 96,962 (37.7) | 47.9 | 50.7 | 48.8 | 41.9 | 35.2 | 28.6 |
| Transverse | 27,367 (10.6) | 11.2 | 9.6 | 9.6 | 10.0 | 10.7 | 11.7 |
| Unknown | 3,927 (1.5) | 1.8 | 1.7 | 1.5 | 1.4 | 1.5 | 1.6 |
Characteristics differ by age group, Chi-square tests; all P < .0001. Abbreviations: LNE = number of lymph nodes examined.
Association of age and LN positivity
| OR (95%CI) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | T1 | T2 | T3 | T4 | ||||||||||
| Age at diagnosis, y | Unadjusted | Adjusted for covariates | Unadjusted | Adjusted for covariates | Unadjusted | Adjusted for covariates | Unadjusted | Adjusted for covariates | Unadjusted | Adjusted for covariates | ||||
| 20-39 | 2.13 (2.01 to 2.26) | 1.83 (1.72 to 1.94) | 3.81 (2.62 to 5.54) | 3.06 (2.09 to 4.48) | 2.75 (2.24 to 3.37) | 2.46 (2.00 to 3.02) | 1.94 (1.79 to 2.12) | 1.77(1.62 to 1.93) | 1.97 (1.78 to 2.18) | 1.68 (1.51 to 1.86) | ||||
| 40-49 | 1.80 (1.74 to 1.87) | 1.67 (1.61 to 1.74) | 2.28 (1.77 to 2.93) | 1.97 (1.53 to 2.55) | 2.22 (1.97 to 2.50) | 2.06 (1.83 to 2.33) | 1.84 (1.75 to 1.94) | 1.75(1.66 to 1.85) | 1.58 (1.49 to 1.69) | 1.47 (1.37 to 1.57) | ||||
| 50-59 | 1.55 (1.50 to 1.59) | 1.49 (1.45 to 1.54) | 1.84 (1.55 to 2.19) | 1.67 (1.40 to 1.99) | 1.80 (1.65 to 1.96) | 1.72 (1.58 to 1.88) | 1.66 (1.60 to 1.73) | 1.61(1.55 to 1.68) | 1.51 (1.44 to 1.58) | 1.46 (1.39 to 1.53) | ||||
| 60-69 | 1.29 (1.26 to 1.32) | 1.27 (1.24 to 1.30) | 1.44 (1.22 to 1.69) | 1.38 (1.17 to 1.62) | 1.54 (1.43 to 1.66) | 1.51 (1.40 to 1.63) | 1.35 (1.31 to 1.40) | 1.35(1.30 to 1.39) | 1.28 (1.23 to 1.33) | 1.27 (1.22 to 1.32) | ||||
| 70-79 | 1.09 (1.07 to 1.12) | 1.09 (1.06 to 1.11) | 1.11 (0.94 to 1.30) | 1.09 (0.93 to 1.27) | 1.22 (1.14 to 1.31) | 1.21 (1.13 to 1.30) | 1.16 (1.12 to 1.19) | 1.16(1.13 to 1.20) | 1.08 (1.04 to 1.12) | 1.09 (1.05 to 1.13) | ||||
| 80+ | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
Chi-square tests for age group variable in each logistic regression model; all P < .0001. Abbreviations: CI = confidence interval; Ref = referent; OR = odds ratio.
Proportion of patients with at least one positive LN by age and T stage. (A) All patients; (B) Group with number of LN examined < 12; (C) Group with number of number of LN examined ≥ 12. Trend was estimated using Cochran Armitage trend tests for all stages and each T stage. P < .0001 for all stages and each T stage.
Association of age and number of positive LNs in patients with at least one positive LN for all stages. (A) Mean number of positive LNs with standard deviation in different age groups; (B) Smoothed restricted cubic spline plot of the rate ratio (RR) versus age. RRs were estimated from Poisson regression models adjusting for covariates including number of LN examined, race, grade, histological type, tumor location, and year of diagnosis. The upper- and lower-most curves (dashed lines) represent the 95% CIs about the RRs (middle solid line).
For colorectal cancer, LN status is one of the most relevant factors dictating the need for adjuvant chemotherapy [18, 19]. Our findings suggest that, when adequately staged, older patients are less likely to have colon cancer with positive LNs. It has been reported that the cancer-specific survival was similar regardless of age in adequately staged patients [8, 9]. Thus, a diligent oncologic resection and pathologic LN examination may spare the older subsequent recommendation for adjuvant chemotherapy because of less LN positivity. Our study also suggests that younger patients with early-stage colon cancer may also be at an increased risk of harboring occult metastatic disease in the regional LNs. This finding may affect the preoperative workup for these patients being considered for polypectomy and local excision, as these procedures does not include LN dissection [20]. However, our study could not be interpreted as justification for a change in management of older patients. Clinical staging, typically done with CT or MRI, is not available for the patients in this dataset, thereby limiting the direct application of these data to clinical practice.
Our study has a number of strengths and novel components. First, this analysis included more recent SEER data up to 2013. We are among the first to evaluate population-based incidence trends applying the new AJCC 8th edition tumor staging to our analysis, which allowed us to add new comments on prognosis under these new guidelines. The large sample size of SEER 17 allowed for more precise risk estimates than previous studies of colon cancer that used SEER 9 to 12; this allowed us to include several potential covariates in our multivariate analysis. Our study is limited by the possibility of coding errors of the SEER database. However, any such miscoding would be expected to be random and not introduce any systematic bias [21]. Data regarding the extent of LN involvement, such as size of the LN metastases and LN capsular invasion were not available, nor were details on LN compartmental dissection. Thus, conclusions regarding extent of LN examined or exact location of the LNs cannot be drawn. The present study also has drawbacks resulting from long study interval (approximately 25 years) and retrospective nature. The operation that patients received varied depending on the year of diagnosis and other clinical factors, such as the experience and judgment of each surgeon. Most of these factors were accounted for in the multivariable analysis, but it is possible that the clinical approach to a young patient may be more aggressive than one who is older, introducing systematic bias.
In conclusion, our analysis of the most recent years of data available in the SEER database show that younger patients with colon cancer have increased rates of positive LNs, after accounting for other known predictive factors. This persists in each T stage, and young patients with at least one positive LN have higher LN ratios. Our results may guide clinicians in optimal treatment selection, considering the different aggressiveness of nodal staging in patients with different age groups. LN examination and resection could aid younger patients more with detection and removal of metastasis. Guidelines that define postdetection interventions may be needed to limit the overtreatment of older patients, who may be vulnerable to unnecessary tests and treatments. Further study is needed to identify which age-subsets of patients derive most potential benefit from current management recommendations.
Supplementary Material
Supplementary tables.
Abbreviations
LN: Lymph node; ORs: Odds ratios; RR: Rate ratio.
Acknowledgements
This work was supported by grants from Australian National Health and Medical Research Council (APP1125794) and National Natural Science Foundation of China (81402002).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
2. Miller KD, Siegel RL, Lin CC. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-89
3. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS. et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;8:109-14
4. Ahnen DJ, Wade SW, Jones WF. et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216-24
5. Sarli L, Bader G, Iusco D. et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272-9
6. Ostadi MA, Harnish JL, Stegienko S. et al. Factors affecting the number of lymph nodes retrieved in colorectal cancer specimens. Surg Endosc. 2007;21:2142-6
7. Domergue J, Ismail M, Astre C. et al. Colorectal-Carcinoma in Patients Younger Than 40-Years-of-Age - Montpellier-Cancer-Institute Experience with 78 Patients. Cancer. 1988;61:835-40
8. Wang L, Hollenbeak CS, Stewart DB. Node yield and node involvement in young colon cancer patients: is there a difference in cancer survival based on age? J Gastrointest Surg. 2010;14:1355-61
9. Khan H, Olszewski AJ, Somasundar P. Lymph node involvement in colon cancer patients decreases with age; a population based analysis. Eur J Surg Oncol. 2014;40:1474-80
10. Glasgow SC, Bleier JIS, Burgart LJ. et al. Meta-analysis of Histopathological Features of Primary Colorectal Cancers that Predict Lymph Node Metastases. J Gastrointest Surg. 2012;16:1019-28
11. Baxter NN, Ricciardi R, Simunovic M. et al. An Evaluation of the Relationship Between Lymph Node Number and Staging in pT3 Colon Cancer Using Population-Based Data. Dis Colon Rectum. 2010;53:65-70
12. Parsons HM, Tuttle TM, Kuntz KM. et al. Virnig BA. Association Between Lymph Node Evaluation for Colon Cancer and Node Positivity Over the Past 20 Years. JAMA. 2011;306:1089-97
13. Meyer JE, Cohen SJ, Ruth KJ. et al. Young Age Increases Risk of Lymph Node Positivity in Early-Stage Rectal Cancer. J Natl Cancer Inst. 2016;108:284-290
14. You YN, Xing Y, Feig BW. et al. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch Intern Med. 2012;172:287-9
15. Fleming M, Ravula S, Tatishchev SF. et al. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-73
16. Deen KI, Silva H, Deen R. et al. Colorectal cancer in the young, many questions, few answers. World J Gastrointest Oncol. 2016;8:481-8
17. Buecher B, Cacheux W, Rouleau E. et al. Role of microsatellite instability in the management of colorectal cancers. Dig Liver Dis. 2013;45:441-9
18. Sargent DJ, Andre T, Grothey A. Further Evaluating the Benefit of Adjuvant Chemotherapy for Colon Cancer. J Clin Oncol. 2016;34:3711-2
19. Norderval S, Solstad OB, Hermansen M. et al. Increased lymph node retrieval decreases adjuvant chemotherapy rate for stage II colon cancer. Scand J Gastroenterol. 2016;51:949-55
20. Bhangu A, Brown G, Nicholls RJ. et al. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg. 2013;258:563-9 discussion 9-71
21. Adam MA, Pura J, Goffredo P. et al. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol. 2015;33:2370-66
Author contact
![]() Corresponding author: Xinxing Li, MD, Department of General Surgery, Changzheng Hospital, The Second Military Medical University, Shanghai, 200003, China. (telephone: 61-07-3443-8033; email: xingxin123456com).
Corresponding author: Xinxing Li, MD, Department of General Surgery, Changzheng Hospital, The Second Military Medical University, Shanghai, 200003, China. (telephone: 61-07-3443-8033; email: xingxin123456com).

 Global reach, higher impact
Global reach, higher impact