Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(10):2243-2249. doi:10.7150/jca.31473 This issue Cite
Research Paper
Perineural and lymphovascular invasion predicts for poor prognosis in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery
1. Department of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China
2. Zhejiang Key Laboratory of Radiation Oncology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China
3. Department of Colorectal Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China
4. Department of Pathology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China
5. Department of Radiology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang Province, China
* These authors contributed equally to this work
Received 2018-11-13; Accepted 2019-3-30; Published 2019-5-21
Abstract
Background: Perineural invasion (PNI) and lymphovascular invasion (LVI) are associated with poor prognosis in colorectal cancer, but their clinical significance is still controversial for patients with locally advanced rectal cancer (LARC) who had received neoadjuvant chemoradiotherapy (nCRT) and surgical resection. The aim of this study was to confirm the correlation between PNI and/or LVI and clinical prognosis and to further confirm whether PNI and/or LVI can be used as potential prognostic indicators of adjuvant chemotherapy after nCRT and surgery in LARC.
Methods: From February 2002 to December 2012, a total of 181 patients with LARC who had received nCRT and surgical resection were retrospectively reviewed. Overall survival (OS) and disease-free survival (DFS) were determined by the Kaplan-Meier method, log-rank test, and Cox proportional hazard regression model.
Results: The median follow-up time was 48 months (range, 3 to 162 months). All the PNI-positive and/or LVI-positive patients showed adverse DFS and OS (P<0.001). In multivariate analysis, PNI and LVI were independent prognostic factors for DFS. PNI, rather than LVI, was also an independent prognostic factor for OS. In a subgroup analysis, PNI-positive, rather than LVI-positive, may benefit from adjuvant chemotherapy.
Conclusion: For patients with LARC undergoing nCRT and surgery, PNI-positive and/or LVI positive were associated with poorer DFS and OS. And PNI-positive, rather than LVI-positive, may benefit from adjuvant chemotherapy.
Keywords: locally advanced rectal cancer, perineural invasion, lymphovascular invasion, neoadjuvant therapy
Introduction
Preoperative neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) was the standard of care for locally advanced rectal cancer (LARC, T3-4/N+) in the past decade [1,2]. Fluorouracil-based postoperative adjuvant chemotherapy has been recommended for all patients undergoing preoperative chemoradiotherapy by National Comprehensive Cancer Network (NCCN) guidelines [3]. However, the benefit of adjuvant chemotherapy for all patients has been controversial over the past few years. EORTC 22921 trial demonstrated no significant benefit for chemotherapy in LARC after nCRT or preoperative radiation alone [4]. Other studies reported significantly increased survival with postoperative chemotherapy [5]. ESMO guidelines suggested that the decision of postoperative chemotherapy should balance the risk of relapse for a particular patient and the predicted toxicity [6]. Thus, identifying the certain subsets of patients who may benefit from adjuvant chemotherapy will help to optimize the modality of treatment as well as diminish the toxicities caused by chemotherapy.
Two pathologic features that have been shown to be correlated with outcome in CRC are perineural invasion (PNI) and lymphovascular invasion (LVI). PNI is a pathologic process of tumor invasion of nervous structures, and it spreads along nerve sheaths [7]. LVI is defined as tumor cells invasion in the small lymphatic or blood vessels [8]. Studies have indicated that both of these pathologic features are the prognostic factors in rectal cancer [9-11]. Rectal cancer patients with PNI or LVI positive usually have a high incidence of local recurrence and distant metastasis [11,12]. However, the role of these two pathologic variables in predicting the prognosis of LARC patients has been still controversial. Several studies demonstrated that PNI or LVI is not related markedly to the poor prognosis after curative resection of colorectal cancers [13,14]. In this study, we retrospectively evaluated the clinical significance of PNI and LVI as indicators of adjuvant chemotherapy after nCRT and surgery in LARC.
Methods
Patient selection
The records of 204 consecutive patients with LARC (clinically T3-4 and/or node positive) who underwent nCRT followed by surgical resection at the Zhejiang Cancer Hospital between February 2002 and December 2012 were retrospectively reviewed. Data pertaining to demographics, staging, tumor markers, pathology, treatment, and outcomes were collected for each patient. Twelve patients were removed because metastatic cancer was found during the course of surgery. Eleven patients were excluded due to the limited follow-up information. Finally, a total of 181 patients were available for analysis. This study was approved by the Independent Ethics Committee of Zhejiang Cancer Hospital.
Staging and treatment
All patients were restaged according to the AJCC 2010 staging system. Chest, abdominal, and pelvic CT scan, pelvic MRI with contrast or endorectal ultrasound were performed for tumor, nodal, and metastasis staging. The main neoadjuvant for chemotherapy was 5-Fu or capecitabine-based chemotherapeutic regimen. A dose of 40-45 Gy radiotherapy was delivered to the whole pelvis in 1.8 Gy daily fractions, followed by a 5.4 Gy boost to the primary tumor in 3 fractions. Radical proctectomy, including abdominoperineal resection (APR), low anterior resection (LAR), and Hartmann's procedure, was performed 6 to 10 weeks after completion of nCRT. XELOX or FOLFOX6 postoperative adjuvant chemotherapeutic regimen was recommended for all patients 4 weeks after surgery. However, only 102 patients (56.4%) received adjuvant chemotherapy. Due to postoperative complications, economic problems or other reasons, the other 79 patients (43.6%) did not receive adjuvant chemotherapy.
Histopathologic analysis
For each patient included in this study, standard pathologic analysis by a pathologist with expertise in colorectal cancer was performed on all surgical specimens. Pathologic features, such as tumor size, tumor infiltration, number of positive lymph nodes, histologic grade, extranodal extension, PNI, LVI, as well as other features included in the standard protocol were documented. The widely accepted definition of PNI-positive is inclusive of two aspects: at least one-third of the nerve is surrounded by tumor cells without invasion of the nerve sheath (SS-PNI), as well as any of the three layers of the nerve sheath is invaded by tumor cells (TS-PNI) [15,16]. Studies have indicated that patients in the SS-PNI subgroup and TS-PNI subgroup have a similar 5-year local recurrence rate [17]. In our study, both SS-PNI and TS-PNI were defined as PNI-positive. LVI was defined as tumor invasion in the vascular and lymphatic structure. Since it is infeasible to distinguish histologically between lymphatic and venous vessels, the term LVI usually refers to any of these structures [18].
Statistical analysis
Associations between clinical and pathological characteristics of the tumor were assessed by the chi-square test. Disease-free survival (DFS) was defined as the time from the completion of surgery to any relapse or death. Overall survival (OS) was calculated from the date of diagnosis to the date of death. DFS was censored at the time of the last follow-up for disease-free or non-cancer-related death patients. OS was censored at the time of the last follow-up for alive patients. The influence of PNI or LVI on DFS and OS was conducted using the Kaplan-Meier survival analysis and log-rank tests. Multivariate analysis was performed using Cox proportional hazard regression. A P-value< 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL).
Results
Patient characteristics
The characteristics of the 181 LARC patients are shown in Table 1. The median follow-up time was 48 months (range, 3 to 162 months). Median patient age at the time of diagnosis was 51-year-old (range, 23 to 76-year-old). In our cohort, 44 patients (24.3%) and 137 patients (75.7%) were PNI positive and PNI negative, respectively, and 26 patients (14.4%) and 155 patients (85.6%) were LVI positive and negative, respectively. There were statistically significant differences in nodal status between PNI+ and PNI- groups (P=0.033) or LVI+ and LVI- groups (P=0.020). No significant differences were observed in other demographic and treatment-related characteristics of PNI or LVI groups.
Clinical and Treatment-related Characteristics by PNI and LVI status
| Characteristic | PNI+ N=44 (24.3%) | PNI- N=137 (75.7%) | P | LVI+ N=26 (14.4%) | LVI- N=155 (85.6%) | P | |
|---|---|---|---|---|---|---|---|
| Age (median, 51) | 0.153 | 0.193 | |||||
| ≤51 | 18 (40.9) | 73 (53.3) | 10 (38.5) | 81 (52.3) | |||
| >51 | 26 (59.1) | 64 (46.7) | 16 (61.5) | 74 (47.7) | |||
| Sex | 0.603 | 0.826 | |||||
| Male | 30 (68.2) | 99 (72.3) | 19 (73.1) | 110 (71.0) | |||
| Female | 14 (31.8) | 38 (27.7) | 7 (26.9) | 45 (29.0) | |||
| Tumor location | 0.860 | 0.776 | |||||
| ≤5cm | 26 (59.1) | 83 (60.6) | 15 (57.7) | 94 (60.6) | |||
| >5cm | 18 (40.9) | 54 (39.4) | 11 (42.3) | 61 (39.4) | |||
| CEA (ng/ml) | 0.768 | 0.524 | |||||
| ≤5 | 22 (50.0) | 72 (52.6) | 12 (46.2) | 82 (52.9) | |||
| >5 | 22 (50.0) | 65 (47.4) | 14 (53.8) | 73 (47.1) | |||
| Radiation dose (Gy) | 0.685 | 0.905 | |||||
| ≤45.0 | 3 (6.8) | 12 (8.8) | 2 (7.7) | 13 (8.4) | |||
| >45.0 | 41 (93.2) | 125 (91.2) | 24 (92.3) | 142 (91.6) | |||
| Tumor stage | 0.537 | 0.053 | |||||
| T3 | 16 (36.4) | 57 (41.6) | 6 (23.1) | 67 (43.2) | |||
| T4 | 28 (63.6) | 80 (58.4) | 20 (76.9) | 88 (56.8) | |||
| Nodal status | 0.033 | 0.020 | |||||
| Negative | 15 (34.1) | 72 (52.6) | 7 (26.9) | 80 (51.6) | |||
| Positive | 29 (65.9) | 65 (47.4) | 19 (73.1) | 75 (48.4) | |||
| Adjuvant Chemotherapy | 0.119 | 0.238 | |||||
| No XELOX FOLFOX | 22 (50.0) 18 (40.9) 4 (9.1) | 57 (41.6) 48 (35.0) 32 (23.4) | 15 (57.7) 6 (23.1) 5 (19.2) | 64 (41.3) 60 (38.7) 31 (20.0) |
PNI, perineural invasion; LVI, Lymphovascular Invasion; CEA, carcinoembryonic antigen.
Pathologic characteristics after neoadjuvant chemoradiotherapy
Pathologic features of our cohort are listed in Table 2. There were statistically significant differences in ypT stage, ypN stage, AJCC stage, and resection level completeness between PNI+ and PNI- groups or LVI+ and LVI- groups (all P<0.05). We observed a statistically significant difference between PNI+ and PNI- groups, rather than LVI+ or LVI- groups, with regard to tumor differentiation (P=0.002). There were no statistically significant differences in pathologic type between PNI or LVI groups.
Pathologic Features After Neoadjuvant Chemoradiation by PNI and LVI status
| Characteristic | PNI+ N=44 (24.3%) | PNI- N=137 (75.7%) | P | LVI+ N=26 (14.4%) | LVI- N=155 (85.6%) | P | |
|---|---|---|---|---|---|---|---|
| ypT stage | <0.001 | 0.001 | |||||
| T0-2 | 5 (11.4) | 71 (51.8) | 3 (11.5) | 73 (47.1) | |||
| T3-4 | 39 (88.6) | 66 (48.2) | 23 (88.5) | 82 (52.9) | |||
| ypN stage | <0.001 | <0.001 | |||||
| N0 | 6 (13.6) | 95 (69.3) | 4 (15.4) | 97 (62.6) | |||
| N1 | 24 (54.5) | 31 (22.6) | 12 (46.2) | 43 (27.7) | |||
| N2 | 14 (31.8) | 11 (8.0) | 10 (38.5) | 15 (9.7) | |||
| AJCC stage | <0.001 | <0.001 | |||||
| 0 | 0 (0.0) | 37 (27.0) | 0 (0.0) | 37 (23.9) | |||
| 1 | 0 (0.0) | 20 (14.6) | 0 (0.0) | 20 (12.9) | |||
| 2 | 7 (15.9) | 38 (27.7) | 4 (15.4) | 41 (26.5) | |||
| 3 | 37 (84.1) | 42 (30.7) | 22 (84.6) | 57 (36.8) | |||
| Pathologic type | 0.756 | 0.466 | |||||
| Adenocarcinoma | 37 (84.1) | 118 (86.1) | 22 (84.6) | 133 (85.8) | |||
| Mucinous adenocarcinoma | 6 (13.6) | 14 (10.2) | 4 (15.4) | 16 (10.3) | |||
| Signet-ring cell carcinoma | 1 (2.3) | 5 (3.6) | 0 (0.0) | 6 (3.9) | |||
| Differentiation | 0.002 | 0.081 | |||||
| Well | 0 (0.0) | 3 (2.2) | 0 (0.0) | 3 (1.9) | |||
| Moderately | 21 (47.7) | 76 (55.5) | 11 (42.3) | 86 (55.5) | |||
| Poorly | 23 (52.3) | 37 (27.0) | 14 (53.8) | 46 (29.7) | |||
| Other | 0 (0.0) | 21 (15.3) | 1 (3.8) | 20 (12.9) | |||
| Resection level completeness | 0.011 | 0.041 | |||||
| R0 | 40 (90.9) | 136 (99.3) | 24 (92.3) | 152 (98.1) | |||
| R1 | 3 (6.8) | 1 (0.7) | 1 (3.8) | 3 (1.9) | |||
| R2 | 1 (2.3) | 0 (0.0) | 1 (3.8) | 0 (0.0) |
PNI, perineural invasion; LVI, Lymphovascular Invasion.
Role of PNI and LVI as prognostic factors of outcome in LARC patients
Univariate analyses were performed to investigate the prognostic significance of PNI and LVI as well as other pathologic and clinical variables. As shown in Table 3, PNI or LVI positive was significantly associated with poorer DFS and OS (all P-value<0.001). In addition, patients with ypT3-4 stage had poorer DFS (P<0.001) and OS (P<0.001). Likewise, patients with positive pN status also had poorer DFS (P<0.001) and OS (P<0.001). The other variables investigated in our univariate analysis did not significantly affect the outcome.
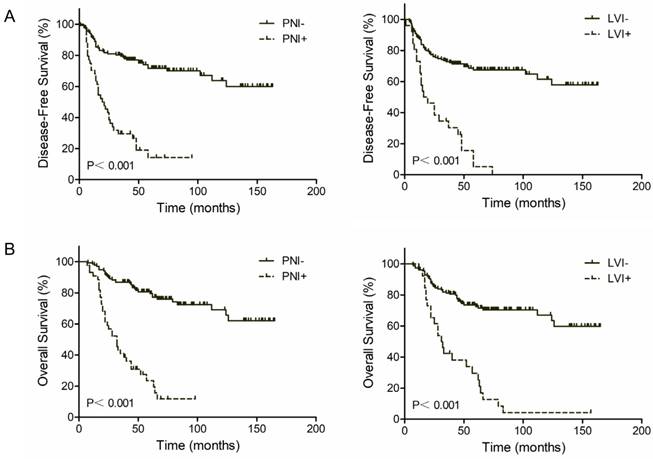
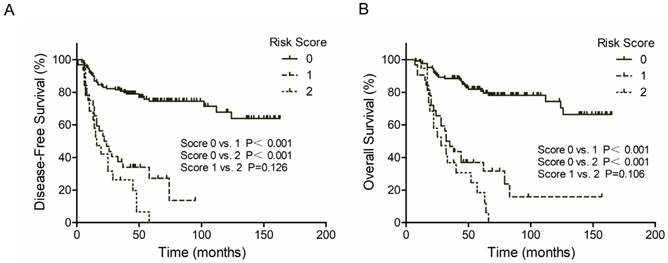
The 3-year DFS rate was 76.8% for PNI negative patients and 26.2% for PNI positive patients (P<0.001, Figure 1A, Left). The 3-year OS rate was 82.8% for PNI negative patients and 31.0% for PNI positive patients (P<0.001, Figure 1B, Left). Similar results were found in LVI groups. Patients with LVI negative status exhibited a two-fold increase in 3-year DFS and OS compared with patients with LVI positive status (71.2% vs. 25.0% and 75.5% vs. 38.3%, respectively; P<0.001, Figure 1A and 1B, Right). However, patients with both PNI and LVI positive did not get a further worse outcome. The 3-year DFS rate was 33.6% for PNI-LVI+ or PNI+LVI- patients and 18.9% for both PNI and LVI positive patients (P=0.126, Figure 2A). The 3-year OS rate was 36.8% for PNI-LVI+ or PNI+LVI- patients and 31.2% for both PNI and LVI positive patients (P=0.106, Figure 2B). The 3-year DFS and OS for both PNI and LVI negative patients were 78.8% and 84.1%, respectively (Figure 2).
Univariate analysis for disease-free and overall survival
| Univariate analysis | Disease-free survival | Overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| PNI | |||||
| Positive vs. negative | 4.865 (3.023-7.831) | <0.001 | 6.350 (3.852-10.467) | <0.001 | |
| LVI | |||||
| Positive vs. negative | 4.506 (2.738-7.413) | <0.001 | 4.634 (2.808-7.645) | <0.001 | |
| ypT stage | |||||
| T0-2 vs. T3-4 | 3.556 (2.042-6.191) | <0.001 | 4.950 (2.595-9.441) | <0.001 | |
| ypN stage | |||||
| N0 vs. N+ | 3.767 (2.300-6.170) | <0.001 | 5.079 (2.972-8.679) | <0.001 | |
| Tumor location | |||||
| ≤5cm vs. >5cm | 1.189 (0.752-1.880) | 0.458 | 1.220 (0.757-1.964) | 0.414 | |
| CEA (ng/ml) | |||||
| ≤5 vs. >5 | 1.359 (0.862-2.141) | 0.186 | 1.449 (0.901-2.331) | 0.126 | |
| Adjuvant Chemotherapy | |||||
| Yes vs. No | 0.758 (0.481-1.193) | 0.231 | 0.702 (0.437-1.129) | 0.144 | |
PNI, perineural invasion; LVI, Lymphovascular Invasion; CEA, carcinoembryonic antigen.
PNI or LVI is an independent prognostic factor of outcome in LARC patients
A Cox proportional hazard regression model was used to evaluate the influence of PNI, LVI, and other relevant covariates on survival. After controlling age, ypT stage, ypN stage, tumor distance from the anal verge, CEA level, and use of adjuvant chemotherapy, multivariate analysis indicated that PNI and LVI were independent prognostic factors for DFS (all P-value<0.05). PNI, rather than LVI, was also an independent prognostic factor for OS (P<0.001) (Table 4). ypT stage and ypN stage also remained independent prognostic factors for DFS (all P-value<0.05) and OS (all P-value<0.05) (Table 4).
Multivariate analysis for disease-free and overall survival
| Multivariate analysis | Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| PNI | ||||||
| Positive vs. negative | 2.115 (1.163-3.848) | 0.014 | 2.936 (1.669-5.164) | <0.001 | ||
| LVI | ||||||
| Positive vs. negative | 1.932 (1.096-3.405) | 0.023 | — | — | ||
| ypT stage | ||||||
| T0-2 vs. T3-4 | 3.149 (1.614-6.143) | 0.001 | 3.006 (1.538-5.876) | 0.001 | ||
| ypN stage | ||||||
| N0 vs. N+ | 2.322 (1.238-4.355) | 0.009 | 2.561 (1.387-4.729) | 0.003 | ||
PNI, perineural invasion; LVI, Lymphovascular Invasion.
Kaplan-Meier survival curves according to perineural (PNI) and lymphovascular invasion (LVI). (A) DFS; (B) OS.
Kaplan-Meier survival curves according to risk score. (A) DFS; (B) OS. Score 0 represent both PNI and LVI negative; Score 1 represent PNI+/LVI- or PNI-/LVI+; Score 2 represent both PNI and LVI positive.
Kaplan-Meier survival curves according to PNI, LVI and adjuvant chemotherapy. (A) DFS; (B) OS.
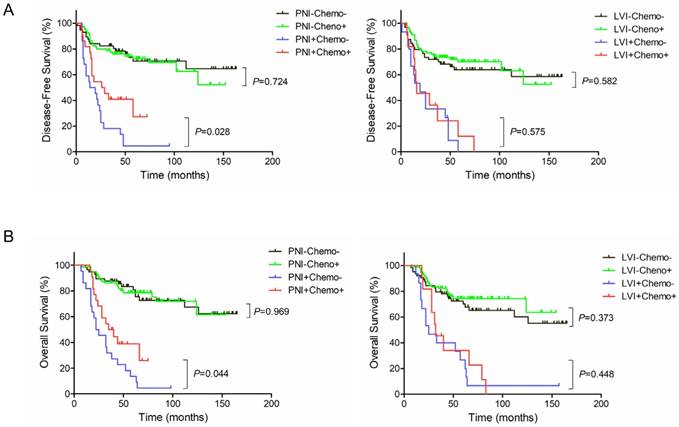
Adjuvant chemotherapy in a subgroup by PNI or LVI
In PNI-positive subgroup, postoperative adjuvant chemotherapy improved 3-year DFS rate (40.3% vs. 13.6%, P=0.028) and 3-year OS rate (39.5% vs. 22.7%, P=0.044) (Figure 3A and 3B, Left). In PNI-negative subgroup, no statistically significant differences were observed between with and without adjuvant chemotherapy in 3-year DFS (75.9% vs. 78.2%, P=0.724) and 3-year OS (80.6% vs. 85.8%, P=0.969) (Figure 3A and 3B, Left). However, in LVI-positive subgroup, postoperative adjuvant chemotherapy did not significantly improve 3-year DFS rate (23.6% vs. 25.9%, P=0.575) or 3-year OS rate (35.4% vs. 40.0%, P=0.448) (Figure 3A and 3B, Right). Likewise, there were no statistically significant differences between with and without adjuvant chemotherapy in 3-year DFS (73.5% vs. 68.0%, P=0.582) and 3-year OS (76.2% vs. 74.5%, P=0.373) in LVI-negative subgroup (Figure 3A and 3B, Right).
Discussion
In our study, we evaluated the significance of PNI and LVI as prognostic indicators in LARC after nCRT and surgery. Positive incisal margin, advanced ypT and ypN positive status were associated with PNI and LVI. Patients with PNI and/or LVI positive had a significantly higher risk of recurrence and poorer survival. Multivariate analysis indicated that PNI and LVI were independent prognostic factors of outcome in LARC patients. In addition, PNI-positive, rather than LVI-positive, may benefit from postoperative adjuvant chemotherapy.
PNI refers to tumor invasion of the space surrounding a nerve and has been investigated in many cancers, including prostate, head and neck cancer, and colorectal cancer [10,19-21]. LVI is defined as tumor invasion in the vascular and lymphatic structure. Since it is infeasible to distinguish histologically between lymphatic and venous vessels, the term LVI usually refers to any of these structures [18]. In the current study, the incidence of PNI and LVI was 24.3% and 14.4%, respectively, which is in-line with previous reports in preoperative CRT patients [22-24]. However, in untreated tumors, higher incidence of PNI and LVI of 30% and 70.6%, respectively, were reported [25,26]. In these previous studies, multivariate analysis indicated that PNI or LVI was an independent negative prognosticator of survival. However, other two studies demonstrated that PNI had an impact on the DFS, but not the OS, based on multivariate analysis [11,27]. In our univariate analysis, we found that patients with PNI-positive or LVI-positive had poorer DFS and OS. In our multivariate analysis, PNI was a significant prognosticator affecting the DFS and OS, and LVI had an impact only on the DFS. In addition, subgroup analysis showed that in PNI-positive group, postoperative adjuvant chemotherapy improved DFS and OS. However, in LVI-positive group, postoperative adjuvant chemotherapy had no impact on DFS or OS. Lower incidence of LVI or the small sample size of our study may account for these results.
In our analysis, advanced ypT and ypN stage were significant factors associated with PNI and LVI in LARC after nCRT. However, studies have indicated that LVI was not significantly associated with the pT or pN stage in rectal cancer patients after preoperative radiotherapy rather than nCRT [28]. Thus, further studies may be conducted to address this controversial problem.
The present study has certain limitations, such as its retrospective nature, and therefore, the treatment delivery could not be controlled for the heterogeneity. Furthermore, the pathologists did not evaluate the pathologic specimens independently, which might lead to a potential error of evaluating PNI and LVI. Nevertheless, clinicopathologic and survival data were collected by reviewing the record of the individual patient. All patients were received standard treatment of nCRT and TME as recommended.
In summary, our study indicated that PNI and/or LVI following nCRT were poor prognosticators, and found the survival benefit of postoperative adjuvant chemotherapy to LARC patients with PNI.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (NO. 81502646 and NO. 81502647), Zhejiang Provincial Natural Science Foundation of China (NO. LY14H160009), and the Zhejiang Medical and Health Science and Technology Project (NO. 2016KYB042 and NO. 2018KY298).
Competing Interests
The authors have declared that no competing interest exists.
References
1. van Gijn W, Marijnen CA, Nagtegaal ID. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582
2. Gerard JP, Conroy T, Bonnetain F. et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625
3. Benson AR, Venook AP, Al-Hawary MM. et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901
4. Collette L, Bosset JF, den Dulk M. et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386
5. Valentini V, van Stiphout RG, Lammering G. et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163-3172
6. Glynne-Jones R, Wyrwicz L, Tiret E. et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:v22-v40
7. Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94:426-427
8. Compton CC, Fielding LP, Burgart LJ. et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994
9. Cienfuegos JA, Rotellar F, Baixauli J. et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol. 2015;22:916-923
10. Liebig C, Ayala G, Wilks J. et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131-5137
11. Lee JH, Jang HS, Kim JG. et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol. 2012;19:1213-1221
12. Chablani P, Nguyen P, Pan X. et al. Perineural Invasion Predicts for Distant Metastasis in Locally Advanced Rectal Cancer Treated With Neoadjuvant Chemoradiation and Surgery. Am J Clin Oncol. 2015
13. Ting WC, Chen LM, Huang LC. et al. Impact of interleukin-10 gene polymorphisms on survival in patients with colorectal cancer. J Korean Med Sci. 2013;28:1302-1306
14. Ptok H, Meyer F, Steinert R. et al. No prognostic impact of isolated lymphovascular invasion after radical resection of rectal cancer-results of a multicenter observational study. Int J Colorectal Dis. 2007;22:749-756
15. Fagan JJ, Collins B, Barnes L. et al. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:637-640
16. Bockman DE, Buchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219-230
17. Peng J, Sheng W, Huang D. et al. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer-Am Cancer Soc. 2011;117:1415-1421
18. Hoda SA, Hoda RS, Merlin S, Shamonki J, Rivera M. Issues relating to lymphovascular invasion in breast carcinoma. Adv Anat Pathol. 2006;13:308-315
19. Beard CJ, Chen MH, Cote K. et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 2004;58:19-24
20. Harnden P, Shelley MD, Clements H. et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer-Am Cancer Soc. 2007;109:13-24
21. Mendenhall WM, Amdur RJ, Hinerman RW. et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol. 2007;30:93-96
22. Ceyhan GO, Liebl F, Maak M. et al. The severity of neural invasion is a crucial prognostic factor in rectal cancer independent of neoadjuvant radiochemotherapy. Ann Surg. 2010;252:797-804
23. Poeschl EM, Pollheimer MJ, Kornprat P. et al. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol. 2010;28:e358-e360 e361-e362
24. Park IJ, You YN, Agarwal A. et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770-1776
25. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer-Am Cancer Soc. 2009;115:3379-3391
26. Sato T, Ueno H, Mochizuki H. et al. Objective criteria for the grading of venous invasion in colorectal cancer. Am J Surg Pathol. 2010;34:454-462
27. Lim SB, Yu CS, Hong YS. et al. Long-term outcomes in patients with locally advanced rectal cancer treated with preoperative chemoradiation followed by curative surgical resection. J Surg Oncol. 2012;106:659-666
28. Ptok H, Meyer F, Steinert R. et al. No prognostic impact of isolated lymphovascular invasion after radical resection of rectal cancer-results of a multicenter observational study. Int J Colorectal Dis. 2007;22:749-756
Author contact
![]() Corresponding author: Prof. Luying Liu, Department of Radiation Oncology, Zhejiang Cancer Hospital, Banshan Road East, Hangzhou 310022, Zhejiang, China. Email: luyingliucom; and Yuan Zhu, Email: zhuyuan63com
Corresponding author: Prof. Luying Liu, Department of Radiation Oncology, Zhejiang Cancer Hospital, Banshan Road East, Hangzhou 310022, Zhejiang, China. Email: luyingliucom; and Yuan Zhu, Email: zhuyuan63com

 Global reach, higher impact
Global reach, higher impact