Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(10):2288-2298. doi:10.7150/jca.30680 This issue Cite
Research Paper
Association between Sedative-hypnotics and Subsequent Cancer in Patients with and without Insomnia: A 14-year Follow-up Study in Taiwan
1. Department of Nursing, Taipei Medical University Hospital, Taipei, Taiwan
2. School of Nursing, College of Nursing, Taipei Medical University, Taipei, Taiwan
3. Microbiome Research Centre, St George & Sutherland Clinical School, University of New South Wales, Sydney, Australia
4. School of Gerontology Health Management, College of Nursing, Taipei Medical University, Taipei, Taiwan
5. Department of Nursing, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
*These authors contributed equally to this work.
Received 2018-10-15; Accepted 2019-3-21; Published 2019-5-21
Abstract
Background: The aim of this population-based 14-year historical and prospective study was to determine the relationships between the usage of sedative-hypnotics, including benzodiazepines and nonbenzodiazepines, and the risk of subsequent cancer in patients with or without insomnia among the Taiwanese population.
Methods: A total of 43,585 patients were recruited, 21,330 of whom had been diagnosed with insomnia and 8,717 who had been prescribed sedative-hypnotics during this study's following period of 2002 to 2015. Information from the claims data, namely basic demographic details, drug prescriptions, comorbidities, and patients' survival, was extracted from the National Health Insurance Research Database for χ2 analysis. A Cox proportional hazards model was used to compute the 14-year cancer-free survival rates after adjustment for confounding factors.
Results: Patients with insomnia who used sedative-hypnotics had an adjusted hazard ratio of 1.49 compared with patients with insomnia who did not use any sedative-hypnotics, and patients without insomnia who used sedative-hypnotics had an adjusted hazard ratio of 1.68 compared with patients without insomnia who did not use any sedative-hypnotics. Regarding site-specific risk, patients with insomnia who used sedative-hypnotics had an increased risk of oral and breast cancers, and patients without insomnia who received sedative-hypnotics prescriptions had an increased risk of liver and breast cancers. The cancer-free survival rate of patients who had used sedative-hypnotics was significantly lower than that of patients who had never used sedative-hypnotics.
Conclusions: The use of sedative-hypnotics in patients either with or without insomnia was associated with subsequent cancer development in the Taiwanese population. Increased risks of oral, liver, and breast cancer were found in the patients with the use of sedative-hypnotics. The use of sedative-hypnotics should be discouraged for treating patients with or without insomnia in Taiwan.
Keywords: Sedative-hypnotics, Insomnia, Cancer, Cancer-free survival rate, Immune function, Viral infection
Introduction
The prevalence of insomnia in Asian and Western countries ranges from 5% to 40% [1-7]. It has been found to correlate with a number of psychological diseases such as depression [5] and physical illnesses such as diabetes [8], hypertension, and cardiovascular disease [9-12]. Thus, insomnia is a major public health concern with considerable personal and socioeconomic burdens [13]. Typical treatments for patients with insomnia include psychological therapy and the use of medications [14]. The two most commonly prescribed medications to treat insomnia are benzodiazepines (BZDs) and non-benzodiazepines (non-BZDs), and other drugs such as gamma-aminobutyric acid agonists, melatonin receptor agonists, sedating antidepressants, antihistamines, and eugeroic drugs are also used but less frequently [15]. Sedative-hypnotics are also prescribed to patients with diseases such as generalized anxiety disorder, addiction, agitation, neurological disorders, muscle spasticity, involuntary movement disorder, alcohol withdrawal, and anxiety associated with cardiovascular or gastrointestinal conditions [16]. However, associations between the usage of sedative-hypnotics and potential side effects, such as cancer and death, have not yet been clearly established.
Concerns about the possible side effects of sedative-hypnotics in humans have existed since the Cancer Prevention Study I of the American Cancer Society first documented that the consumption of hypnotics was associated with excessive deaths in 1979 [17, 18]. Although a few population-based studies have investigated the correlation between the use of sedative-hypnotics and corresponding mortality [19-31], no consistent relationships have been established. Few local studies have attempted to determine the correlations between the use of sedative-hypnotics and potential carcinogenicity in the Taiwanese population, but the influence of both insomnia and the use of sedative-hypnotics has not been explored [32-35]. Moreover, most studies have analyzed either the use of BZDs [33, 34] or non-BZDs [32] alone, and thus the overall influence of how the use of sedative-hypnotics, including both BZDs and non-BZDs, correlates with carcinogenicity in the general Taiwanese population remains to be elucidated. To our knowledge, our study is the first to specifically investigate the correlations between subsequent cancer development, the use of sedative-hypnotics, and insomnia in the Taiwanese population.
Materials and Methods
Data Sources
The National Health Insurance (NHI) Project was initiated and maintained by the Health and Welfare Data Science Center of the Ministry of Health and Welfare. It captures all the reimbursable items of consolidated health insurance claims from every single payer. The NHI program has covered more than 96% of the Taiwanese population and it has already established contact with 97% of the local clinics and hospitals since the end of 1996 [34]. The full population file from the NHI database was used to analyze the risk of subsequent cancer in a more accurate manner. Information from each of the medical claims records included 1) the patient's demographic details, 2) clinical details concerning diseases (according to the International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-9-CM), and 3) details of health care service such as drug prescription and cost. This study was reviewed and approved by the Joint Institutional Review Board of Taipei Medical University (N201611010).
Study samples
This study examined the risk of cancer in three sedative-hypnotics-exposed groups and one sedative-hypnotics-unexposed group. Patients who had been diagnosed with insomnia (ICD-9-CM Codes 307.40-307.42, 307.44, 307.49, or 780.52) and prescribed sedative-hypnotic medications were classified as the first exposure group (Inso-Hyp group). The date each patient was first diagnosed with insomnia was within January 1, 2002 and December 31, 2004. To avoid coding errors, only patients with at least two diagnosis records for insomnia within the same year were recruited. The index date was defined as the first outpatient visit for insomnia treatment. Prescription of sedative-hypnotic drugs was defined as at least 30 defined daily dose (DDD) per year [36] until the first diagnosis of cancer, death, or endpoint of the follow-up period. Patients with any two consecutive prescription intervals greater than three months were classified as having discontinued the prescription and were excluded [37]. In the second exposed group, all patients were diagnosed with insomnia but were not prescribed any sedative-hypnotics during the entire follow-up period (Inso-NonHyp). The third exposed group consisted of patients who had received a sedative-hypnotics prescription for any reason other than insomnia (NonInso-Hyp). The definition of insomnia for the Inso-NonHyp and NonInso-Hyp was same as for the Inso-Hyp group. Any patients in these three exposure groups were excluded if they had either been diagnosed with insomnia or prescribed a sedative-hypnotics drug between 2000 and 2001. The remaining beneficiaries without diagnosis of insomnia or sedative-hypnotic prescription between 2000 and 2013 were recruited into the unexposed group (NonInso-NonHyp), and the first record of medical service use was defined as their index visit. Common exclusion criteria for all four groups included any patients who had (1) a previous diagnosis of cancer between 2000 and 2001 or (2) a previous diagnosis of cancer before the index date, (3) who were aged under 18 years on the index date, or (4) who had a latency period less than two years. Patients for inclusion in the NonInso-NonHyp group were selected by matching to those in the NonInso-Hyp group according to age, sex, index year, and Charlson comorbidity score (CCI; four for each patient). Patients for the Inso-NonHyp group were selected to match with those in the Inso-Hyp group according to same way. Finally, 43,585 patients were included in this retrospective study.
Variables of Interest
The risk of subsequent cancer incidence in each cohort (codes according to the International Classification of Diseases for Oncology, third edition, in the Cancer Registry file) was evaluated. From the respective index dates to the day with first diagnosis of cancer, date of death, or the end of the follow-up period on December 31, 2015, the patients were individually followed for 2 to 14 years. We retrospectively investigated the risk of nine major cancer types in Taiwan, namely oral cancer (C00-C06, C09-C10, C12-C14), stomach cancer (C16), colon cancer (C18-C21), liver cancer (C22), lung cancer (C33-C34), skin cancer (C44), breast cancer (C50), corpus cancer (C54), and prostate cancer (C61) [38]. Only female subjects were included in the analysis of the associated risk of subsequent breast cancer.
Sedative-hypnotics were classified as either BZDs or non-BZDs. Alprazolam, bromazepam, brotizolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, fludiazepam, flunitrazepam, flurazepam, lorazepam, medazepam, midazolam, nitrazepam, nordazepam, oxazepam, and triazolam were classified as BZDs, and zolpidem, zopiclone, and zaleplon were classified as non-BZDs [39]. Patients who received a prescription for both BZDs and non-BZDs during the study period were excluded from the drug type analysis. Three groups of different half-life duration of sedative-hypnotic drugs were further analyzed. We defined a drug as short acting, intermediate acting, or long acting if it had a half-life of less than 10 hours, 10 to 35 hours, or more than 35 hours, respectively [40]. Moreover, patients who had ever switched from using a long-acting drug to a short-acting drug were sorted into the long-acting group. Low-dose users were patients with a prescribed yearly mean DDD of 7 to 30 during the follow-up period, medium-dose users were those with a prescribed yearly mean DDD of 31 to 90, and high-dose users were patients with a prescribed yearly mean DDD of ≥ 91 [36].
Statistical Analysis
Demographic information for each patient was obtained from the Registry for Beneficiaries file. There were four groups for patient's age: younger than 30 years old, 30-44 years old, 45-64 years old, and 65 years or older. Patients were divided into three groups according to their average monthly income: < US$640 (New Taiwan Dollars [NT$]20,000), US$640-$1,280 (NT$20,000-39,999), and ≥ US$1,281 (≥ NT$40,000). Northern, Central, Southern, and Eastern administrative regions of Taiwan were used as criteria for classifying residential area. The urbanization level of patient's residence was defined as urban, suburban, or rural. CCI score was used to evaluate any preexisting comorbidities [41]. Patients were subsequently stratified into three groups according to the sum of points (range: 0 to 6): 0, 1, ≥ 2 points [42]. The chi-squared test was used to analyze differences in the demographic information of four patient groups. The incidence density rate of each at-risk population was defined as the number of incident cases divided by the sum of the person-years. The crude hazard ratios (CHRs) for developing cancer were estimated using univariate Cox proportional hazards model. The adjusted hazard ratio (AHR) represented the differing risks for the study groups after controlling for demographic information of patient. The between-group differences in the survival rates and curves of survival distributions for developing cancer were assessed by Kaplan-Meier method and log-rank test. SAS statistical package (SAS systems for Windows, Version 9.2; Cary, NC, USA) was used for all statistical analyses. The statistically significant p value was set at < 0.05.
Results
The cohort of this study consisted of a total of 43,585 patients from Taiwan's National Health Insurance Research Database (NHIRD); 4,266 patients were assigned to the Inso-Hyp group, 17,064 patients were sorted into the Inso-NonHyp group, 4,451 patients comprised the NonInso-Hyp group, and 17,804 patients constituted the Noninso-NonHyp group (Table 1). Significant differences were identified in the demographic characteristics of the patients in these four groups. In general, most of the subjects had a monthly income under $640 (66.4%-78.8%) and lived in the Northern (48.6%-57.3%) and urban region (56.9%-57.7%). Compared with patients without insomnia, more patients in the Inso-Hyp and Inso-NonHyp groups were younger than 30 (4.4% and 4.6%, respectively) or were female (48.1%). In addition, patients who had not been prescribed any sedative-hypnotics exhibited significantly lower CCIs than those who had.
Table 2 displays the CHRs of subsequent cancer incidence in our study cohort. The patients with exposure to sedative-hypnotic prescriptions exhibited higher hazard ratios for cancer compared with those without sedative-hypnotic prescriptions: The CHR was 1.47 (CI: 1.21-1.79) for the Inso-Hyp group compared with the Inso-NonHyp group and 1.65 (CI: 1.36-2.01) for the NonInso-Hyp group compared with the NonInso-NonHyp group. In addition, patients aged 45 years or older were associated with higher densities of cancer incidence than those who were younger than 30 years old. The densities of cancer incidence were significantly higher in male patients (CHR: 1.55, 95% confidence interval (CI): 1.37-1.76), patients with a monthly income < $640 (CHR: 1.34, 95% CI: 1.07-1.67), or who lived in the Southern region of Taiwan. Subjects with a CCI score equal to or greater than 1 were also more likely to subsequently develop cancer when compared with patients whose CCI score was zero.
As Table 3 presents, for patients receiving a diagnosis of insomnia, those prescribed long-acting sedative-hypnotic drugs exhibited a higher risk of developing cancer (AHR: 1.73, CI: 1.37-2.19) than those without sedative-hypnotic prescriptions. Patients having a prescribed mean DDD of at least 7 per year exhibited a higher risk of cancer than those without any sedative-hypnotic prescriptions. In particular, patients with a low dosage (AHR: 2.70, 95% CI: 1.12-6.54) had a 2.7-fold increased risk of a subsequent cancer incidence. Noninsomniac patients with a prescription of BZD had a higher risk of cancer compared with those without sedative-hypnotic drugs (AHR: 1.51, CI: 1.17-1.95). With respect to the half-life of the prescribed sedative-hypnotics, it was found that the risk of subsequently developing cancer among the patients with either short-, intermediate-, or long-acting drugs was comparable (AHR: 1.75, 95% CI: 1.09-2.82; AHR: 1.65, 95% CI: 1.21-2.26; and AHR: 1.69, 95% CI: 1.31-2.17, respectively). Patients having a prescribed mean DDD of at least 7 per year exhibited a higher risk of cancer than those without any sedative-hypnotic prescription, especially for those receiving a low dosage (AHR: 5.47, CI: 4.10-7.30).
The incidence densities of each type of cancer for each of the four groups within the follow-up period are summarized in Table 4. In general, after adjustment for individual age, sex, income, residential region, urbanization, and CCI scores, patients who received sedative-hypnotic prescriptions exhibited higher hazard ratios for cancer compared with those without sedative-hypnotic prescriptions: AHR 1.49 (CI: 1.22-1.82) in Inso-Hyp group compared with the Inso-NonHyp group and 1.68 (CI: 1.38-2.05) in the NonInso-Hyp group compared with NonInso-NonHyp group (Table 4). In particular, the Inso-Hyp group exhibited a higher probability of developing oral and breast cancer than the Inso-NonHyp group did, and the NonInso-Hyp group exhibited a significantly higher risk of developing liver and breast cancers than the NonInso-NonHyp group did.
Demographic characteristics of patients in the Inso-Hyp, Inso-NonHyp, NonInso-Hyp, and NonInso-NonHyp groups
| Variable | Inso-Hyp (N = 4,266) | Inso-NonHyp (N = 17,064) | NonInso-Hyp (N = 4,451) | NonInso-NonHyp (N = 17,804) | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | p value | |
| Age (y) | <0.001 | ||||||||
| Younger than 30 | 188 | 4.4 | 789 | 4.6 | 102 | 2.3 | 411 | 2.3 | |
| 30-44 | 1464 | 34.3 | 5813 | 34.1 | 1078 | 24.2 | 4306 | 24.2 | |
| 45-64 | 1987 | 46.6 | 7957 | 46.6 | 2258 | 50.7 | 9036 | 50.8 | |
| 65 or older | 627 | 14.7 | 2505 | 14.7 | 1013 | 22.8 | 4051 | 22.8 | |
| Sex | <0.001 | ||||||||
| Female | 2051 | 48.1 | 8204 | 48.1 | 1816 | 40.8 | 7264 | 40.8 | |
| Male | 2215 | 51.9 | 8860 | 51.9 | 2635 | 59.2 | 10540 | 59.2 | |
| Income (US$) | <0.001 | ||||||||
| ≥1281 (≥NT$40000) | 362 | 8.5 | 2029 | 11.9 | 408 | 9.2 | 1259 | 7.1 | |
| 640-1280 (NT$20000-$39999) | 699 | 16.4 | 3706 | 21.7 | 707 | 15.9 | 2516 | 14.1 | |
| <640 (<NT$20000) | 3205 | 75.1 | 11329 | 66.4 | 3336 | 75.0 | 14029 | 78.8 | |
| Region | <0.001 | ||||||||
| Northern | 2074 | 48.6 | 8865 | 52.0 | 2212 | 49.7 | 10193 | 57.3 | |
| Central | 946 | 22.2 | 3600 | 21.1 | 876 | 19.7 | 3003 | 16.9 | |
| Southern | 1171 | 27.5 | 4265 | 25.0 | 1277 | 28.7 | 4185 | 23.5 | |
| Eastern | 75 | 1.8 | 334 | 2.0 | 86 | 2.0 | 423 | 2.4 | |
| Urbanization | <0.001 | ||||||||
| Urban | 2426 | 56.9 | 9832 | 57.6 | 2558 | 57.5 | 10272 | 57.7 | |
| Suburban | 1450 | 34.0 | 5589 | 32.8 | 1460 | 32.8 | 5517 | 31.0 | |
| Rural | 390 | 9.1 | 1643 | 9.6 | 433 | 9.7 | 2015 | 11.3 | |
| CCI | <0.001 | ||||||||
| 0 | 1701 | 39.9 | 7760 | 45.5 | 1565 | 35.2 | 7932 | 44.6 | |
| 1 | 1124 | 26.4 | 4479 | 26.3 | 1123 | 25.2 | 3548 | 19.9 | |
| ≥2 | 1441 | 33.8 | 4825 | 28.3 | 1763 | 39.6 | 6324 | 35.5 | |
CCI, Charlson comorbidity index; Inso-Hyp, patients with insomnia and sedative-hypnotic prescriptions; Inso-NonHyp, patients with insomnia and without sedative-hypnotic prescriptions; NonInso-Hyp, patients with sedative-hypnotics and without insomnia; NonInso-NonHyp, patients with neither insomnia nor sedative-hypnotic prescriptions.
Crude hazard ratios for cancer in patients in the Inso-Hyp, Inso-NonHyp, NonInso-Hyp, and NonInso-NonHyp groups
| Variable | Cancer | |||
|---|---|---|---|---|
| Cases | PY | Incidence | CHR (95% CI) | |
| Age (y) | ||||
| Younger than 30 | 7 | 10782.79 | 0.65 | 1.00 |
| 30-44 | 100 | 83935.98 | 1.19 | 1.88 (0.88-4.05) |
| 45-64 | 494 | 137295.32 | 3.60 | 5.75 (2.73-12.12)** |
| 65 or older | 469 | 53457.42 | 8.77 | 14.00 (6.64-29.52)** |
| Sex | ||||
| Female | 368 | 127644.11 | 2.88 | 1.00 |
| Male | 702 | 157827.40 | 4.45 | 1.55 (1.37-1.76)** |
| Income (US$) | ||||
| ≥1281 (≥NT$40000) | 84 | 27310.87 | 3.08 | 1.00 |
| 640-1280 (NT$20000-$39999) | 144 | 50524.91 | 2.85 | 0.93 (0.71-1.22) |
| <640 (<NT$20000) | 842 | 207635.73 | 4.06 | 1.34 (1.07-1.67)* |
| Region | ||||
| Northern | 530 | 152126.20 | 3.48 | 1.00 |
| Central | 224 | 55922.83 | 4.01 | 1.14 (0.98-1.33) |
| Southern | 294 | 71482.77 | 4.11 | 1.18 (1.02-1.36)* |
| Eastern | 22 | 5939.71 | 3.70 | 1.06 (0.69-1.63) |
| Urbanization | ||||
| Urban | 583 | 164425.75 | 3.55 | 1.00 |
| Suburban | 362 | 92145.41 | 3.93 | 1.11 (0.97-1.26) |
| Rural | 125 | 28900.35 | 4.33 | 1.23 (1.01-1.49)* |
| CCI | ||||
| 0 | 285 | 117425.26 | 2.43 | 1.00 |
| 1 | 272 | 68558.74 | 3.97 | 1.59 (1.35-1.88)** |
| ≥2 | 513 | 99487.52 | 5.16 | 2.04 (1.76-2.36)** |
| Group1 | ||||
| Inso-NonHyp | 392 | 119187.82 | 3.29 | 1.00 |
| Inso-Hyp | 133 | 28097.40 | 4.73 | 1.47 (1.21-1.79)** |
| Group2 | ||||
| NonInso-NonHyp | 410 | 113218.87 | 3.62 | 1.00 |
| NonInso-Hyp | 135 | 24967.32 | 5.41 | 1.65 (1.36-2.01)** |
Incidence, incidence density (1000 per person-years). *: p < .05. **: p < .001. CHR, crude hazard ratio; CCI, Charlson comorbidity index; CI, confidence interval; PY, person years.
Adjusted hazard ratios for cancer in the Inso-Hyp and NonInso-Hyp groups according to the type, half-life, and doses of sedative-hypnotics drugs
| Variable | Cancer | |||||
|---|---|---|---|---|---|---|
| N | Cases | PY | Incidence | CHR (95% CI) | AHR (95% CI) | |
| Inso-Hyp vs. Inso-NonHyp | ||||||
| Typea | ||||||
| NonHyp | 17064 | 392 | 119187.92 | 3.29 | 1.00 | 1.00 |
| NonBZD | 354 | 8 | 2305.16 | 3.47 | 1.08 (0.54-2.18) | 1.11 (0.55-2.24) |
| BZD | 840 | 22 | 5825.99 | 3.78 | 1.15 (0.75-1.77) | 1.02 (0.67-1.57) |
| Half-lifeb | ||||||
| NonHyp | 17064 | 392 | 119187.92 | 3.29 | 1.00 | 1.00 |
| Short-acting | 483 | 12 | 3158.77 | 3.80 | 1.18 (0.67-2.10) | 1.22 (0.68-2.16) |
| Intermediate-acting | 1232 | 33 | 8074.47 | 4.09 | 1.27 (0.89-1.81) | 1.16 (0.81-1.65) |
| Long-acting | 2551 | 88 | 16864.17 | 5.22 | 1.62 (1.28-2.04)** | 1.73 (1.37-2.19)** |
| Dosec | ||||||
| NonHyp | 17064 | 392 | 119187.92 | 3.29 | 1.00 | 1.00 |
| Low | 138 | 5 | 621.57 | 8.04 | 3.13(1.29-7.56)* | 2.70(1.12-6.54)* |
| Medium | 474 | 15 | 2130.92 | 7.04 | 2.75(1.64-4.61)** | 2.07(1.23-3.49)* |
| High | 5485 | 157 | 25001.66 | 6.28 | 2.43(2.01-2.94)** | 2.46(2.02-3.00)** |
| NonInso-Hyp vs. NonInso-NonHyp | ||||||
| Typed | ||||||
| NonHyp | 17804 | 410 | 113218.87 | 3.62 | 1.00 | 1.00 |
| NonBZD | 278 | 9 | 1462.55 | 6.15 | 1.97 (1.01-3.81)* | 1.93 (1.00-3.75) |
| BZD | 2492 | 70 | 13899.56 | 5.04 | 1.56 (1.21-2.01)** | 1.51 (1.17-1.95)* |
| Half-lifee | ||||||
| NonHyp | 17804 | 410 | 113218.87 | 3.62 | 1.00 | 1.00 |
| Short-acting | 592 | 18 | 3114.44 | 5.78 | 1.83 (1.14-2.94)* | 1.75 (1.09-2.82)* |
| Intermediate-acting | 1481 | 44 | 8075.53 | 5.45 | 1.70 (1.24-2.32)** | 1.65 (1.21-2.26)* |
| Long-acting | 2378 | 73 | 13780.34 | 5.30 | 1.59 (1.24-2.04)** | 1.69 (1.31-2.17)** |
| Dosec | ||||||
| NonHyp | 17804 | 410 | 113218.87 | 3.62 | 1.00 | 1.00 |
| Low | 957 | 56 | 3555.39 | 15.75 | 6.79(5.11-9.02)** | 5.47(4.10-7.30)** |
| Medium | 1764 | 60 | 6157.22 | 9.74 | 4.57(3.46-6.03)** | 3.77(2.85-5.00)** |
| High | 5393 | 168 | 20844.67 | 8.06 | 3.32(2.75-4.00)** | 3.42(2.82-4.14)** |
aOverall, 3,072 patients prescribed BZDs and nonBZDs were excluded. bIn total, 3,227 patients (75.64%) switched from short-acting to intermediate-acting (N=920) or long-acting (N=2307) sedative-hypnotic drugs, and 198 patients (4.64%) switched from intermediate-acting to long-acting sedative-hypnotic drugs. cLow, year mean defined daily dose (DDD) of 7 to 30 during follow-up period; Medium, year mean DDD of 31 to 90 in during follow-up period; High, mean DDD of at least 91 during follow-up period. dOverall, 1,681 patients prescribed BZDs and nonBZDs were excluded. eIn total, 2,027 patients (45.54%) switched from short-acting to intermediate-acting (N=672) or long-acting (N=1355) sedative-hypnotic drugs, and 571 patients (12.83%) switched from intermediate-acting to long-acting sedative-hypnotic drugs. *p < 0.05. **p < 0.001. AHR, adjusted hazard ratio (adjusted for patient age, sex, income, residential region, urbanization, and Charlson comorbidity index); BZD, benzodiazepine; CI, confidence interval.
Incidence and AHR for cancer in patients in the Inso-Hyp, Inso-NonHyp, NonInso-Hyp, and NonInso-NonHyp groups
| Cancer (n, %) | Inso-Hyp vs. Inso-NonHyp | NonInso-Hyp vs. NonInso-NonHyp | Inso-Hyp vs. NonInso-Hyp | Inso-NonHyp vs. NonInso-NonHyp |
|---|---|---|---|---|
| All | 133 (3.12%) vs. 392 (2.30%) | 135 (3.03%) vs. 410 (2.30%) | 133 (3.12%) vs. 135 (3.03%) | 392 (2.30%) vs. 410 (2.30%) |
| Incidence | 4.73 vs. 3.29 | 5.41 vs. 3.62 | 4.73 vs. 5.41 | 3.29 vs. 3.62 |
| AHR (95% CI) | 1.49 (1.22-1.82)** | 1.68 (1.38-2.05)** | 1.04 (0.82-1.33) | 1.08 (0.94-1.25) |
| Oral cancer | 16 (0.38%) vs. 27 (0.16%) | 12 (0.27%) vs. 30 (0.17%) | 16 (0.38%) vs. 12 (0.27%) | 27 (0.16%) vs. 30 (0.17%) |
| Incidence | 0.56 vs. 0.22 | 0.47 vs. 0.26 | 0.56 vs. 0.47 | 0.22 vs. 0.26 |
| AHR (95% CI) | 2.44 (1.31-4.54)* | 1.92 (0.97-3.81) | 1.25 (0.58-2.68) | 0.93 (0.55-1.59) |
| Stomach cancer | 8 (0.19%) vs. 21 (0.12%) | 4 (0.09%) vs. 19 (0.11%) | 8 (0.19%) vs. 4 (0.09%) | 21 (0.12%) vs. 19 (0.11%) |
| Incidence | 0.28 vs. 0.17 | 0.16 vs. 0.17 | 0.28 vs. 0.16 | 0.17 vs. 0.17 |
| AHR (95% CI) | 1.60 (0.71-3.63) | 1.32 (0.44-4.02) | 2.32 (0.69-7.75) | 1.52 (0.81-2.87) |
| Colon cancer | 22 (0.52%) vs 72 (0.42%) | 19 (0.43%) vs. 75 (0.42%) | 22 (0.52%) vs. 19 (0.43%) | 72 (0.42%) vs. 75 (0.42%) |
| Incidence | 0.77 vs. 0.60 | 0.75 vs. 0.66 | 0.77 vs. 0.75 | 0.60 vs. 0.66 |
| AHR (95% CI) | 1.35 (0.83-2.17) | 1.25 (0.75-2.09) | 1.33 (0.71-2.49) | 1.13 (0.81-1.57) |
| Liver cancer | 16 (0.38%) vs. 42 (0.25%) | 22 (0.49%) vs. 40 (0.22%) | 16 (0.38%) vs. 22 (0.49%) | 42 (0.25%) vs. 40 (0.22%) |
| Incidence | 0.56 vs. 0.35 | 0.87 vs. 0.35 | 0.56 vs. 0.87 | 0.35 vs. 0.35 |
| AHR (95% CI) | 1.54 (0.86-2.74) | 2.53 (1.48-4.32)** | 0.84 (0.44-1.62) | 1.21 (0.78-1.88) |
| Lung cancer | 10 (0.23%) vs. 53 (0.31%) | 17 (0.38%) vs. 51 (0.29%) | 10 (0.23%) vs. 17 (0.38%) | 53 (0.31%) vs. 51 (0.29%) |
| Incidence | 0.35 vs. 0.44 | 0.67 vs. 0.45 | 0.35 vs. 0.67 | 0.44 vs. 0.45 |
| AHR (95% CI) | 0.83 (0.42-1.63) | 1.71 (0.98-3.00) | 0.65 (0.29-1.43) | 1.37 (0.93-2.03) |
| Skin cancer | 4 (0.09%) vs. 9 (0.05%) | _ | _ | 9 (0.05%) vs. 8 (0.04%) |
| Incidence | 0.14 vs. 0.07 | _ | _ | 0.07 vs. 0.07 |
| AHR (95% CI) | 1.94 (0.59-6.41) | _ | _ | 1.42 (0.54-3.75) |
| Breast cancera | 21 (1.02%) vs. 33 (0.40%) | 11 (0.61%) vs. 26 (0.36%) | 21 (1.02%) vs. 11 (0.61%) | 33 (0.40%) vs. 26 (0.36%) |
| Incidence | 1.53 vs. 0.57 | 1.06 vs. 0.56 | 1.56 vs. 1.06 | 0.57 vs. 0.56 |
| AHR (95% CI) | 2.85 (1.64-4.96)** | 2.08 (1.01-4.28)* | 1.67 (0.80-3.48) | 0.97 (0.54-1.64) |
| Corpus cancer | _ | _ | _ | 9 (0.05%) vs. 6 (0.03%) |
| Incidence | _ | _ | _ | 0.07 vs. 0.05 |
| AHR (95% CI) | _ | _ | _ | 1.04 (0.36-3.00) |
| Prostate cancer | 4 (0.09%) vs. 26 (0.15%) | 5 (0.11%) vs. 21 (0.12%) | 4 (0.09%) vs. 5 (0.11%) | 26 (0.15%) vs. 21 (0.12%) |
| Incidence | 0.14 vs. 0.22 | 0.20 vs. 0.18 | 0.14 vs. 0.20 | 0.22 vs. 0.18 |
| AHR (95% CI) | 0.70 (0.24-2.01) | 1.55 (0.57-4.23) | 0.94 (0.24-3.71) | 1.70 (0.94-3.05) |
Incidence, incidence density (1000 per person-years); AHR: adjusted hazard ratio using Cox proportional hazards regression model after controlling for age, sex, income, region, area, and CCI; *: p < .05; **: p < .001; ICD-O-3 codes: Oral cancer: C00-C06, C09-C10, C12-C14; Stomach cancer: C16; Colon cancer: C18-C21; Liver cancer: C22; Lung cancer: C33-C34; Skin cancer: C44; Breast cancer: C50; Corpus cancer: C54; Prostate cancer: C61. _: not applicable because of the sample size; a: only data of female patients were analyzed.
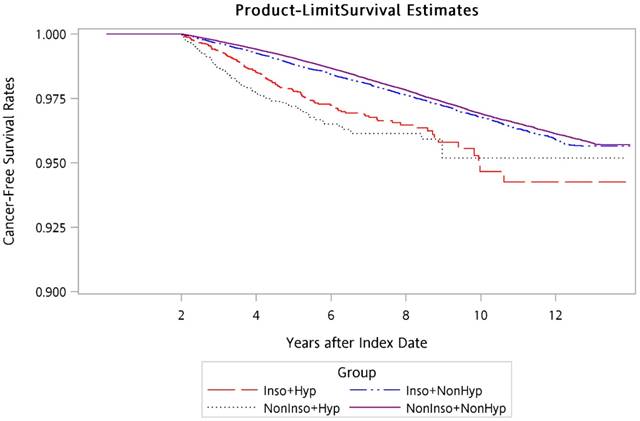
Using the Kaplan-Meier method, the overall 14-year cancer-free survival curves for the four groups were calculated and are presented in Figure 1. Log-rank testing revealed that there were significantly lower cancer-free survival rates for Inso-Hyp and NonInso-Hyp groups than those for Inso-NonHyp and NonInso-NonHyp groups (p < 0.001).
Discussion
In this 14-year cohort study, we historically and prospectively analyzed the relationship between sedative-hypnotics usage and subsequent cancer risk on the basis of 21,330 patients with insomnia and 22,255 patients without insomnia collected from the NHIRD in Taiwan. The findings of the present study provided evidence that the use of sedative-hypnotics in patients either with insomnia or without was associated with an increased risk of subsequent cancer development in the Taiwanese population. Thus, the use of sedative-hypnotics to treat patients with or without insomnia is discouraged. For patients who clinically require sedative-hypnotics, they should be advised and warned of the potential cancer risks before receiving a prescription.
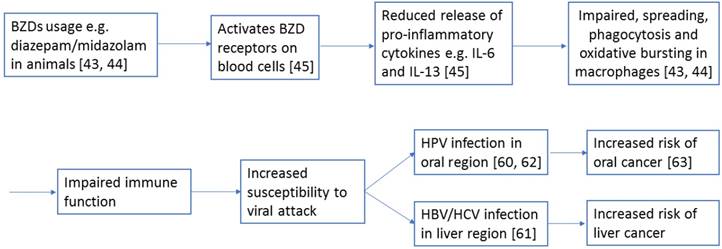
The underlying mechanisms and reasons for the evident relationship between sedative-hypnotics and cancer incidence remain to be determined. A report has suggested that sedative-hypnotics usage leads to weakened immune function. For instance, some animal studies have discovered that BZDs such as diazepam and midazolam impaired the processes of spreading, phagocytosis, and oxidative bursting of macrophages [43, 44]. These impairments can be partially explained by the reduced release of the proinflammatory cytokines interleukin-6 and interleukin-13 in blood cells because of the activation of their BZD receptors [45]. Figure 2 shows how BZDs may contribute to increased risk of subsequent cancer through the above mechanism.
Cancer-free survival rates of patients in Inso-Hyp, Inso-NonHyp, NonInso-Hyp, and NonInso-NonHyp groups. Inso-Hyp, patients with insomnia and sedative-hypnotic prescriptions; Inso-NonHyp, patients with insomnia and without sedative-hypnotic prescriptions; NonInso-Hyp, patients with sedative-hypnotics and without insomnia; NonInso-NonHyp, patients with neither insomnia nor sedative-hypnotic prescriptions
A diagram showing a potential mechanism how BZDs, such as diazepam or midazolam, contribute to the increased risk of subsequent cancers, such as oral and liver cancers, in this study. The numbers refer to the corresponding supporting references.
Sedative-hypnotics are also prescribed to patients with various types of psychiatric illnesses [46]. For instance, BZDs are commonly prescribed to patients with bipolar disorder [46] or schizophrenia [47] in Taiwan, and non-BZD drugs are prescribed to patients with depression [48]; this could account for the sedative-hypnotics prescribed to patients without insomnia in our study cohort (Table 3). Psychological parameters may alter immune function [49]. Moreover, individuals who regularly use sedative-hypnotics also tend to have more psychological problems. Indeed, one of our previous studies demonstrated that the use of sedative-hypnotics, including both BZDs and non-BZDs, increased the risk of developing various types of psychiatric disorders such as depressive disorder, bipolar disorder, and anxiety disorder [46]. Moreover, randomized controlled trials have demonstrated that the use of sedative-hypnotics increased the incidence of depression [50]. Non-BZDs such as zolpidem have been associated with a relatively high incidence of depression [50]. Taken together, these study findings suggest that the increased subsequent cancer risk can at least be partially accounted for by the weakened immune function that results from both intrinsic psychiatric weaknesses and extrinsic usage of sedative-hypnotics. Figure 3 shows the relationships between psychological illnesses, sedative-hypnotics and the impaired immune function. It has been recently suggested that sedative-hypnotics are clastogens that can transform normal cells into cancer through disruption or breakages of chromosomes [51, 52]. The author pointed out that non-BZDs such as zopiclone, zaleplon and ramelteon, based on the findings from the Center for Drug Evaluation and Research, are clastogenic in cell models [51]. Although both zopiclone and zaleplon usage were included in the non-BZDs group in our study, there was no significant increase in the risk of having subsequent cancers in subjects either with insomnia or without insomnia (Table 3). The conflict may be due to the fact that zolpidem was also included in the non-BZDs group in our analysis. Further in-vitro studies on the effect of non-BZDs in inducing cell transformation are warranted. Figure 4 depicted one of the possible mechanisms how non-BZDs induce carcinogenesis.
A diagram showing the relationships between psychological illnesses, sedative-hypnotics and impaired immune function.

A diagram showing a potential mechanism how non-BZDs such as zopiclone or zaleplon contribute to increased risk of subsequent cancers. The number refers to the corresponding supporting reference.

The use of long-acting sedative-hypnotics, but not short- or intermediate-acting ones, increased the risk of subsequent cancer development among patients with insomnia in our study. This suggests that the sedative-hypnotics having a half-life longer than 35 hours should be restricted and not prescribed to patients with insomnia. A possible reason for this is that longer duration of sleep that results from the use of short- or intermediate-acting sedative-hypnotics may help restore their normal immune function, which could compensate for the risk of subsequent cancer. By contrast, the use of long-acting sedative-hypnotics by patients with insomnia results in more than half the dose consumed the previous night remaining in the patient's system until just before the next dose is consumed. Thus, the negative effect of this carry-over effect may significantly interrupt the circadian rhythm of these patients. Disruption of the normal circadian rhythm has been reported to increase the risk of developing cancer [53]. Furthermore, Thompson et al has recently proposed that disruption of the circadian rhythm and suppression of nocturnal production of melatonin, which has been shown to promote DNA repair in animal models [54-56], could be potential mechanisms that contribute to an increased risk of colorectal adenomas due to shorter sleep duration in a group of subjects who were having colonoscopy screening [57]. In contrast, no significant increase in the risk of colon cancer was observed in the Inso-NonHyp group when compared with the NonInso-NonHyp group. A possible reason to explain this is that the risk of colon/colorectal malignancy had been reduced because of the implementation of the Nationwide Colorectal Cancer Screening Program since 2004 in Taiwan [58]. Colon/colorectal malignancy can be prevented if early detection of precancerous stage and subsequent intervention can be achieved [59]. Thus, we suggest that more frequent cancer screening (e.g. for colon cancer) could be provided to individuals who are having sedative-hypnotics.
The use of sedative-hypnotics resulted in a 2.4-fold increase in the risk of oral cancer in patients with insomnia in our study, and a 2.5-fold risk of future liver cancer was evident in patients without insomnia who used sedative-hypnotics. As we discussed earlier, patients who use sedative-hypnotics are more likely to have weakened immune function; thus, they are more susceptible to various types of viral attack. The viral infection hypothesis has been suggested to explain the high risk of oral and liver cancers that is associated with sedative-hypnotics usage [60-62]. For example, human papillomavirus (HPV) has been determined to contribute to the development of oral cancer [63], and hepatitis B virus (HBV) and hepatitis C virus (HCV) infections account for the majority of primary liver cancer cases in Taiwan [64].
Experimental studies have suggested that diazepam, one of the BZDs family members, may promote breast cancer growth [65, 66]. However, this hypothesis was rejected by Kleinerman et al., who concluded that the breast cancer growth was not associated with the use of diazepam [67]. In addition, two other groups of researchers also failed to discern a significant association between the use of sedative-hypnotics and breast cancer risk [68, 69]. Pottegard et al. concluded that the odds ratio for breast cancer among patients in their Danish cohort comprised of approximately 1.3 million individuals who used BZDs or BZD-related drugs was close to unity (AHR: 1.01, 95% CI 0.90-1.14) [70]. In Halapy's study, BZDs usage did not correlate with an increased breast cancer rate in their Canadian cohort [68]. However, we discovered that female patients in our study who used sedative-hypnotics had at least a 2.2-fold increased risk of developing subsequent breast cancer (Table 4). This finding is partially supported by Kao et al., who determined that the use of Zolpidem, the most commonly used non-BZDs drug, was associated with a 1.84-fold increase in breast cancer risk in Taiwanese women [32]. Further investigation into this area should be undertaken in the future. Different mechanisms of breast carcinogenesis may vary among patients of different ethnicities, which could account for the discrepant research findings.
Pottegard et al have recently concluded that the long term usage of either BZDs and BZD-related drugs did not significantly increase the overall risk of subsequent cancer [71]. However, significant increases (at least 1.35 folds) in the risks of site-specific cancers such as cancers of esophagus, stomach, liver, pancreas, lung, bronchus and pleura and melanoma of skin could still be demonstrated. In particular, a 1.8 folds increase in the risk of having liver cancer for patients taking sedative-hypnotics was observed. Although the author suggested that heavy alcohol usage could be a confounding factor which contributed to the increased risk [71], BZDs such as diazepam and oxazepam were CYP4A inducers in mice that could account for their ability to promote tumorigenesis in liver [72]. Furthermore, liver is the organ responsible for drug metabolism. Thus, the potential side effect of prolonged use of sedative-hypnotics on liver may be similar to that of the prolonged use of acetaminophen, which was associated with slightly increased risk of liver cancer [73], possibly due to liver glutathione depletion, increased oxidative stress, mitochondrial dysfunction [74]. Taking these together, it is possible that the ability of sedative-hypnotics to initiate/promote tumor growth may be different on different organs and it may exert a more direct and stronger carcinogenic effect on liver. Mechanistic study of hepatocarcinogenic effect exerted by sedative-hypnotics is warranted.
Compared with a recent Taiwanese study, Lan et al have shown that the use of Zolpidem was also associated with increased cancer risk [35]. Since this study targeted at investigating the relationships between the use of BZDs and non-BZDs and various mortality, no further information was provided on the potential risk of subsequent cancer after prolonged usage of BZDs and non-BZDs in the general Taiwanese population. Thus, our study provided the most recent findings to indicate that the overall cancer risk was increased with prolonged usage of sedative-hypnotics in Taiwanese population (Table 4). Although prescription of sedative-hypnotics is still the main treatment regimen for patients with insomnia [14], cognitive behavior therapy (CBT) appears to be an alternative treatment option [75]. In consideration of the time and costs associated with the conventional face-to-face treatment session, a randomized controlled trial has suggested that video-based CBT (V-CBT) can also produces significant and sustainable treatment effects in a group of breast cancer patients with insomnia symptoms [76]. Therefore, the feasibility of the use of V-CBT to substitute sedative-hypnotics medication should be investigated.
This study has a few strengths. To the best of our knowledge, it is the first study to investigate the correlations between sedative-hypnotics usage (including both BZDs and non-BZDs) and subsequent cancer risk in patients with or without insomnia in the Taiwanese population. Moreover, the cohort in this study was derived from the NHIRD, which has a national coverage rate of approximately 96%, and thus this study is representative of the total population in Taiwan. The data included in this study encompass a period of 14 years (2002-2015), which is relatively longer than other local researches that have been conducted; thus, any potential bias could be minimized. In addition, patients enrolled in this study were carefully matched in terms of age, sex, index year, and CCI score. Last, only patients who received a cumulative DDD prescription of more than 30 per year were included for most of the statistical analyses with the exception of dosage analysis. This was done to eliminate the effect of sedative-hypnotics usage that was unlikely to have a significant effect on the development of subsequent cancers. Several limitations have also been identified. First, data from the NHIRD do not include factors that are potentially related to sedative-hypnotics usage and cancer risk such as drinking habit, smoking habit, exercise habit, and family history of cancer [77]. These are major risk factors for multiple cancers and thus these may have constituted residual confounding effects in this study. Second, the use of sedative-hypnotics in the claims data was inferred from prescription data. Thus, it might not accurately represent the actual usage among patients. The cancer risk associated with the potential use of sedative-hypnotics could have been underestimated. However, the correlated cancer risk could also have been overestimated if some patients had obtained sedative-hypnotics from sources other than NHI, but this situation was unlikely because all sedative-hypnotics require a prescription in Taiwan. Third, a causal relationship could not be established because observational data was used in this study. Last, main findings from this current study have not been validated in another independent cohort due to limited number of subjects currently available.
Conclusions
This study presented evidence that the use of sedative-hypnotics in patients either with or without insomnia was associated with subsequent cancer development in the Taiwanese population. The use of long-acting and high dose of sedative-hypnotics increased the risk of subsequent cancer development. The use of sedative-hypnotics resulted in increased risks of oral, liver, and breast cancer in patients. The increased risk of cancer should be explained to patients who require sedative-hypnotics before a prescription is given. The use of sedative-hypnotics is discouraged in the long term, and other treatments, such as cognitive behavior therapy, should be considered to substitute the prescription of sedative-hypnotics in Taiwan. Cancer screening can also be provided to individuals who are receiving sedative-hypnotics in a more frequent manner.
Abbreviations
BZDs: benzodiazepines; non-BZDs: nonbenzodiazepines; NHI: National Health Insurance; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; Inso-Hyp: patients with insomnia and sedative-hypnotic prescriptions; Inso-NonHyp: patients with insomnia and without sedative-hypnotic prescriptions; NonInso-Hyp: patients with sedative-hypnotics and without insomnia; NonInso-NonHyp: patients with neither insomnia nor sedative-hypnotic prescriptions; DDD: defined daily dose; CCI: Charlson comorbidity score; CHR: crude hazard ratio; AHR: adjusted hazard ratio; NHIRD: National Health Insurance Research Database; CI: confidence interval.
Acknowledgements
The study was granted by Taipei Medical University Hospital (105TMU-TMUH-24).
Ethics Committee Approval and Patient Consent
The present study was approved by Joint Institutional Review Board of Taipei Medical University (N201611010).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41-7
2. Li RH, Wing YK, Ho SC, Fong SY. Gender differences in insomnia-a study in the Hong Kong Chinese population. J Psychosom Res. 2002;53:601-9
3. Mai E, Buysse DJ. Insomnia: Prevalence, Impact, Pathogenesis, Differential Diagnosis, and Evaluation. Sleep Med Clin. 2008;3:167-74
4. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97-111
5. Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002;53:593-600
6. Ohayon MM, Partinen M. Insomnia and global sleep dissatisfaction in Finland. J Sleep Res. 2002;11:339-46
7. Kao CC, Huang CJ, Wang MY, Tsai PS. Insomnia: prevalence and its impact on excessive daytime sleepiness and psychological well-being in the adult Taiwanese population. Qual Life Res. 2008;17:1073-80
8. Green MJ, Espie CA, Popham F, Robertson T, Benzeval M. Insomnia symptoms as a cause of type 2 diabetes Incidence: a 20 year cohort study. BMC Psychiatry. 2017;17:94
9. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36:985-95
10. Thomas SJ, Calhoun D. Sleep, insomnia, and hypertension: current findings and future directions. J Am Soc Hypertens. 2017;11:122-9
11. Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19:2409-19
12. Javaheri S, Redline S. Sleep, slow-wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14:442-8
13. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1:227-47
14. Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56:332-43
15. Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361-71
16. Hollister LE, Muller-Oerlinghausen B, Rickels K, Shader RI. Clinical uses of benzodiazepines. J Clin Psychopharmacol. 1993;13:1S-169S
17. Hammond EC. Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr. 1966;19:127-204
18. Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103-16
19. Isacson D, Carsjo K, Bergman U, Blackburn JL. Long-term use of benzodiazepines in a Swedish community: an eight-year follow-up. J Clin Epidemiol. 1992;45:429-36
20. Thorogood M, Cowen P, Mann J, Murphy M, Vessey M. Fatal myocardial infarction and use of psychotropic drugs in young women. Lancet. 1992;340:1067-8
21. Kojima M, Wakai K, Kawamura T, Tamakoshi A, Aoki R, Lin Y. et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87-93
22. Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131-6
23. Ahmad R, Bath PA. Identification of risk factors for 15-year mortality among community-dwelling older people using Cox regression and a genetic algorithm. J Gerontol A Biol Sci Med Sci. 2005;60:1052-8
24. Fukuhara S, Green J, Albert J, Mihara H, Pisoni R, Yamazaki S. et al. Symptoms of depression, prescription of benzodiazepines, and the risk of death in hemodialysis patients in Japan. Kidney Int. 2006;70:1866-72
25. Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Saf. 2007;16:913-8
26. Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245-53
27. Winkelmayer WC, Mehta J, Wang PS. Benzodiazepine use and mortality of incident dialysis patients in the United States. Kidney Int. 2007;72:1388-93
28. Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10:279-86
29. Belleville G. Mortality hazard associated with anxiolytic and hypnotic drug use in the National Population Health Survey. Can J Psychiatry. 2010;55:558-67
30. Rod NH, Vahtera J, Westerlund H, Kivimaki M, Zins M, Goldberg M. et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300-9
31. Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850
32. Kao CH, Sun LM, Liang JA, Chang SN, Sung FC, Muo CH. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc. 2012;87:430-6
33. Iqbal U, Nguyen PA, Syed-Abdul S, Yang HC, Huang CW, Jian WS. et al. Is long-term use of benzodiazepine a risk for cancer? Medicine (Baltimore). 2015;94:e483
34. Kao CH, Sun LM, Su KP, Chang SN, Sung FC, Muo CH. et al. Benzodiazepine use possibly increases cancer risk: a population-based retrospective cohort study in Taiwan. J Clin Psychiatry. 2012;73:e555-60
35. Lan TY, Zeng YF, Tang GJ, Kao HC, Chiu HJ, Lan TH. et al. The Use of Hypnotics and Mortality-A Population-Based Retrospective Cohort Study. PLoS One. 2015;10:e0145271
36. Chen PL, Lee WJ, Sun WZ, Oyang YJ, Fuh JL. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PloS one. 2012;7:e49113
37. Wang MT, Su CY, Chan AL, Lian PW, Leu HB, Hsu YJ. Risk of digoxin intoxication in heart failure patients exposed to digoxin-diuretic interactions: a population-based study. British journal of clinical pharmacology. 2010;70:258-67
38. Health Promotion Administration of Ministry of Health and Welfare in Taiwan. Cancer Registry Annual Report, 2015 Taiwan. 2017. p. 500-1.
39. Fang SY, Chen CY, Chang IS, Wu ECH, Chang CM, Lin KM. Predictors of the incidence and discontinuation of long-term use of benzodiazepines: a population-based study. Drug & Alcohol Dependence. 2009;104:140-6
40. Nomura K, Nakao M, Sato M, Yano E. Regular prescriptions for benzodiazepines: a cross-sectional study of outpatients at a university hospital. Internal Medicine. 2006;45:1279-83
41. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC medical research methodology. 2011;11:83
42. Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS one. 2013;8:e62422
43. Massoco C, Palermo-Neto J. Effects of midazolam on equine innate immune response: a flow cytometric study. Vet Immunol Immunopathol. 2003;95:11-9
44. Massoco CO, Palermo-Neto J. Diazepam effects of peritoneal macrophage activity and corticosterone serum levels in Balb/C mice. Life Sci. 1999;65:2157-65
45. Torres SR, Frode TS, Nardi GM, Vita N, Reeb R, Ferrara P. et al. Anti-inflammatory effects of peripheral benzodiazepine receptor ligands in two mouse models of inflammation. Eur J Pharmacol. 2000;408:199-211
46. Chung KH, Li CY, Kuo SY, Sithole T, Liu WW, Chung MH. Risk of psychiatric disorders in patients with chronic insomnia and sedative-hypnotic prescription: a nationwide population-based follow-up study. J Clin Sleep Med. 2015;11:543-51
47. Wu CS, Lin YJ, Liu SK. Benzodiazepine use among patients with schizophrenia in Taiwan: a nationwide population-based survey. Psychiatr Serv. 2011;62:908-14
48. Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10:329-36
49. Schwarz S, Messerschmidt H, Doren M. [Psychosocial risk factors for cancer development]. Med Klin (Munich). 2007;102:967-79
50. Kripke DF. Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry. 2007;7:42
51. Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res. 2016;5:918
52. Emerit I. Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic Biol Med. 1994;16:99-109
53. Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9:1097-103
54. Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179-88
55. Reiter RJ. Mechanisms of cancer inhibition by melatonin. J Pineal Res. 2004;37:213-4
56. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705-6
57. Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841-7
58. Chiu HM, Chen SL, Yen AM, Chiu SY, Fann JC, Lee YC. et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121:3221-9
59. Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-96
60. Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: an Indian perspective. J Cancer Res Ther. 2009;5:71-7
61. Pecic V, Stankovic-Djordjevic D, Nestorovic M, Radojkovic M, Marjanovic H, Ilic B. et al. Hepatitis C virus-related hepatocellular carcinoma and liver cirrhosis. J BUON. 2011;16:277-81
62. Bosch FX. Human papillomavirus: science and technologies for the elimination of cervical cancer. Expert Opin Pharmacother. 2011;12:2189-204
63. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108:1098-103
64. Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32(Suppl):S66-81
65. Karmali RA, Volkman A, Muse P, Louis TM. The influence of diazepam administration in rats bearing the R3230AC mammary carcinoma. Prostaglandins Med. 1979;3:193-8
66. Horrobin DF, Trosko JE. The possible effect of diazepam on cancer development and growth. Med Hypotheses. 1981;7:115-25
67. Kleinerman RA, Brinton LA, Hoover R, Fraumeni JF Jr. Diazepam use and progression of breast cancer. Cancer Res. 1984;44:1223-5
68. Halapy E, Kreiger N, Cotterchio M, Sloan M. Benzodiazepines and risk for breast cancer. Ann Epidemiol. 2006;16:632-6
69. Chiu HY, Huang CJ, Fan YC, Tsai PS. Insomnia But Not Hypnotics Use Associates with the Risk of Breast Cancer: A Population-Based Matched Cohort Study. J Womens Health (Larchmt). 2018;27:1250-6
70. Pottegard A, Friis S, Hallas J. Cancer risk in long-term users of vitamin K antagonists: a population-based case-control study. Int J Cancer. 2013;132:2606-12
71. Pottegard A, Friis S, Andersen M, Hallas J. Use of benzodiazepines or benzodiazepine related drugs and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol. 2013;75:1356-64
72. Parkinson A, Leonard N, Draper A, Ogilvie BW. On the mechanism of hepatocarcinogenesis of benzodiazepines: evidence that diazepam and oxazepam are CYP2B inducers in rats, and both CYP2B and CYP4A inducers in mice. Drug Metab Rev. 2006;38:235-59
73. Yang B, Petrick JL, Chen J, Hagberg KW, Sahasrabuddhe VV, Graubard BI. et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016;43:105-11
74. Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010:369-405
75. National Institutes of H. National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142:1003-13
76. Savard J, Ivers H, Savard MH, Morin CM. Long-Term Effects of Two Formats of Cognitive Behavioral Therapy for Insomnia Comorbid with Breast Cancer. Sleep. 2016;39:813-23
77. Shen M, Shi Y, Xiang P. CYP3A4 and CYP2C19 genetic polymorphisms and zolpidem metabolism in the Chinese Han population: a pilot study. Forensic Sci Int. 2013;227:77-81
Author contact
![]() Corresponding authors: Min-Huey Chung, RN, PhD, School of Nursing, College of Nursing, Taipei Medical University, No.250, Wu-Xing Street, Taipei, Taiwan, 110, R.O.C. E-mail address: minhuey300edu.tw. Mei-Ju Chi, PhD, School of Gerontology Health Management, College of Nursing, Taipei Medical University, No.250, Wu-Xing Street, Taipei, Taiwan, 110, R.O.C. E-mail address: mjchiedu.tw
Corresponding authors: Min-Huey Chung, RN, PhD, School of Nursing, College of Nursing, Taipei Medical University, No.250, Wu-Xing Street, Taipei, Taiwan, 110, R.O.C. E-mail address: minhuey300edu.tw. Mei-Ju Chi, PhD, School of Gerontology Health Management, College of Nursing, Taipei Medical University, No.250, Wu-Xing Street, Taipei, Taiwan, 110, R.O.C. E-mail address: mjchiedu.tw

 Global reach, higher impact
Global reach, higher impact