Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(10):2350-2356. doi:10.7150/jca.30474 This issue Cite
Research Paper
Is Routine Subcarinal Lymph Node Dissection Necessary in Superficial Esophageal Squamous Cell Carcinoma? A Propensity Score Matching Analysis
Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, Shanghai, 200032, China
Received 2018-10-7; Accepted 2019-4-13; Published 2019-5-26
Abstract
Background: The purpose of this study was to investigate the impact of subcarinal lymph node dissection on short-term and long-term outcomes after esophagectomy in patients with superficial esophageal squamous cell carcinoma (ESCC).
Methods: From January 2010 to December 2015, 490 patients with pT1 ESCC were enrolled in the study. Patients in subcarinal dissection or non-dissection group were matched randomly in a 2:1 ratio, eventually, 255 patients were selected for further statistical analysis.
Results: The metastasis rate of subcarinal lymph nodes in superficial ESCC was 1.24% and significantly lower than the other stations (7.14-9.96%). Compared with dissection group, non- dissection group had shorter operation time (193±35 vs. 204±39, P=0.016), less blood loss (157±48 vs. 178±29, P=0.011) as well as lower incidence of pulmonary complications (9.4 vs. 20%, P=0.032). At a median follow-up of 46 months, the recurrent rate in each group was similar (16.5 vs. 15.3%, P=0.809). Survival analysis revealed no overall survival (P=0.992) and disease-free survival (P=0.665) reductions in non-dissection group. In univariate and multivariate analyses, subcarinal lymph node dissection was not a predictive factor of overall and disease-free survival in superficial ESCC.
Conclusion: Subcarinal lymph node dissection was not beneficial and could be omitted in superficial ESCC.
Keywords: subcarinal lymph node dissection, superficial esophageal squamous cell carcinoma, complication, recurrence, survival
Introduction
Esophageal cancer is one of the most common malignant tumors, accounting for approximately 455,800 new cases and 400,200 deaths in 2012 worldwide [1], most of which is reported from China. In China, esophageal squamous cell carcinoma (ESCC) is the predominant pathological type and tends to metastasize in a multidirectional fashion through the extensive submucosal lymphatics, even when the disease is a superficial lesion. Superficial esophageal cancer is categorized as intraepithelial (Tis), mucosal (T1a), or submucosal (T1b) cancer. It is reported that the incidence of lymph node metastasis in superficial ESCC ranged from 0 to 50%[2-5] and multiple regions of lymph nodes from cervix to abdomen may be involved. Thus, the importance of radical esophagectomy with 2- or 3-field lymph node dissection to achieve optimal local control has been emphasized [6]. However, extensive lymphadenectomy may lead to huge trauma, there is still fierce debate over the optimal extent of lymphadenectomy for superficial esophageal cancer.
Subcarinal lymph nodes is regarded as one of regional node stations for esophageal cancer and is suggested to be dissected by the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual[7] and the 11th edition of the Japanese Classification of Esophageal Cancer[8, 9]. However, the metastatic rate of subcarinal nodes in ESCC is relatively low, no higher than 12.9%[10-12]. What's more, considering the procedure reportedly increasing pulmonary complications[13], subcarinal node dissection had better be performed for those at high risk for metastasis. In recent years, some investigators proposed that lymph node dissection for some regions in specific patients can be omitted, such as common hepatic nodes and splenic nodes[14-16], however, no literatures have reported the outcomes of subcarinal node dissection in superficial ESCC. It remains unclear whether subcarinal node dissection confers any survival benefit to patients with superficial ESCC.
This retrospective study was performed to compare the short-term and long-term outcomes between the subcarinal nodes dissection group and non-dissection group and aimed at illustrating the value of subcarinal node dissection in superficial ESCC.
Patients and Methods
Patients
From January 2010 to December 2015, 1940 patients with esophageal cancer were performed esophagectomy at our unit. Among these patients, we retrospectively reviewed 490 patients diagnosed with pT1 ESCC without neoadjuvant therapy (Figure. 1). Preoperative routine examinations including endoscopic biopsy, barium meal, computed tomography (CT) of the chest and abdomen, positron emission tomography-computed tomography (PET-CT) and endoscopic ultrasound (EUS), pulmonary function and blood tests were performed. This study was approved by the Ethics Committee of our hospital and complied with the standards of the Declaration of Helsinki and current ethical guidelines. Informed consent was obtained from each patient.
Surgery
The surgical approaches included McKeown, Ivor-Lewis and Sweet procedures. Surgeons made the personalized operative plan and chose the most suitable procedure for individuals according to preoperative evaluation of primary tumor and potential metastatic lymph node location. Generally, McKeown procedure was used for upper or middle esophageal tumors, Ivor-Lewis procedure for middle or lower tumors and Sweet procedure for lower tumors. In this cohort of patients, en bloc lymph node dissection was performed, including the paraesophageal, subcarinal, paratracheal and tracheobronchial, pulmonary ligament, diaphragmatic, and paracardial, as well as those located along the lesser gastric curvature, the origin of the left gastric artery and the common hepatic artery. If tumors located in the upper third of esophagus, cervical lymph nodes were also dissected. Subcarinal node dissection was performed in patients with swollen nodes located under the carina, but not performed in patients without swollen nodes. According to the dissection status of subcarinal nodes, patients were divided into two groups: subcarinal node dissection group (dissection group) and subcarinal node non-dissection group (non-dissection group). Pathological staging was recorded according to the 8th edition of the TNM classification[17].
Follow-up
Follow-up visits were carried out every 3 months in the first two years and every 6 months in the following years. Regular follow-up included physical examination, tumor markers, CT of thorax and abdomen and neck ultrasonography. Follow-up was terminated in December 31st, 2016. The median follow-up time for the entire cohort was 46 months.
Flow chart of selection of patients
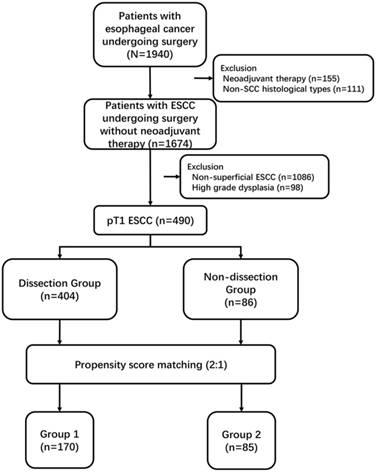
Study cohort and statistical analysis
The medical records were used to collect demographic data, tumor factors and hospital course. The classification of complications was recorded according to International Consensus of Esophagectomy Complications Consensus Group (ECCG)[18]. To control for potential differences in the characteristics of patients between the two groups, nearest neighbor propensity-score matching based on logistic regression was applied using a 1:2 ratios according to 8 baseline variables: gender, age, ASA grade, tumor location, surgical approach, cTNM, pT and pN status[19, 20]. The caliper definition was set at 0.10. Eventually, 170 cases in dissection group paired with 85 cases in non-dissection group were enrolled in the cohort, classified as group 1 and group 2, respectively (Figure 1).
Statistical analyses were performed according to the intent-to-treat principle. The SPSS 22.0 software (SPSS Inc, Chicago, Ill) was used for data analysis. The clinical and pathological parameters were described as mean ± standard eviation for continuous variables and as frequency (%) for categorical variables. Categorical variables in any two groups were compared by χ2 test or Fisher's exact test and continuous variables by Mann-Whitney U test. The Kaplan-Meier method and log-rank tests were used to compare the survival. A parameter was included in a Cox regression model if the p value was <0.1 in the univariate analysis. All p values were derived from two-tailed tests. The significance level was set at 0.05.
Results
Prevalence of Subcarinal Lymph Node Metastasis
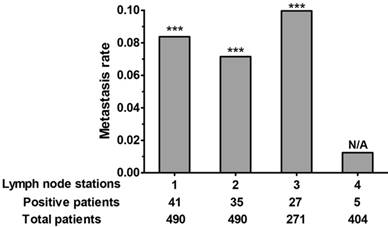
In the whole cohort, subcarinal nodes were dissected in 404 patients, 5 of which have positive subcarinal nodes. The metastasis rate of subcarinal nodes was quite low (1.24%) and significantly lower than the other stations (7.14-9.96%) (Figure. 2). In these 5 cases, subcarinal node metastasis was accompanied with synchronous multiregion node metastases, including nodes along esophagus, stomach or bilateral recurrent laryngeal nerves.
Operative Features, Complications and Pathological Data
The demographic data reached a balance after matching (Table 1). Compared with group 1, group 2 had shorter operation time (193±35 vs. 204±39, P=0.016) and less blood loss (157±48 vs. 178±29, P=0.011) (Table 2). Group 2 had significantly lower incidence of pulmonary complications than group 1 (9.4 vs. 20%, P=0.032), resulting in the lower rate of total complications (30.6 vs 41.8%, P=0.083). The incidence of other complications, such as cardiac complications, anastomotic leakage and hoarseness were comparable between the two groups (Table 2). Regarding the pathological outcomes (Table 2), there were no differences in R0 resection, tumor length, differentiation, lymphovascular invasion and TNM stage. The number of dissected lymph nodes was significantly lower in group 2 than that in group 1 (20.7±8.8 vs. 24.5±9.5, P=0.002).
Recurrence and Survival Analysis
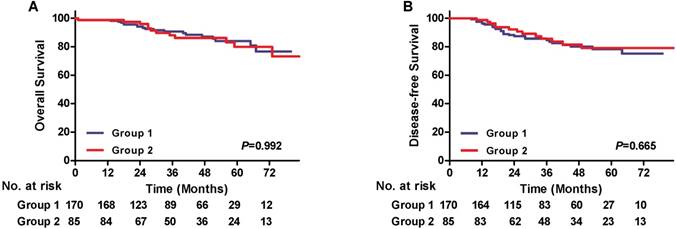
The median follow-up time was 45.5 in group 1 and 47 months in group 2, respectively, which were similar between two groups (P=0.965). There were no differences in the total recurrent rate between the groups (16.5 vs. 15.3%, P=0.809) (Table 3). And loco-regional, distant or combined recurrence was also comparable. The 3-year overall survival (OS) rate of group 1 and 2 was 89.7%, 90.4%, respectively, correspondingly, the 3-year disease-free survival (DFS) was 83.5% and 88.5%. The Kaplan-Meier and log-rank analyses revealed no OS and DFS reduction of patients in group 2 compared with those in group 1 (P=0.992, P=0.665, respectively) (Figure 3). Subgroup analyses stratified by pT and pN status were also performed. In pT1a or pT1b subgroups, the OS curves and DFS curves between the two groups showed no significant differences (Figure S1). As for pN0 or pN+ subgroup, the OS and DFS were also comparable (Figure S2). In univariate analysis, pT1b, pN+ and lymphovascular invasion were adverse risk factors for OS and DFS, while subcarinal node dissection procedure as well as other factors, including age and surgery, was not associated with survival. A multivariate Cox regression model adjusted for statistically significant prognostic factors showed that pN+ (HR = 3.365, 95% CI: 1.532-7.392, p = 0.003) was an independent prognostic factor for OS, and pT1b (HR = 3.222, 95% CI: 1.243-8.353, p = 0.016), pN+ (HR = 3.203, 95% CI: 1.645-6.234, p = 0.001) and lymphovascular invasion (HR = 3.185, 95% CI:1.400-7.245, p = 0.006) were independent adverse risk factors for DFS (Table 4).
Metastasis rate of each lymph node station (station 1, perigastric; station 2, paraesophageal; station 3, upper paratracheal/bilateral recurrent laryngeal nerves (106recR/L); station 4, subcarinal). A chi-square test was used to compare the metastasis rate of station 4 with other stations. ***p < 0.001.
Patients demographic characteristics
| Variables | Before matching | P | After matching | P | ||
|---|---|---|---|---|---|---|
| Dissection Group (n=404) | Non-dissection Group (n=86) | Dissection Group (Group 1) (n=170) | Non-dissection Group (Group 2) (n=85) | |||
| Age | 61.48±7.38 | 61.70±7.14 | 0.798 | 61.50±7.46 | 61.60±7.16 | 0.918 |
| Gender | 0.991 | 0.922 | ||||
| Male | 291(72.0) | 62(66.7) | 121(71.2) | 61(71.8) | ||
| Female | 113(28.0) | 24(33.3) | 49(28.8) | 24(28.2) | ||
| ASA grade | 0.894 | 0.845 | ||||
| I | 206(51.0) | 42(48.8) | 87(51.2) | 41(48.2) | ||
| II | 177(43.8) | 40(46.5) | 77(45.3) | 40(47.1) | ||
| III | 21(5.2) | 4(4.7) | 6(3.5) | 4(4.7) | ||
| Tumor location | 0.982 | 0.813 | ||||
| Upper | 30(7.4) | 6(7.0) | 52(30.6) | 23(27.1) | ||
| Middle | 269(66.6) | 57(66.3) | 108(63.5) | 56(65.9) | ||
| Lower | 105(26) | 23(26.7) | 10(5.9) | 6(7.0) | ||
| Surgery | 0.867 | 0.577 | ||||
| McKeown | 240(59.4) | 49(57) | 93(54.7) | 49(57.6) | ||
| Ivor-Lewis | 53(13.1) | 13(15.1) | 19(11.2) | 12(14.1) | ||
| Sweet | 111(27.5) | 24(27.9) | 58(34.1) | 24(28.3) | ||
| cTNM | 0.794 | 0.772 | ||||
| I | 314(77.7) | 65(75.6) | 136(80) | 65(76.5) | ||
| II | 73(18.1) | 16(18.6) | 26(15.3) | 16(18.8) | ||
| III | 17(4.2) | 5(5.8) | 8(4.7) | 4(4.7) | ||
| pT | 0.196 | 0.777 | ||||
| pT1a | 104(25.7) | 28(32.6) | 57(33.5) | 27(31.8) | ||
| pT1b | 300(74.3) | 58(67.4) | 113(66.5) | 58(68.2) | ||
| pN status | 0.990 | 0.530 | ||||
| pN0 | 338(83.7) | 72(83.7) | 147(86.5) | 71(83.5) | ||
| pN+ | 66(16.3) | 14(16.3) | 23(13.5) | 14(16.5) | ||
Values are expressed as mean ± standard deviation or number of patients (n, %); ASA, American Society of Anesthesiologists; *p<0.05
Survival curves. A) Overall survival (OS) according to dissection of subcarinal lymph node, 3-year OS rates: Group 1, 89.7%; Group 2, 90.4%. B) Disease-free survival (DFS) according to dissection of subcarinal lymph node, 3-year DFS rates: Group 1, 83.5%; Group 2, 88.5%.
Discussion
Esophageal cancer tended to have “upward and downward” metastases due to the unique, extensive lymphatics draining the esophagus. Some surgeons advocated wide range of mediastinal lymph node dissection for thoracic ESCC, such as 3-field lymphadenectomy[21] or extended lymphadenectomy[22]. However, extensive lymphadenectomy was reported to increase the incidence of complications, and was not likely to offer improved prognosis for all patients with thoracic ESCC[23, 24]. Therefore, the concept of individualized specific lymphadenectomy was proposed to improve postoperative quality of life and reduce the incidence of complications on the basis of ensuring oncological outcomes.
Subcarinal lymph nodes were suggested to be dissected radically as the regional nodes for thoracic esophageal cancer. But we found subcarinal node metastasis was infrequent in patients with superficial ESCC, which was consistent with previous reports[10, 12, 25]. In addition, it was hard to detect solitary subcarinal node metastasis in superficial ESCC, indicating subcarinal nodes may be not the first metastatic station. From the perspective of anatomy, subcarinal nodes mainly received lymph flow from the right middle and lower lobes of the lung, and drained into the paratracheal node chain and anterior mediastinal nodes[26]. Although lymphatic vessels coursing directly from the esophagus to the subcarinal nodes have occasionally been observed in cadavers, the afferent vessels usually originated at the intermuscular layer[27]; thus, subcarinal nodes had a low probability of being the first station involved in metastasis as a result of superficial esophageal cancer[26].
Previous studies reported subcarinal node metastasis indicated worse prognosis[10, 11, 28, 29]. Given that subcarinal node metastasis was more likely associated with an advanced disease status, the value of subcarinal node dissection in thoracic ESCC was also investigated. Hu et al[28] found the prognosis of the subcarinal non-dissection group was significantly worse than the dissection group, especially in the N0 subgroup. However, another propensity score matched study[13] showed there were no significant differences in OS and DFS between dissection and non-dissection groups and subcarinal node dissection might be futile for patients with thoracic ESCC. The concept of eliminating subcarinal node dissection is aggressive, but it may be suitable and sensible for a specific group. The newly published article[30] showed low efficacy index for the subcarinal nodes in patients with upper and lower thoracic ESCC, indicating subcarinal node dissection could be omitted for patients with upper and lower ESCC. Additionally, T stage (T2-T4) was the independent predictive factor for subcarinal node metastasis, implying that T1 ESCC may be candidates for omission of subcarinal node dissection. To be note, our study was the first research directly comparing the long-term survival between non-dissection and dissection groups in superficial ESCC, and indicated subcarinal node dissection had few contributions to better prognosis.
Another important issue was the short-term outcomes following subcarinal node dissection. It was reported subcarinal node dissection not only increased blood loss, operation time and postoperative pleural drainage volume[28], but also increased the incidence of postoperative complications significantly, especially pulmonary complications[13]. Our study also revealed the higher risk of pulmonary complications in subcarinal dissection group. Two reasons may account for such results. Firstly, the branches of the vagus nerve in the pulmonary plexus were often injured during subcarinal node dissection, resulting in decreased ability to expectorate sputum and thus induced pulmonary infection. Secondly, the heat conduction of electronic devices may damage the tracheobronchial wall as a result of increasing respiratory secretion and decreasing tracheobronchial blood flow. Given this, patients were more likely to suffer from the procedure rather than achieve benefits, although it was not difficult to perform such procedure technically. Nowadays, enhanced recovery after surgery (ERAS) has become the hotspot in multiple disciplines, and subcarinal node dissection in superficial ESCC could be left out for the implementation of ERAS.
Intraoperative, postoperative and pathological data according to preoperative treatment
| Variables | After matching | P | |
|---|---|---|---|
| Group 1 (n=170) | Group 2 (n=85) | ||
| Operative features | |||
| Operation time (min) | 204±39 | 193±35 | 0.016* |
| Blood loss (ml) | 178±29 | 157±48 | 0.011* |
| Length of stay (days) | |||
| ICU median (range) | 2(0-38) | 2(0-13) | 0.962 |
| Hospital median(range) | 11(7-97) | 10(7-64) | 0.825 |
| Complications | |||
| Total complications | 71(41.8) | 26(30.6) | 0.083 |
| Pulmonary complications | 34(20) | 8(9.4) | 0.032* |
| Cardiac complications | 5(2.9) | 2(2.4) | 1.000 |
| Digestive complications | 23(13.5) | 9(10.6) | 0.504 |
| Anastomotic leakage | 18(10.6) | 7(8.2) | 0.551 |
| Delayed gastric emptying | 5(2.9) | 2(2.4) | 1.000 |
| Surgical complications | 14 (8.2) | 8(9.4) | 0.752 |
| Postoperative bleeding | 1(0.6) | 0 | |
| Chylothorax | 1(0.6) | 1(1.2) | |
| Hoarseness | 7(4.1) | 3(3.5) | |
| Wound infection | 5(2.9) | 4(4.7) | |
| 30-day mortality | 2(1.2) | 1(1.2) | 1.000 |
| Pathological data | |||
| R0 resection | 165(97.1) | 83(97.6) | 1.000 |
| Tumor length (cm) | 1.78±0.88 | 1.74±0.71 | 0.712 |
| Differentiation | 0.813 | ||
| Well | 25(14.7) | 10(11.8) | |
| Moderate | 108(63.5) | 56(65.9) | |
| Poor | 37(21.8) | 19(22.3) | |
| Total lymph node number | 24.5±9.5 | 20.7±8.8 | 0.002* |
| Lymphovascular invasion | 10(5.9) | 5(5.9) | 1.000 |
| Pathological stage | 0.540 | ||
| pT1aN0 | 53(31.2) | 27(31.8) | |
| pT1aN1 | 3(1.8) | 0 | |
| pT1aN2 | 1(0.6) | 0 | |
| pT1bN0 | 94(55.3) | 44(51.8) | |
| pT1bN1 | 11(6.5) | 10(11.7) | |
| pT1bN2 | 8(4.7) | 4(4.7) | |
Values are expressed as mean ± standard deviation or number of patients (n, %) unless indicated otherwise; ICU, intensive care unit; *P<0.05
Surveillance data
| Variables | After matching | P | |
|---|---|---|---|
| Group 1 (n=170) | Group 2 (n=85) | ||
| Median follow-up time (months) | 45.5 | 47 | 0.965 |
| Recurrence | |||
| Total recurrence | 28(16.5) | 13(15.3) | 0.809 |
| Loco-regional | 19(11.2) | 8(9.4) | 0.666 |
| Distant | 3(1.8) | 3(3.5) | 0.661 |
| Combined | 6(3.5) | 2(2.4) | 0.899 |
| Death | |||
| Total death | 20(11.8) | 12(14.1) | 0.593 |
| Cancer-related | 14(8.2) | 9(10.6) | 0.536 |
| Intercurrent disease | 5(2.9) | 2(2.4) | 1.000 |
| Unknown | 1(0.6) | 1(1.2) | 1.000 |
Values are expressed as mean ± standard deviation or number of patients (n, %) unless indicated otherwise; *P<0.05
Univariate and multivariate analyses for overall survival and disease-free survival
| Overall Survival HR (95% CI) | Disease-free Survival HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | P | Multivariate | P | Univariate | P | Multivariate | P | ||
| Age | 1.028(0.981-1.076) | 0.245 | 0.985(0.945-1.027) | 0.480 | |||||
| Group 2 (vs Group 1) | 1.037(0.505-2.128) | 0.922 | 0.861(0.446-1.664) | 0.657 | |||||
| Surgery | |||||||||
| Sweet (ref.) | - | - | - | - | |||||
| Ivor-Lewis | 1.544(0.541-4.404) | 0.417 | 1.552(0.625-3.852) | 0.343 | |||||
| McKeown | 0.943(0.440-2.021) | 0.881 | 0.965(0.486-1.915) | 0.919 | |||||
| Number of lymph nodes | 1.020(0.982-1.058) | 0.309 | 1.017(0.985-1.050) | 0.314 | |||||
| pT1b (vs. pT1a) | 2.582(1.059-6.296) | 0.037* | 3.627(0.886-5.373) | 0.099 | 4.068(1.595-10.397) | 0.003 | 3.222(1.243-8.353) | 0.016* | |
| pN+ (vs pN0) | 3.878(1.801-8.352) | 0.001* | 3.365(1.532-7.392) | 0.003* | 4.031(2.101-7.731) | <0.001 | 3.203(1.645-6.234) | 0.001* | |
| Lymphovascular invasion | 2.563(0.981-6.698) | 0.055 | 2.581(0.987-6.751) | 0.053 | 3.512(1.552-7.944) | 0.003 | 3.185(1.400-7.245) | 0.006* | |
HR, hazard ratio; CI, confidence interval. *P<0.05
The present study was the first available analysis investigating values of subcarinal node dissection focusing on superficial ESCC. Our results showed the metastatic rate of subcarinal nodes in superficial ESCC was extremely low, indicating subcarinal nodes may have a low probability of being the first lymph node station involved in metastasis. Then the comparison between non-dissection and dissection groups revealed subcarinal node dissection did not contribute to improved survival for superficial ESCC, whereas may increase the risk of postoperative pulmonary complications as well as intraoperative blood loss and operation duration. Therefore, subcarinal node dissection could be omitted for patients with superficial ESCC.
The research also had limitations. It was a retrospective study from single medical center with a relative small sample size, which could lead to selection bias, though propensity score matching method has been performed. Therefore, new prospective randomized controlled trials in multiple centers are needed to validate these findings.
Conclusions
Subcarinal lymph node dissection might have little value for patients with superficial ESCC and could be omitted in this population.
Supplementary Material
Supplementary figures.
Abbreviations
ESCC, esophageal squamous cell carcinoma; AJCC, American Joint Committee on Cancer; CT, computed tomography; PET-CT, positron emission tomography-computed tomography; EUS, endoscopic ultrasound; ECCG, Esophagectomy Complications Consensus Group; OS, overall survival; DFS, disease- free survival; ERAS, enhanced recovery after surgery.
Acknowledgements
This work was supported by the Science and Technology Commission of Shanghai Municipality (grant number: 16411965900); and Zhongshan Hospital (grant number: 2016ZSLC15).
Ethics Committee Approval and Patient Consent
This study has been approved by the Ethics Committee of Zhongshan Hospital of Fudan University. Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
2. Amano T, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y. et al. Subepithelial extension of squamous cell carcinoma in the esophagus: histopathological study using D2-40 immunostaining for 108 superficial carcinomas. Pathology international. 2007;57:759-64
3. Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C. et al. Prediction of lymph node status in superficial esophageal carcinoma. Annals of surgical oncology. 2008;15:3278-88
4. Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y. et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:475-80
5. Gockel I, Domeyer M, Sgourakis GG, Schimanski CC, Moehler M, Kirkpatrick CJ. et al. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining. Journal of surgical oncology. 2009;100:191-8
6. Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R. et al. Surgical treatment of esophageal cancer in the era of multimodality management. Annals of the New York Academy of Sciences. 2018
7. Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017;67:304-17
8. Society JE. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus: official journal of the Japan Esophageal Society. 2017;14:37-65
9. Society JE. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus: official journal of the Japan Esophageal Society. 2017;14:1-36
10. Liu J, Hu Y, Xie X, Fu J. Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. The Annals of thoracic surgery. 2012;93:423-7
11. Ma H, Li Y, Ding Z, Liu X, Xu J, Qin J. The clinical significance of subcarinal lymph node dissection in the radical resection of oesophageal cancer. Interactive cardiovascular and thoracic surgery. 2013;16:839-43
12. Gotohda N, Nishimura M, Yoshida J, Nagai K, Tanaka N. The pattern of lymphatic metastases in superficial squamous cell carcinoma of the esophagus. Hepato-gastroenterology. 2005;52:105-7
13. Hu W, Liang Y, Zhang S, Hu Y, Liu J. Impact of subcarinal dissection on short-term outcome and survival following esophagectomy. American journal of surgery. 2013;206:314-9
14. Liu J, Liu X, Zhang J, Liu Q, Hu W. Impact of splenic node dissection on short-term outcome and survival following esophagectomy. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2017;43:440-4
15. Ma X, Li B, Yang S, Guo W, Zhu X, Li H. et al. Extent of lymph node dissection: common hepatic artery lymph node dissection can be omitted for esophageal squamous cell carcinoma. Journal of thoracic disease. 2014;6(Suppl 3):S325-32
16. Shim YM, Park JS, Lee M, Kim D, Kim K. Can common hepatic artery lymph node dissection be safely omitted in surgery for clinical T1N0 thoracic esophageal squamous cell carcinoma? Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2013;26:272-5
17. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017;12:36-42
18. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D'Journo XB. et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Annals of surgery. 2015;262:286-94
19. Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Statistical Science. 2008;2:219-36
20. Ho DE, Imai K, King G. et al. Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software. 2011:42
21. Ma GW, Situ DR, Ma QL, Long H, Zhang LJ, Lin P. et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World journal of gastroenterology. 2014;20:18022-30
22. Li B, Hu H, Zhang Y, Zhang J, Miao L, Ma L. et al. Extended Right Thoracic Approach Compared With Limited Left Thoracic Approach for Patients With Middle and Lower Esophageal Squamous Cell Carcinoma: Three-year Survival of a Prospective, Randomized, Open-label Trial. Annals of surgery. 2017
23. Fujita H, Sueyoshi S, Tanaka T, Fujii T, Toh U, Mine T. et al. Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: comparing the short- and long-term outcome among the four types of lymphadenectomy. World journal of surgery. 2003;27:571-9
24. Tachibana M, Kinugasa S, Yoshimura H, Dhar DK, Nagasue N. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Archives of surgery. 2003;138:1383-9 discussion 90
25. Kato H, Tachimori Y, Mizobuchi S, Igaki H, Ochiai A. Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer. 1993;72:2879-82
26. C B. Lymphatics of the mediastinum, esophagus and lungs: thoracic surgeon's point of view. Jurnalul de Chirurgie. 2014;10:101
27. Kuge K, Murakami G, Mizobuchi S, Hata Y, Aikou T, Sasaguri S. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. The Journal of thoracic and cardiovascular surgery. 2003;125:1343-9
28. Hu W, Liang Y, Zhang S, Hu Y, Liu J. The significance of subcarinal dissection in esophageal cancer surgery. Asia-Pacific journal of clinical oncology. 2014;10:183-9
29. Feng JF, Zhao Q, Chen QX. Prognostic value of subcarinal lymph node metastasis in patients with esophageal squamous cell carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2013;14:3183-6
30. Niwa Y, Koike M, Hattori M, Iwata N, Takami H, Hayashi M. et al. The Prognostic Relevance of Subcarinal Lymph Node Dissection in Esophageal Squamous Cell Carcinoma. Annals of surgical oncology. 2016;23:611-8
Author contact
![]() Corresponding author: Lijie Tan, Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Xuhui District, Shanghai, 200032, People's Republic of China. E-mail: tan.lijiesh.cn; Phone number: +86-2164041990; Fax number: +86-2164041990
Corresponding author: Lijie Tan, Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Xuhui District, Shanghai, 200032, People's Republic of China. E-mail: tan.lijiesh.cn; Phone number: +86-2164041990; Fax number: +86-2164041990

 Global reach, higher impact
Global reach, higher impact