Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(13):3012-3020. doi:10.7150/jca.30669 This issue Cite
Research Paper
Triptolide sensitizes cisplatin-resistant human epithelial ovarian cancer by inhibiting the phosphorylation of AKT
1. Department of Obstetrics & Gynecology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, PR China
2. Institute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330031, PR China
Received 2018-10-14; Accepted 2019-4-26; Published 2019-6-2
Abstract
Advanced and chemotherapy-resistant ovarian cancer causes high mortality of ovarian cancer, and it is important to find safe and effective drugs to reduce the chemotherapeutic resistance of ovarian cancer. In our study, we attempted to clarify the resistance mechanisms of SKOV3/DDP cells in vitro and evaluated the sensitization to triptolide (TPL) in vivo. Our results indicated that the overexpression of AKT and p-AKT greatly enhanced the cisplatin (DDP) tolerance of SKOV3/DDP, and the combination of DDP+TPL had a significant tumour inhibition effect compared to DDP treatment (p<0.05), via reducing the expressions of p-PI3K, p-Akt, Survivin, VEGF and MMP-2, and the increase of Caspase-3. Collectively, these results suggest that the synergistic anticancer effect of TPL and DDP warrants their potential clinical applications in further.
Keywords: Triptolide, Cisplatin, PI3K/Akt, SKOV3/DDP, Sensitisation
Introduction
Ovarian cancer is the main cause of death and accounts for substantial morbidity and mortality worldwide [1]. Epithelial ovarian cancer (EOC) belongs to one of the most common type of ovarian cancer, and is usually diagnosed at an advanced stage for the ineffective screening strategies. Though a combination of cisplatin‑based chemotherapy and surgical resection has prolonged clinical remission for EOC patients, while only a 30% overall survival is obtained for patients with advanced disease, mainly caused by the chemoresistance [2]. Moreover, the severe side effects of cisplatin (e.g., severe toxicity, functional impairment) have also limited its clinical applications [3-5]. Therefore, it is important to find a suitable sensitizer to cisplatin for EOC patients, and clarify its mechanisms of action.
As the important source of emerging preventive and therapeutic agents, phytochemicals play a key role in cancer treatment, and triptolide (TPL), purified from the Thundergod vine, Tripterygium wilfordii Hook. f, have been regarded as one of the most promising anti-inflammatory agents, and has been widely used to treat a variety of cancers and rheumatoid arthritis [4, 6-10]. However, little study has been done to evaluate its sensitisation effect on cisplatin-resistant EOC.
In previous work, our group indicated that TPL had synergistically enhanced the cytotoxicity of cisplatin, and promoted the apoptosis of the SKOV3PT cells through mitochondria - derived ROS accumulation [9]. However, little work was done to explore the potential role of PI3K/Akt pathway in cisplatin-resistant EOC though silencing the PI3K/Akt signal channel showed a positive role in the treatments of other cancers [11]. As we know, the PI3K/Akt pathway plays a key role in regulating the cell cycle, and it can directly regulate tumourigenesis, metabolism, cell growth, proliferation, angiogenesis, survival and apoptosis [12, 13]. In various cancers, PI3K/Akt pathway is overactive, therefore this pathway shows an important role in the development of various therapies [14].
In our work, we investigated the role of the PI3K/Akt pathway in cisplatin-resistance EOC using SKOV3/DDP cells and further studied the therapeutic and sensitisation effects of TPL by reducing the production of pathway-related proteins using animal model.
Materials and Methods
Cell culture
The SKOV3 cell line (human ovarian carcinoma‑derived) and platinum-resistant SKOV3 /DDP cell line (human ovarian carcinoma‑derived) were cultured using RPMI‑1640 medium supplemented with 10% foetal bovine serum and 100 U/ml penicillin / streptomycin in a 5% humidified CO2 atmosphere at 37 ˚C, and 0.3 μg/ml cisplatin was added into the SKOV3 /DDP culture media to maintain the acquired resistance to cisplatin.
Cell growths were done by seeding 50,000 cells in 6-well plates and cultured for 1 day, 2 day, 3 day, 4 day, 5 day, 6 day, 7 day, 8 day and 9 day (n=5), and cell growth was determined using a TC10 Automated Cell Counter (Bio-Rad).
siRNA transfection
SKOV3/DDP cells were cultured at density of 19,000 cells/cm2. Twenty-four hours after plating, the scramble siRNAs or negative control FAM were mixed with RNAi-Max transfection reagent, and the best transfection concentration and siRNA fragments were determined using a flow cytometry assay.
Quantitative real-time PCR
SKOV3 and SKOV3 /DDP cells were plated into 24-well plates (50,000 cells per well) for 24 h, and their RNAs were isolated using Trizol solution (Life Technologies, Grand Island, NY). When removing genomic DNA using DNAse I (Ambion), 2.5 μg of the total RNA isolated from SKOV3 and SKOV3 /DDP cells were reverse transcribed to cDNA by a commercially available kit (Applied Biosystems). Then, quantitative real-time PCR was done using a 7900HT fast real-time PCR system (ABI, Foster City, CA) with 2×SYBR Green master mix (Bio-Rad). Forty cycles were performed as follows: 95 ºC for 30 s, 60 ºC for 30 s, preceded by 1 min at 95 ºC for polymerase activation using the following primers (Q-PCR PI3K: sense primer 5'- GTAAAGGAGCCCAAGAATGC -3', antisense primer 5'- GAGCCAAGCATCATTGAGAA -3'; Q-PCR Akt: sense primer 5'- GTGGACCAACGTGAGGCTC, antisense primer 5'- GAAGGTGCGTTCGATGACAG -3'; Q-PCR β-actin: sense primer 5'- CATCGAGCACGGCATCGTCA -3', antisense primer 5'- TAGCACAGCCTGGATAGCAAC -3').
Western Blotting
Whole-cell lysates of samples were prepared by cell lysis buffer, containing 1 mM PMSF and protease inhibitor cocktail [4]. Protein concentrations were determined using polyacrylamide gel electrophoresis. After the protein was electrotransfered to polyvinylidene difluoride membranes, 5% nonfat milk in TBST was used to block nonspecific binding sites for 1 h at RT. Primary antibodies were added and incubated with membranes overnight at 4 °C, and then washed using TBST, then appropriate HRP-conjugated secondary antibody was added and incubated for 1 h.
Distribution of cell cycle
The 70% ice-cold ethanol was used to fix cells, and PI solution (0.1% Triton X-100, 30 mg/mL polyethylene glycol, 25 μg/mL PI and 180 U/mL RNase in 4 mM citrate buffer, pH 7.8; Sigma Chemical) was used to stain cells. Then, a FACScan flow cytometer (BectonDickinson, San Jose, CA) was used to determine the DNA content, and flowJo software (Treestar, Inc., San Carlos, CA) was used to analysis the cell cycle distribution.
Cell apoptosis
Treated cells were obtained and washed twice using cold phosphate‑buffered saline (PBS, pH=7.6), and then suspended with a binding buffer containing PI and Annexin V‑FITC and was incubated for 15 min at RT in the dark. Then, fluorescence‑activated cell sorting (FACS) cater‑plus flow cytometry (Becton Dickinson, San Jose, CA) was used to determine cell apoptosis.
Ovarian cancer modeling
To develop primary tumour xenografts, an insulin syringe was used and injected in the BALB/c-nu nude mouse with 20 μl PBS containing 500,0000 SKOV3 /DDP cells. Mice were checked daily. When the tumour xenografts in mice reached a size of 100 mm3, PBS (50 ml/kg/d every day), DDP (4 mg/kg/d on the 1 day and 8 day), TPL (0.15 mg/kg/day every day), DDP+TPL (4 mg/kg/d of DDP on 1 day and 8 day, 0.15 mg/kg/day of TPL every day) was injected i.p. into the mice. At the end of experiment, the mice were sacrificed and their tumours were removed, measured, and weighed. This study had gotten the approval by the Ethical Committee of the second affiliated hospital of Nanchang University, and all methods used in the present study were in accordance with the approved guidelines.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Sample sections were soaked in xylene and rehydrated in alcohol. Then, samples were covered with 50 μL of proteinase-K solution and incubated at RT for 25 min, and 3 % H2O2 was used to inactivate their endogenous peroxidase activity. The TUNEL assay was done using a kit (Trevigen, MD, USA) based on the manufacturer's protocol. A positive control and negative control were performed, and four randomly chosen fields per sample with at least 100 cells were determined at high magnification to calculate the percentage of TUNEL-positive nuclei.
Immunohistochemical staining
Sample sections were fixed in 10% buffered formalin, and were then embedded in paraffin and were mounted on slides. After deparaffinisation and rehydration, sample sections were soaked in 3% hydrogen peroxide to inactive endogenous peroxidase activity, and 10-mM sodium citrate (pH 6.0) was used for antigen retrieval by microwaving. Samples were then incubated 2.5% horse serum and with antibodies overnight at 4 ºC, and were detected using the Avidin-Biotin Complex System (Vector Laboratories).
Statistical analysis
Data were presented as mean ± SD and analysed using Student's t test, and p < 0.05 was considered statistically significant.
Results
Possible mechanism of SKOV3/DDP cells
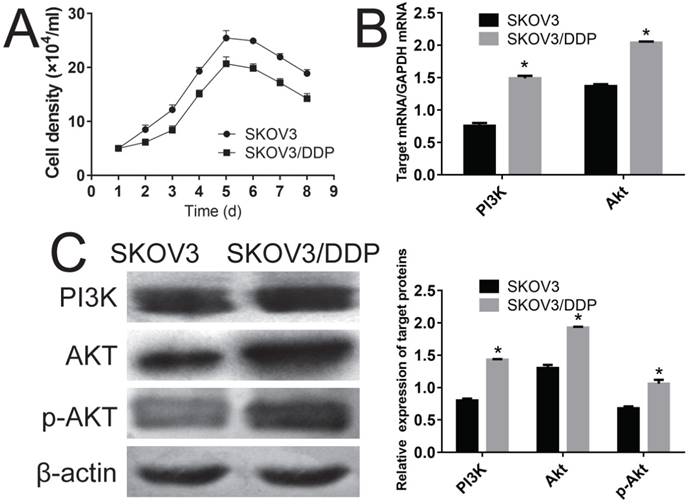
First, the growth curve for SKOV3 and SKOV3 /DDP was compared. Fig. 1A shows that both the SKOV3 and SKOV3 /DDP cells gotten their plateau on 5th-6th days, and SKOV3 /DDP cells grown slower than that of SKOV3 cells throughout the experiment. Q-PCR analyses clearly indicated that the PI3K and Akt in SKOV3 /DDP cells were significantly higher than those of SKOV3 cells at mRNA level (Fig. 1B, P<0.05), and the overexpression of PI3K, Akt and p-Akt in SKOV3 /DDP cells showed that the persistently activated PI3K/Akt channel might cause the cisplatin resistance of SKOV3 /DDP cells (Fig 1C, P<0.05).
Evaluation of the possible mechanism of cisplatin resistance using SKOV3 and SKOV3/DDP cell lines. A, comparison of the growth curve of SKOV3 and SKOV3/DDP cell lines. B, comparison of genes of PI3K and Akt in SKOV3 and SKOV3/DDP cells using RT-PCR. C, comparison of proteins of PI3K and Akt in SKOV3 and SKOV3/DDP cells using Western-blot. *: p<0.05.
Verification of the mechanism of SKOV3/DDP cells
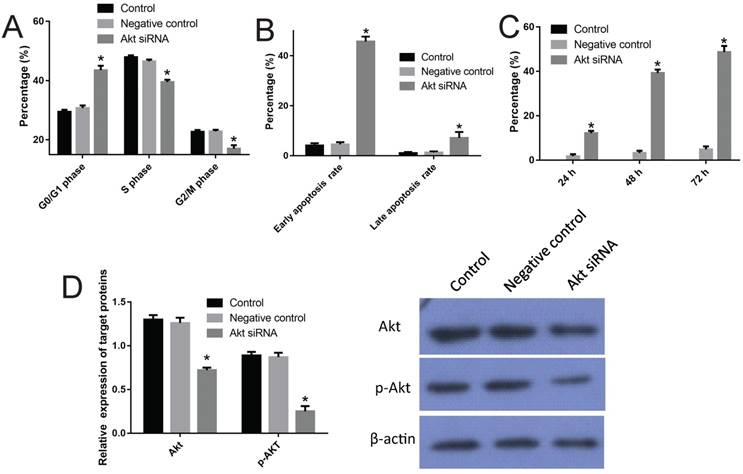
To confirm the role of PI3K/Akt channel in the cisplatin resistance of SKOV3/DDP cells, specific siRNA was performed to interfere with the production of AKT in SKOV3/DDP cell line. First, three different transfection concentrations of 50 nM, 80 nM and 100 nM were tested; 50 nM was chosen for the analysis of comparable transfection efficiency (Fig S1). Three siRNA fragments named as siRNA-1, siRNA-2 and siRNA-3 were used, and siRNA-2 was chosen for its significant inhibition efficiency with respect to Akt expression (85%, P<0.05) (Fig S2). Just as expected, the transfection of siRNA-2 arrested SKOV3/DDP cells in the G0/G1 phase (up to 44.9% from 29.2% in control) and made a typical subdiploid peak (up to 45.7% from 4.2%) on the DNA histogram (Fig 2, P<0.05). Moreover, the Western blot results indicated that transfection of siRNA-2 significantly reduced the overexpression of Akt and p-Akt in SKOV3/DDP cells (Fig 2, P<0.05).
Verification of the mechanism of cisplatin resistance of SKOV3/DDP cells using Akt siRNA. A, effect of the Akt siRNA on the cell cycle of SKOV3/DDP cells (also see Figure S3). B, effect of the Akt siRNA on the apoptosis rate of SKOV3/DDP cells (also see Figure S3). C, inhibition rate of Akt siRNA on SKOV3/DDP cells. D, effect of the Akt siRNA on expressions of AKT and p-AKT. *: p<0.05.
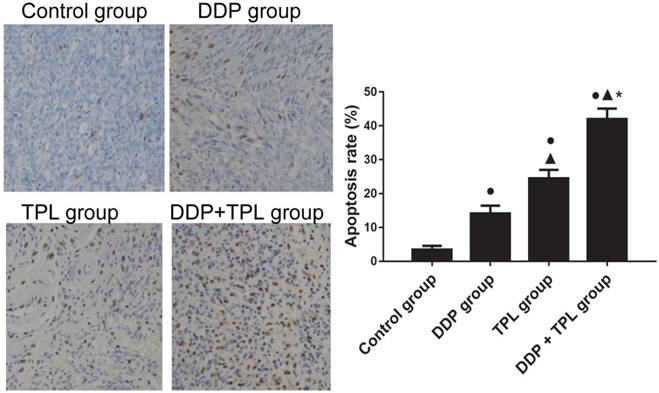
Effect of DDP, TPL and DDP+TPL on the apoptosis rate in tumor tissues using TUNEL assay. ●P<0.05 (compare with control group); ▲P<0.05 (compare with DDP group); *P<0.05 (compare with TPL group). (n=8).
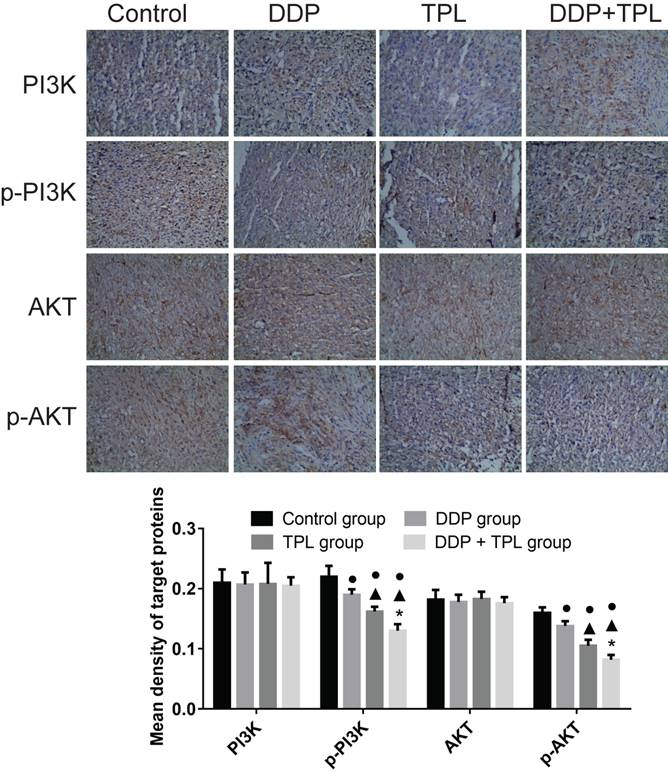
Effect of DDP, TPL and DDP+TPL on the expression of PI3K, p-PI3K, AKT and p-AKT using immunohistochemical method. ●P<0.05 (compare with control group); ▲P<0.05 (compare with DDP group); *P<0.05 (compare with TPL group). (n=8).
TPL treatment prohibits tumour growth in mice model
To study the sensitisation effects of TPL on cisplatin-resistant tumours, DDP, TPL and DDP+TPL were given to mice via intraperitoneally injection. In Table 1, DDP, TPL and DDP+TPL markedly reduced the weight and volume of tumours (P<0.05), and the volume/weight in the DDP+TPL group was 210.81±73.84 mm3/0.28±0.05 g, demonstrating inhibition that was significantly stronger than that in the DDP group (1060.44±376.58 mm3/0.94±0.07 g) and TPL group (572.00±193.15 mm3/0.60±0.04 g) (P<0.05). Moreover, the immunohistochemistry indicated that the level of cell apoptosis in the DDP+TPL group (42.02%) was higher than that in the DDP group (14.14%) and TPL group (24.48%) (Fig 3, P<0.05).
TPL inhibits p-PI3K phosphorylation and p-Akt phosphorylation
To better understand the sensitisation of mechanism of TPL with respect to DDP, protein production of PI3K, p-PI3K, Akt and p-Akt was compared among the control group, DDP group, TPL group and DDP+TPL group. In Fig 4, the protein productions of PI3K and AKT remained stable, while the levels of phosphorylated PI3K and AKT significantly decreased after DDP, TPL, or DDP+TPL treatment (P<0.05).
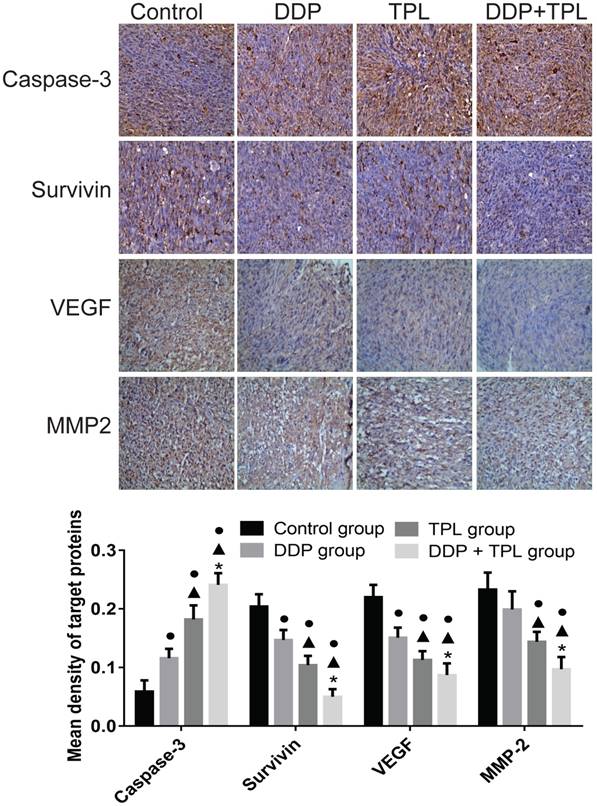
TPL affects expression of Caspase-3, Survivin, VEGF and MMP-2
Tumour development is a complex physiological process, therefore the cancer-related proteins (e.g., Caspase-3, Survivin, VEGF and MMP-2) were also tested. In Fig 5, the DDP, TPL and DDP+TPL markedly increased the expression of apoptosis-promoting protein Caspase-3 (DDP+TPL group > TPL group > DDP group, P<0.05) and obviously decreased the expression of apoptosis-inhibiting proteins (Survivin, VEGF and MMP-2) (DDP+TPL group < TPL group < DDP group, P<0.05).
Comparision of the tumor parameters among control group, DDP group, TPL group and DDP + TPL group (mean ± SD, n=8)
| Group | longest diameter (mm) | shortest diameter (mm) | volume (mm3) | weight (g) | inhibition rate (%) |
|---|---|---|---|---|---|
| Control group | 17.13±2.53 | 13.38±1.30 | 1567.44±536.17 | 1.36±0.03 | 0 |
| DDP group | 15.25±1.83● | 11.63±1.41● | 1060.44±376.58● | 0.94±0.07● | 44.5 |
| TPL group | 13.00±1.41● | 9.25±1.034●▲ | 572.00±193.15●▲ | 0.60±0.04●▲ | 56.7 |
| DDP+TPL group | 9.00±1.07●▲* | 6.75±0.89●▲* | 210.81±73.84●▲* | 0.28±0.05●▲* | 84.4 |
Note: ●P<0.05 (compare with control group); ▲P<0.05 (compare with DDP group); *P<0.05 (compare with TPL group)
Effect of DDP, TPL and DDP+TPL on the expression of caspase-3, survivin, VEGF and MMP-2 using immunohistochemical method. ●P<0.05 (compare with control group); ▲P<0.05 (compare with DDP group); *P<0.05 (compare with TPL group). (n=8).
Discussion
Cisplatin has been used as the first line for cancer treatment, including ovarian cancer, small-cell lung cancer, lymphomas, bladder cancer, cervical cancer and germ cell tumours. Cisplatin can bind to DNA and cause the DNA strands to crosslink, which triggers cells to die in a programmed way. Although the combination of platinum‑based chemotherapy and the apparent curative resection has significantly improved survival length of patients, the serious adverse side-effects and drug resistance hindered its application in ovarian cancer therapy [15].
Therefore, the naturally occurring and plant-derived compound is solely studied as potential therapies for cancers [16]. As phytochemicals can influence multiple signalling channels and enhance the efficiency of conventional chemotherapy and radiation therapy, they may present synergistic effects even a lower doses is used [17]. To date, TPL had been investigated for its pleiotropic anticancer activities, via inhibiting the proliferation and inducing apoptosis of different cancers both in vitro and in vivo, and it also shows a sound effect on drug‑resistant multiple myeloma and cervical cancer [6, 9, 18-23].
Currently, various small molecule signal transduction inhibitors have been used to treat cancers, and some of them are undergoing clinical evaluation, and these inhibitors offer significant promise in prostate cancer and have recently become standard therapy in renal cell carcinoma. As one of the most important channels, the PI3K/Akt pathway is thought to be up-regulated in about 40% of prostate cancers, and molecular changes of PI3K/Akt/ signalling channel have been regarded to differentiate benign from malignant prostatic epithelium, and have a strong connection with increasing cancer stage, grade, and risk of biochemical recurrence [24-26].
In our study, we compared the protein production of PI3K and Akt in SKOV3 and SKOV3 /DDP, and the overexpression of PI3K, Akt and p-Akt in SKOV3 /DDP cell line. Our results suggested that the PI3K/Akt signal might be responsible for the cisplatin resistance of SKOV3 /DDP cell line (Fig 1). Then, we silenced AKT expression in SKOV3 /DDP cell line using siRNA technology and the results indicated that the interference of AKT arrested SKOV3/DDP cells in the G0/G1 phase (44.9%), significantly reducing the overexpression of Akt and p-Akt (P<0.05); 45.7% of the cells were in the apoptosis stage, compared with 4.2% of the cells in the control group (P<0.05). Interestingly, 0.3 μg/ml cisplatin in culture media of SKOV3 /DDP cells resulted in a slower growth rate than observed in the SKOV3 cells, although SKOV3/DDP cells overexpressed Akt and p-Akt.
Classically, cancer was regarded as a set of diseases that were caused by progressive genetic abnormalities (include mutations in tumour-suppressor genes, oncogenes and chromosomal abnormalities). Caspase-3 belongs to a member of the cysteine protease family, playing a crucial role in apoptotic channels by cleaving a variety of key cellular proteins [27], while Survivin belongs to a member of the inhibitor of apoptosis (IAP) family that inhibits caspase activation, thereby negatively regulation the apoptosis or programmed cell death [28]. Other proteins, e.g. vascular endothelial growth factor (VEGF), which supplies adequate blood for tumour growth [29], and MMP-2,the altered expression and activity levels of which is strongly implicated in the progression and metastasis of various cancer [30-32], were also evaluated. Similar to the PI3K/Akt pathway, the combination of DDP and TPL significantly increased the expression of Caspase-3 (P<0.05) and decreased the production of Survivin, VEGF and MMP-2 (P<0.05), which indicated the promoting effect of DDP + TPL on caspase-3 expression as well as an inhibitory effect on Survivin, VEGF and MMP-2, contributing to tumour apoptosis and tumour suppression (Fig 4 and Fig 5).
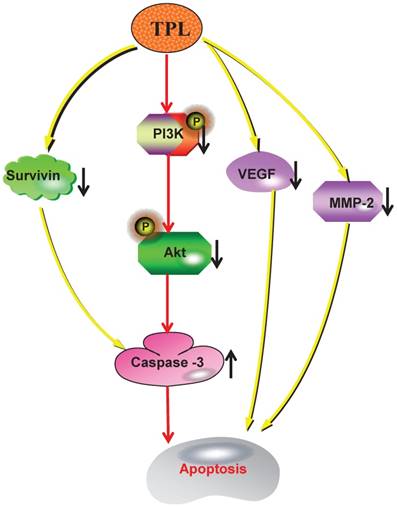
In conclusion, our work indicated that TPL had significantly down-regulated the expression of Survivin and the phosphorylations of PI3K and Akt, which significantly enhanced the production of Caspase-3. And the overexpression of Caspase-3 and the reduction of VEGF and MMP-2 caused by TPL had markedly promoted cell apoptosis and tumour suppression (Fig 6). Therefore, we propose that TPL assists DDP in lowering the resistance of EOC to cisplatin, which indicates that TPL is a promising agent that should be further evaluated in combination with other chemotherapeutic agents for the treatment of human ovarian cancer. However, our findings are based on one cisplatin resistant ovarian cancer cell line derived from SKOV3 cells, which might not be a true representative of high grade serous ovarian cancer - the most common subtype of epithelial ovarian cancer and responsible for most ovarian cancer deaths, and these findings may be cell line specific and not be clinically truly relevant.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81760729, 81260382, 81503364, 31560264) and the Jiangxi Government (20151BAB205001, 20181BBG70028, 20181BCB24003, 20171BCB23028).
The mechanism of TPL to sensitize cisplatin-resistant human epithelial ovarian cancer.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
Buzhen Tan and Tingtao Chen designed the current experiment; Genhua Huang, Hui Hu, Yao Zhang, Yinfang Zhu and Junli Liu performed the experiments; Buzhen Tan and Tingtao Chen analyzed all data and wrote this article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Second Affiliated Hospital of Nanchang University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519-29
2. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nature Reviews Cancer. 2003;3(7):502-16
3. Lux MP, Fasching PA, Beckmann MW. Hereditary breast and ovarian cancer: review and future perspectives. J Mol Med. 2006;84(1):16-28
4. Johnson SM, Wang X, Evers BM. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2011;168(2):197-205
5. Hu H, Luo L, Liu F. et al. Anti-cancer and Sensibilisation Effect of Triptolide on Human Epithelial Ovarian Cancer. J Cancer. 2016;7(14):2093-9
6. Manzo SG, Zhou Z-L, Wang Y-Q. et al. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72(20):5363-73
7. Li H, Pan G-f, Jiang Z-z, Yang J, Sun L-x, Zhang L-y. Triptolide inhibits human breast cancer MCF-7 cell growth via downregulation of the ERα-mediated signaling pathway. Acta Pharmacologica Sinica. 2015;36(5):606-13
8. Ziaei S, Halaby R. Immunosuppressive, anti-inflammatory and anti-cancer properties of triptolide: A mini review. Avicenna Journal of Phytomedicine. 2016:1-11
9. Zhong YY, Chen HP, Tan BZ, Yu HH, Huang XS. Triptolide avoids cisplatin resistance and induces apoptosis via the reactive oxygen species/nuclear factor-κB pathway in SKOV3PT platinum-resistant human ovarian cancer cells. Oncology letters. 2013;6(4):1084-92
10. Hu H, Huang G, Wang H. et al. Inhibition effect of triptolide on human epithelial ovarian cancer via adjusting cellular immunity and angiogenesis. Oncol Rep. 2018;39(3):1191-6
11. Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34(6):462-8
12. King D, Yeomanson D, Bryant HE. PI3King the Lock: Targeting the PI3K/Akt/mTOR Pathway as a Novel Therapeutic Strategy in Neuroblastoma. J Pediatr Hematol Oncol. 2015;37(4):245-51
13. Sandler AD, Knudsen MW, Brown TT, Christian RM. Neurodevelopmental dysfunction among nonreferred children with idiopathic megalencephaly. The Journal of pediatrics. 1997;131(2):320-4
14. Hengst M, Tücke J, Zerres K, Blaum M, Häusler M. Megalencephaly, mega corpus callosum, and complete lack of motor development: delineation of a rare syndrome. American Journal of Medical Genetics Part A. 2010;152(9):2360-4
15. Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. The Journal of reproductive medicine. 2005;50(6):426-38
16. Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102(3):580-92
17. White KL, Rider DN, Kalli KR, Knutson KL, Jarvik GP, Goode EL. Genomics of the NF-κB signaling pathway: hypothesized role in ovarian cancer. Cancer Causes Control. 2011;22(5):785-801
18. Yang M, Huang J, Pan H-z, Jin J. Triptolide overcomes dexamethasone resistance and enhanced PS-341-induced apoptosis via PI3k/Akt/NF-κB pathways in human multiple myeloma cells. Int J Mol Med. 2008;22(4):489-96
19. Chen Y-W, Lin G-J, Chuang Y-P. et al. Triptolide circumvents drug-resistant effect and enhances 5-fluorouracil antitumor effect on KB cells. Anticancer Drugs. 2010;21(5):502-13
20. Carter BZ, Mak DH, Schober WD. et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108(2):630-7
21. Antonoff MB, Chugh R, Borja-Cacho D. et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146(2):282-90
22. Shu B, Duan W, Yao J, Huang J, Jiang Z, Zhang L. Caspase 3 is involved in the apoptosis induced by triptolide in HK-2 cells. Toxicology in vitro. 2009;23(4):598-602
23. Miyata Y, Sato T, Ito A. Triptolide, a diterpenoid triepoxide, induces antitumor proliferation via activation of c-Jun NH 2-terminal kinase 1 by decreasing phosphatidylinositol 3-kinase activity in human tumor cells. Biochem Biophys Res Commun. 2005;336(4):1081-6
24. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003;4(4):257-62
25. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature reviews Drug discovery. 2005;4(12):988-1004
26. Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Current cancer drug targets. 2009;9(2):237
27. Devarajan E, Sahin AA, Chen JS. et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21(57):8843-51
28. Olie RA, Simões-Wüst AP, Baumann B. et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60(11):2805-9
29. Cooper ME, Vranes D, Youssef S. et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48(11):2229-39
30. Rogers GB, Zain NMM, Bruce KD. et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Annals of the American Thoracic Society. 2014;11(4):496-503
31. Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2005;1755(1):37-69
32. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2004;1705(2):69-89
Author contact
![]() Corresponding authors: Dr. Buzhen Tan, Department of Obstetrics & Gynecology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, PR China. tanbuzhencom; Tel: +86-791-86266912 and Dr. Tingtao Chen, Institute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330031, PR China. chentingtao1984com; Tel: +86-791-83827170
Corresponding authors: Dr. Buzhen Tan, Department of Obstetrics & Gynecology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, PR China. tanbuzhencom; Tel: +86-791-86266912 and Dr. Tingtao Chen, Institute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330031, PR China. chentingtao1984com; Tel: +86-791-83827170

 Global reach, higher impact
Global reach, higher impact