Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(13):3028-3036. doi:10.7150/jca.30588 This issue Cite
Research Paper
Estradiol and Progesterone Induced Differentiation and Increased Stemness Gene Expression of Human Fallopian Tube Epithelial Cells
1. Stem Cell Laboratory, Department of Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation; Hualien, Taiwan
2. Department of Pediatrics, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation; Tzu Chi University, Hualien, Taiwan
3. Department of Obstetrics and Gynecology, Hualien Tzu-Chi Hospital, Buddhist Tzu Chi Medical Foundation; Tzu Chi University, Hualien, Taiwan
4. Institute of Medical Sciences, Tzu Chi University; Hualien, Taiwan
Received 2018-10-11; Accepted 2019-5-3; Published 2019-6-2
Abstract
Fallopian tube epithelial cells (FTECs) are thought to be the origin of epithelial ovarian cancer. However, the effect of the hormones on FTECs is unknown, and therefore, this study explored this effect. We successfully derived FTECs from the fallopian tube epithelial layer and treated them with estradiol and progesterone. Reverse transcription polymerase chain reaction was used to evaluate the gene expression of the FTECs' hormone receptors. Confocal and electron microscopy were used to evaluate the morphology of the FTECs after they were treated with hormones. Finally, quantitative PCR was used to evaluate the gene expression of the hormone-treated FTECs. The results showed that the FTECs exhibited cuboidal cell morphology and could be maintained at a constant proliferation rate. Furthermore, flow cytometry revealed that the FTECs expressed stem cell markers, such as SSEA3, SSEA4, and Lgr5. Moreover, the FTECs could express both estrogen and progesterone receptors. In a culture treated with 400 nM estrogen, the FTECs differentiated toward ciliated cells, whereas in a culture treated with estradiol or progesterone, the FTECs increased their expression of certain stem cell markers (SSEA3, SSEA4, and Aldh1) and stemness genes [Wnt (AXIN2, LGR5, LGR6, and OLFM4) and Notch (Hes1) signaling]. In conclusion, hormones may alter the gene expressions of FTECs, and these cells may provide new insights into how FTECs regenerate in response to hormones.
Keywords: Fallopian tube, epithelium, stem cells, differentiation, hormone
Introduction
The fallopian tube epithelium (FTE) comprises two cell types, namely ciliated and secretory cells. Ciliated cells are responsible for sperm and egg transportation [1,2]. Through producing fluid, secretory cells provide a microenvironment in which sperm and eggs can meet [3]. Fallopian tube epithelial cells (FTECs) have been thought to be the origin of high-grade serous ovarian carcinoma (HGSOC). Furthermore, the loss of P53 function was thought to be the necessary event for HGSOC initiation [4]. The fallopian tubes are located adjacent to the ovaries and are exposed to follicular fluid (FF) during ovulation each month [5]. FF contains numerous inflammatory factors, which can lead the epithelium to induce a double-strand break [6]. The accumulation of inflammatory factors (injuries) from repetitive ovulation may induce cancer and cell injuries.
However, because of people's reproductive needs, the regeneration of injured epitheliums is crucial. The presence of “stem cell-like” cells in the FTE has been postulated [7-9]. The Wnt and Notch signaling pathways are responsible for the continuous proliferation and differentiation of organoids [10]. Wnt/beta-catenin is one of the major signaling pathways maintaining pluripotency in stem cells [11,12]. Frizzled receptors and LRP5/6 coreceptors bind to Wnt and inhibit beta-catenin degradation, thereby promoting cell-cycle progression and inhibiting apoptosis [13]. Dkk1 was able to block this signaling pathway through LRP6 coreceptor isolation [14]. In the intestines and ovaries [15], Wnt signaling could also act through R-spondin family proteins [16] and bind to Wnt target genes [17].
In the intestinal tract, skin, liver, and ovaries [10], stemness is maintained by active Wnt signaling and is centered on a subfamily of leucine-rich repeat-containing G protein-coupled receptors called Lgr4, Lgr5, and Lgr6 [10], which are Wnt target genes and have the capacity to greatly amplify Wnt signals; the R-spondin family of proteins acts as LGR receptor agonists [18].
Notch signaling is responsible for regulating genes of known stem cell factors [10]. Notch inhibitors decrease both Notch signaling and Wnt signaling as well as another stemness gene, olfactomedin 4 (OLFM4), in the FTE [10].
Estrogen is a steroid hormone and has an effect on multiple tissues, including the FTE. Estrogen can help the movement and maturation of eggs, sperm, and embryos in the fallopian tube [19]. Furthermore, it can send signals through three types of receptor, including estrogen receptor (ER) alpha, ER beta, and ER G protein-coupled receptors [20,21]. Hormone therapy featuring estrogen was suggested to increase the incidence of ovarian cancer [22]. A relevant study showed that ER alpha was expressed in 80% of HGSOC tissues, and progesterone receptor (PR) was expressed in 30% of HGSOC tissues [23]. Another relevant study showed that the FTE of mice could respond to estrogen stimulation through regulating the expression of a tissue-specific set of target genes [24].
Both estrogen and progesterone have crucial effects on the antiapoptosis, proliferation, contraction, and cytokine production in the FTE [24-27]. A report indicated that progesterone could induce necroptosis in p53-defective FTECs and prevent the occurrence of HGSOC.
Nevertheless, the effect of hormones on the morphology and stemness genes of FTECs remains unknown. Thus, the aim of this study was to evaluate the effect of hormones on morphology changes and expression of genes in derived FTECs.
Materials and Methods
Derivation and culture of primary FTECs
The experimental protocol was approved by the Research Ethics Committee of Buddhist Tzu Chi General Hospital (IRB 104-70B). Surgical specimens of fallopian tubes were obtained from patients under bilateral salpingectomy accompanied by hysterectomy or other ovarian surgery. Only normal fallopian tubes uncontaminated with cancer cells were collected. For the culture of FTECs, we followed a previously published and modified protocol [7]. In brief, the FTE was separated from the underlying stroma, and then a small segment of fallopian tube was washed in 5 mM ethylenediaminetetraacetic acid (EDTA) and incubated in 1% trypsin for 45 min. Subsequently, the FTE was digested at 37°C for 45 min in 0.8 mg/mL of collagenase in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 5 μg/mL of insulin. Next, the FTE was incubated in prewarmed 0.05% trypsin-EDTA (Invitrogen, Grand Island, NY, United States). Then, the FTE was pierced five times for dissociation, and DMEM containing 10% FBS was added to stop the trypsinization. Finally, the resulting FTECs were placed on gelatin-coated wells.
Proliferation assay
The FTECs were seeded in triplicate at a density of 2 × 103 cells/cm2 in a 96-well plate with DMEM, 10 % FBS, and 5 μg/mL of insulin. The FTECs at passage 2 and 3 were used for a proliferation assay. Cells were harvested and counted using a cell proliferation kit (XTT based, Biological Industries Ltd., Kibbustz Beit Haemek, Israel) on days 0, 3, and 7, and a growth curve was generated. XTT solution and N-methyl dibenzo-pyrazine methyl sulfate (PMS) were defrosted immediately prior to use in a 37°C bath. PMS was added to the XTT solution immediately before use, and 50 μL of XTT/PMS was added to each 100-μL culture. After 2-5 h of incubation, the optical density of the wells was determined using a spectrophotometer (ELISA reader, BioTek, Winooski, VT, U.S.A) at a wavelength of 450 nm and a reference wavelength of 650 nm.
Flow cytometry of FTECs
Surface molecules of the FTEC cultures on passage 3 were characterized using flow cytometry. Three FTEC cell lines were enrolled for testing. Cells were detached using 2 mM EDTA in PBS, washed with PBS containing 2% bovine serum albumin and 0.1% sodium azide (Sigma, Saint Louis, MO, United States), and incubated with their respective antibody conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). The FTE stem cell marker Lgr5 (GeneTex, Irvine, CA, United States) and the embryonic stem cell markers SSEA3 (eBioscience, ThermoFisher, Waltham, MA, USA) and SSEA4 (Abcam, Cambridge, England) were used for identification of the FTECs. Cells were analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, United States).
Estrogen and progesterone treatment
Estradiol-17β (Sigma) was prepared as a stock solution at 1 mg/mL in absolute alcohol. This stock solution was diluted in culture medium to final concentrations of 200 and 400 nM to mimic FF estradiol levels [28], and progesterone (Sigma) was diluted in culture medium to a final concentration of 15 μM to mimic FF progesterone levels [29]. FTECs were treated with estradiol for 7 days and then fixed in paraformaldehyde for immunohistochemistry (IHC) staining.
Immunohistochemistry
IHC was used to evaluate the expression of anti-PAX8 antibodies for secretory cells (1:50, Abcam), anti-TUBB4 for ciliated cells (1: 50, Abcam), and DAPI for a nucleus (1:200, GeneTex, Irvine CA, United States) in FTECs. The FTECs were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 4% normal goat serum, and then treated with the abovementioned primary antibodies. The FTECs with antibody staining were analyzed using confocal laser scanning microscopy (LSM 510 META; Zeiss, Jena, Germany).
Scanning electron microscopy
FTECs cultured in culture medium with 0 and 400 nM estradiol for 14 days and grown on collagen inserts were fixed in 4% formaldehyde and 2.5% glutaraldehyde in phosphate buffer for 15 min. The membranes were dehydrated in a series of alcohols and critical-point dried. The mount membranes were coated in an SEM E5000 coating unit and examined using scanning electron microscope (H-7500; Hitachi, Tokyo, Japan). In an additional experiment, FTECs with a fibrillary appearance were quantified after estradiol treatment. First, 200 FTECs were placed in a single well of an eight-well chamber slide (Nunc, Rochester, New York, United States). After treatment with estradiol for 14 days, the FTECs were fixed and pictures were captured using a microscope. FTECs with fibrillary appearance were counted in each group (control, 200 nM E2, and 400 nM E2). Finally, the data were presented as the percentage of FTECs with fibrillary appearance.
Ribonucleic acid extraction and quantitation
Total ribonucleic acid (RNA) was prepared from two endometrial cancer cell lines (Ishikawa and HEC1A) and five FTEC lines using TRIzol solution (Invitrogen). Next, 1 μg of DNase-treated RNA was transcribed into complementary DNA using 200 units of Superscript II reverse transcriptase (Invitrogen). The sequence of primers was ERα: forward: 5'- CCACCAACCAGTGCACCATT-3' and reverse: 5'- GGTCTTTTCGTATCCCACCTTTC-3'; ERβ: forward: 5'- AGAGTCCCTGGTGTGAAGCAAG-3' and reverse: 5'- GACAGCGCAGAAGTGAGCATC-3'; PR: forward: 5'- CGCGCTCTACCCTGCACTC-3' and reverse: 5'- TGAATCCGGCCTCAGGTAGTT-3'; and GAPDH: forward: 5'- GGTCTCCTCTGACTTGAACA-3' and reverse: 5'- GTGAGGGTCTCTCTCTTCCT-3'. All PCR samples were analyzed using electrophoresis on 2% agarose gel (Invitrogen) containing 0.5 μg/mL of ethidium bromide (Sigma-Aldrich).
Total RNA of FTECs at passage 3 with different treatments (control, 200 and 400 nM E2, and 15 μM progesterone) for 7 days was extracted using an RNEasy® kit (Qiagen, Valencia, CA, United States) according to the manufacturer's instructions. Real-time PCR analysis for stemness markers (OLFM4 [forward: 5'-ACTGTCCGAATTGACATCATGG-3' and reverse: 5'-TTCTGAGCTTCCACCAAAACTC-3'] [30], AXIN2 [forward: 5'-CCTGCCACCAAGACCTACAT-3' and reverse: 5'-TTCATTCAAGGTGGGGAGA-3'] [31], and LGR6 [forward: 5'-CCTTCCTCAGCTTCACCTTG-3' and reverse: 5'-CTTCCAGGCCTTTCTCTGTG-3'] [32]); Notch signaling (Hes1 [forward: 5'-TTCTGAGCTTCCACCAAAACTC-3' and reverse: 5'-AGGCGCAATCCAATATGAAC-3']) [33]; SSEA4 synthase (forward: 5'-TGGACGGGCACAACTTCATC-3' and reverse: 5'-GGGCAGGTTCTTGGCACTCT-3') [34]; b3GalT5 (forward: 5'-GCAGATCTATGGCTTTCCCGAAGATG-3' and reverse: 5'-GTCTCGAGTCAGACAGGCGGACAAT-3') [35]; Lgr5 (forward: 5'-ACCCGCCAGTCTCCTACATC-3' and reverse: 5'-GCATCTAGGCGCAGGGATTG-3') [36]; Aldh1 (forward: 5'-CTGCTGGCGACAATGGAGT-3' and reverse: 5'-GTCAGCCCAACCTGCACAG-3') [37] was performed. Complementary DNA was synthesized using a SuperScript III One-Step RT-PCR kit (Invitrogen, Grand Island, NY, United States) and amplified using PCR with an AmpliTaq Gold Kit (Applied Biosystems, Foster City, CA, United States). Real-time PCRs were performed and monitored using a FastStart Universal SYBR Green Master kit (Rox) (Roche, Indianapolis, IN, United States) and a quantitative real-time PCR detection system (ABI Step One Plus system; Applied Biosystems, Foster City, CA, United States). The gene products were analyzed with the GAPDH gene (forward: 5'-TCT CCT CTG ACT TCA ACA GCG AC-3' and reverse: 5'-CCC TGT TGC TGT AGC CAA ATT C-3') as a reference. The expression level of each target gene was then calculated as 2-ΔΔCt as previously described [38]. Four readings of each experimental sample were obtained for each gene of interest, and the experiments were repeated at least in triplicate.
Statistical analysis
The results were expressed as the mean ± standard deviation. Raw data were analyzed using the Student's t-test to compare CD markers between two passages. Additionally, the following tests were performed: analysis of variance (ANOVA), one-way ANOVA with repeated measures, post-hoc tests, and Fisher's least significant difference test for gene expression between four treatment groups, where a p < 0.05 denoted statistical significance.
Results
FTECs isolated from the epithelial layer grew to P9
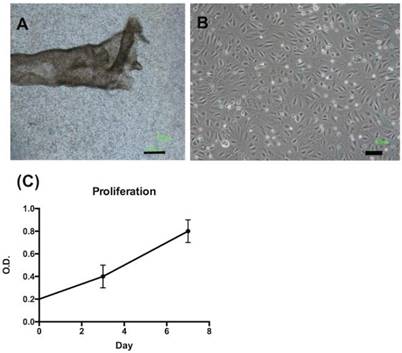
We isolated FTECs from FTE sheets (Fig. 1A). The FTECs from P2 to P6 exhibited the same morphology and had a cobblestone appearance (Fig. 1B). The proliferation pattern of FTECs at P3 exhibited a normal cell growth pattern over 7 days (Fig. 1C).
FTECs expressed the stem cell markers Lgr5, SSEA3, and SSEA4
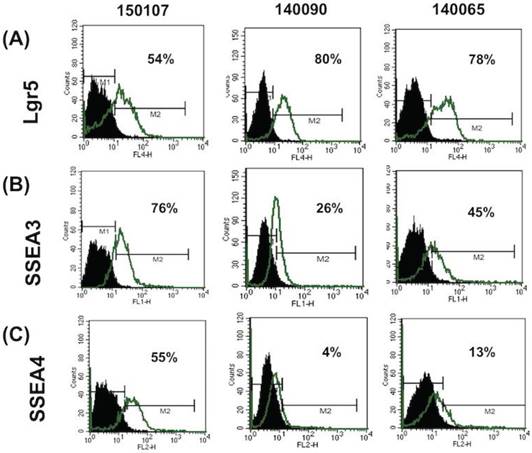
The expression of stem cell markers in FTECs was investigated using flow cytometry. Three stem cell markers, Lgr5 (intestinal and fallopian tube stem cell marker) and SSEA3 and 4 (embryonic and cancer stem cell markers), were selected for evaluation. All three FTEC lines expressed these three stem cell markers at different percentages (Fig. 2). Two cell lines expressed less SSEA4. Isotype IgG was used as a negative control. These results indicated that the isolated FTECs could express various stem cell markers.
Morphology and growth kinetics of fallopian tube epithelial cells (FTECs). (A) The epithelial layer of a fallopian tube after manual extraction and isolation. (B) After dissociation and culturing, FTECs exhibited polygonal morphology; scale bar = 100 µm. (C) After culturing for 7 days, the growth kinetics of FTECs were evaluated using XTT assay on days 3 and 7. O.D. = optical density.
Flow cytometry analysis of the expression of stem cell markers in fallopian tube epithelial cells (FTECs). Flow cytometry of Lgr5, SSEA3, and SSEA4 of three FTEC cell lines (passage 3) are illustrated. FTEC expressed all three stem cell markers with different percentages. IgG was used as a negative control.
FTECs expressed hormone receptors
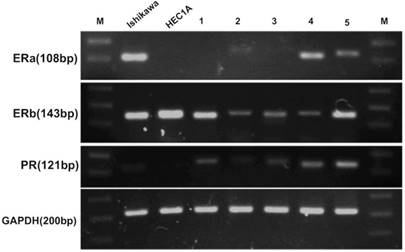
To evaluate whether FTECs could express hormone receptors to received hormone treatment, RT-PCR was used to detect gene expression. The results showed that ERα, β, and PR were expressed by FTECs (Fig. 3).
Fallopian tube epithelial cells (FTECs) could express hormone receptor genes. (A) Real-time polymerase chain reaction revealed that all FTEC cell lines (1-5) expressed estrogen receptor (ER) β and progesterone receptor (PR), and some expressed ERα. Endometrial cancer cells (Ishikawa and HEC1A cell lines) were used as positive controls. M: marker of base-pair numbers.
Hormones did not alter FTECs' morphology
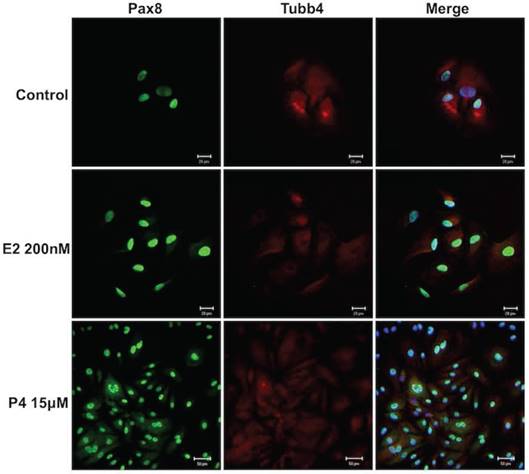
Hormones such as estradiol and progesterone have effects on the reproductive organs [25]. Therefore, estrogen and progesterone were added to the FTEC culture to investigate their effects on the cells. The cell culture was treated with 200 nM estradiol and 15 μM progesterone for 14 days. All FTECs stained positive for PAX8 and TUBB4 (Fig. 4). The results indicated no changes in PAX8 or TUBB4 expression and cell morphology after estradiol and progesterone treatment.
High concentration of estrogen caused fibrillary outgrowth of FTECs
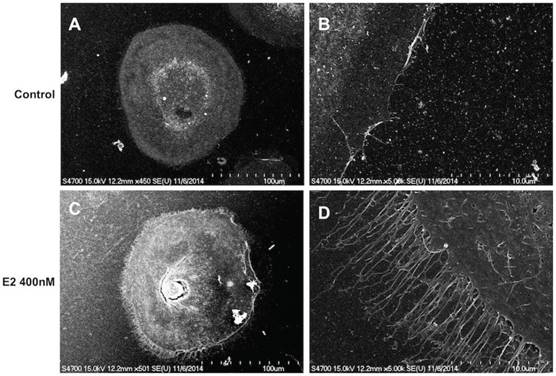
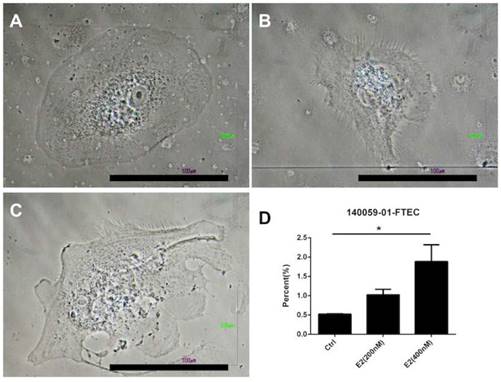
Because estrogen treatment could induce ciliated cell phenotypes [39], we explored the effects of a high concentration of estradiol on FTECs. The FTEC cells, which were grown on collagen inserts, exhibited a cobblestone appearance without villi (Fig. 5A and B). Nevertheless, scanning electron microscopy revealed fibrillary-like structures near the cell membrane after treatment with 400 nM estradiol (Fig. 5C and D). Subsequently, we quantitated the FTECs that had a fibrillary appearance after hormone treatment (Fig. 6), which revealed that in the hormone treatment group (400 nM E2) approximately 2% of cells had a fibrillary appearance. By contrast, only 0.5% of cells in the control group exhibited a fibrillary appearance (Fig. 6). Statistical analysis of the difference between the groups revealed a p-value of <0.05. In summary, a high concentration of estradiol (400 nM) caused fibrillary outgrowth in FTECs.
Estradiol and progesterone treatments had no effect on the morphology of fallopian tube epithelial cells (FTECs). Both 0 nM and 200 nM estradiol (E2) and 15 μM progesterone (P4) had no effect on cell morphology. PAX8: a secretory cell marker; TUBB4: a ciliated cell marker. Scale bar = 20 μm.
Scanning electron micrograph of human fallopian tube epithelial cells (FTECs). FTECs grown on gelatin collagen inserts for 14 days in culture medium without (A, B) or with (C, D) 400 nM estradiol (E2). The FTECs exhibited fibrillary-like structures near the cell membranes after treatment with 400 nM E2 (C, D). Scale = 100 μm in (A, C), 10 μm in (B, D).
Quantification of fallopian tube epithelial cell (FTEC)-differentiated ciliated cells after hormone treatment. (A) Gross picture of the FTECs in the control group. (B) FTECs after treatment with 200 nM estradiol (E2). (C) FTECs after treatment with 400 nM E2. (D) Quantification of hormone-treated FTECs with fibrillary appearance among groups. Scale bar = 100 μm. *p < 0.05.
Both estrogen and progesterone increased stemness gene expression in FTECs
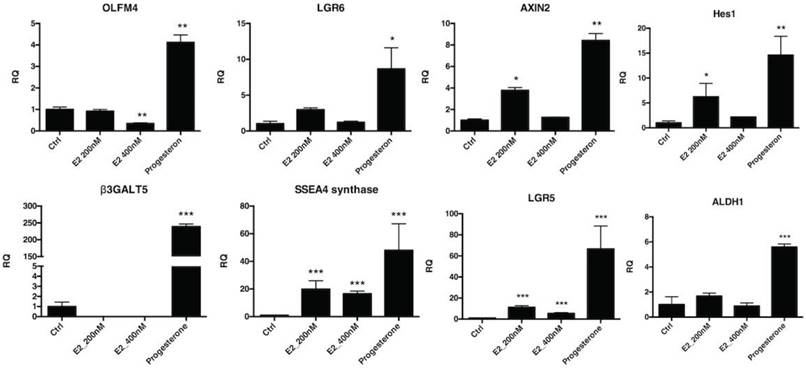
The effect of hormones on FTECs was investigated using quantitative PCR for Wnt- and Notch-related stemness genes. The FTECs were treated with estrogen and progesterone for 7 days. All stemness genes related to the Wnt (OLFM4, AXIN2, and LGR6) and Notch (HES1) signaling pathways, ES cell markers (SSEA3 and SSEA4), Aldh1, and Lgr5 had significantly increased expression after progesterone treatment (Fig. 7). Taken together, after estradiol and progesterone treatment, stemness gene expression in FTECs was significantly increased.
Discussion
In the present study, we successfully derived human FTECs, which were able to express estrogen and PRs. Estradiol treatment caused ciliated differentiation of the FTECs. Furthermore, treatment of FTECs with either estradiol or progesterone in culture medium increased stemness gene expression (both Wnt and Notch signaling pathways) compared with nontreated controls.
Quantitative mRNA expression of fallopian tube epithelial cells (FTECs) after hormone treatment. Passage 3 FTECs after 15 μM progesterone treatment for 7 days increased stemness markers (OLFM4, AXIN2, and LGR6), Notch signaling (Hes1), and stem cell markers (SSEA3, SSEA4, Aldh1, and Lgr5). The FTECs after 200 nM estradiol treatment also increased stemness markers (AXIN2, Hes1, SSEA4, Lgr5, and PAX8). *p < 0.05, **p < 0.001 compared with the controls. E2: estradiol. R.Q.: relative quantification to GAPDH.
Because the fallopian tube is exposed to cyclical hormone changes, mechanisms to ensure its long-term renewal and integrity are critical. In addition, estrogen and progesterone have crucial effects on the antiapoptosis, proliferation, contraction, and cytokine production of the FTE [24-27]. The fallopian tube could express ERβ and PRs [40,41]. A relevant study showed that after 150 ng/mL of estradiol treatment, FTE cells could differentiate into ciliated cells [39]. In the present study, we demonstrated that hormones did not influence the cells' gross morphology. However, after 7 days of exposure to a high concentration of estrogen (400 nM), a fibrillary-like structure near the cell membrane was noted. This indicated that FTECs could differentiate into ciliated cells under estrogen stimulation.
The effects of Wnt inhibition indicated a crucial role of the Wnt pathway in stemness regulation in FTECs. In one study, Notch signaling-related genes were overlapped between human fallopian tubes and mouse intestines [36], which indicated that both may have similar regulatory mechanisms. Lgr5-expressing intestinal cells exhibited a stem cell niche. In our study, after progesterone treatment, Lgr5 expression in FTECs was also increased. Another LGR subfamily, Lgr6, exhibited increased expression after progesterone treatment. These two LGR subfamilies could be the receptor of another crucial factor, R-spondin-1, which regulates the self-renewal of fallopian tube stem cells through Wnt signaling. As reported in both humans and mice, Lgr5 was expressed in ovarian surface epithelial cells but not in FTECs, whereas Lgr6 was expressed in FTECs. Nevertheless, Lgr5 expression was detected at quite high levels in the present study. This phenomenon may be caused by a two-dimensional tissue culture condition.
Studies have shown that OLFM4 is a highly specific marker for intestinal stem cells [42] and colorectal cancer [43]. OLFM4 deletion induced colon cancer [44]. In another study, the inhibition of OLFM4 inhibited tumorigenicity [45]. In our study, we found that OLFM4 exhibited increased expression following progesterone treatment. This indicated that after ovulation, progesterone might increase stemness gene expression in FTECs.
Wnt and Notch signaling are crucial for the organoid growth and differentiation of FTECs [10]. AXIN2 and LGR6 expression are related to the Wnt signaling pathway. A relevant study indicated that AXIN2 inhibition was correlated with type 1 pneumocyte differentiation from type 2 progenitor cells [46]. AXIN2 can respond to tooth damage and regenerate odontoblasts [47]. In our study, both 200 nM estradiol and 200 nM progesterone could increase AXIN2 expression in FTECs, which may indicate that during the menstrual cycle, the AXIN2 expression of FTECs can be a response to stimulation by both hormones.
SSEA3 and SSEA4 are known as embryonic stem cell markers [34,35], and both are known as cancer stem cell markers [35,48]. In our study, FTECs could express SSEA3 and SSEA4. After hormone treatment, expression of both markers was increased in FTECs.
Aldh1 is a known cancer stem cell marker and is correlated with prognosis [49]. Aldh1 is also responsible for enhanced invasiveness and the endothelial-mesenchymal transition process [50]. In our study, after progesterone treatment, Aldh1 expression was increased in FTECs.
In our study, estrogen and progesterone could increase stemness gene expression (Wnt and Notch signaling pathways). In general, less stemness gene expression was noted in the groups treated with 400 nM E2 compared with those treated with 200 nM. A study showed that a high concentration of E2 may downregulate ER and cause decreased gene expression [51]. Moreover, after progesterone treatment, stemness gene expression increased compared with the control and E2-treated groups. Another study indicated that progesterone is often considered an antagonist in modulating the effects of estrogen in the fallopian tubes [52]. In our study, stemness markers increased simultaneously after estrogen or progesterone treatment. We speculated that in the luteal phase, both estrogen and progesterone could be maintained at high concentrations to prepare a suitable environment for embryo implantation. In addition to cilia cells, secretory cells are also involved in the development of tubal secretion. Thus, we observed that stemness markers increased after estrogen and progesterone treatment.
In conclusion, we successfully derived FTECs and evaluated hormone-related changes. Increased stemness gene expression upon hormone treatment was noted. The cells derived in this study may provide new insights into how FTECs regenerate and respond to hormones.
Acknowledgements
The work was supported by the Ministry of Science and Technology, Taiwan, ROC (MOST 104-2314-B-303-MY3) and Buddhist Tzu Chi General Hospital (TCRD 104-08). The authors thank Dr. Jon-Son Kuo for English editing.
Author contributions
D.C.D. designed the experiment; D.C.D. performed experiments; D.C.D. and T.Y.C. analyzed the data; D.C.D. and T.Y.C. wrote the paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mahmood T, Saridogan E, Smutna S, Habib AM, Djahanbakhch O. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod. 1998;13:2991-4
2. Lyons RA, Djahanbakhch O, Mahmood T, Saridogan E, Sattar S, Sheaff MT. et al. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum Reprod. 2002;17:584-8
3. Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001;121:339-46
4. Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49-56
5. King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627-42
6. Huang HS, Chu SC, Hsu CF, Chen PC, Ding DC, Chang MY. et al. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: initiation of fimbria carcinogenesis. Carcinogenesis. 2015;36:1419-28
7. Paik DY, Janzen DM, Schafenacker AM, Velasco VS, Shung MS, Cheng D. et al. Stem-Like Epithelial Cells Are Concentrated in the Distal End of the Fallopian Tube: A Site for Injury and Serous Cancer Initiation. Stem Cells. 2012;30:2487-97
8. Yamamoto Y, Ning G, Howitt BE, Mehra K, Wu L, Wang X. et al. In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells. J Pathol. 2016;238:519-30
9. Wang Y, Sacchetti A, van Dijk MR, van der Zee M, van der Horst PH, Joosten R. et al. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS One. 2012;7:e40691
10. Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989
11. Pera MF, Tam PPL. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713-20
12. Chang YH, Chu TY, Ding DC. WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells. Sci Rep. 2017;7:41913
13. Zaffaroni N, Costa A, Pennati M, De Marco C, Affini E, Madeo M. et al. Survivin is highly expressed and promotes cell survival in malignant peritoneal mesothelioma. Cell Oncol. 2007;29:453-66
14. Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171-8
15. Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ. et al. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol. 2014;16:745-57
16. de Lau W, Barker N, Low TY, Koo BK, Li VSW, Teunissen H. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293-7
17. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-7
18. de Lau WBM, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13:242
19. Menezo Y, Guerin P. The mammalian oviduct: biochemistry and physiology. Eur J Obstet Gynecol Reprod Biol. 1997;73:99-104
20. Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M. et al. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279-85
21. Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715-26
22. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385:1835-42
23. Sieh W, Köbel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT. et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853-62
24. Moyle-Heyrman G, Schipma MJ, Dean M, Davis DA, Burdette JE. Genome-wide transcriptional regulation of estrogen receptor targets in fallopian tube cells and the role of selective estrogen receptor modulators. J Ovarian Res. 2016;9:5
25. Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK. et al. Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod. 2016;22:756-67
26. Eddie SL, Quartuccio SM, Zhu J, Shepherd JA, Kothari R, Kim JJ. et al. Three-dimensional modeling of the human fallopian tube fimbriae. Gynecol Oncol. 2015;136:348-54
27. Bylander A, Gunnarsson L, Shao R, Billig H, Larsson DGJ. Progesterone-mediated effects on gene expression and oocyte-cumulus complex transport in the mouse fallopian tube. Reprod Biol Endocrinol. 2015;13:40
28. Bentov Y, Jurisicova A, Kenigsberg S, Casper RF. What maintains the high intra-follicular estradiol concentration in pre-ovulatory follicles? J Assist Reprod Genet. 2016;33:85-94
29. Wen X, Li D, Tozer AJ, Docherty SM, Iles RK. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod Biol Endocrinol. 2010;8:117
30. Berger E, Rath E, Yuan D, Waldschmitt N, Khaloian S, Allgäuer M. et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat Commun. 2016;7:13171
31. Chai S, Ng KY, Tong M, Lau EY, Lee TK, Chan KW. et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 2016;64:2062-76
32. Kessler M, Zielecki J, Thieck O, Mollenkopf HJ, Fotopoulou C, Meyer TF. Chlamydia trachomatis disturbs epithelial tissue homeostasis in fallopian tubes via paracrine Wnt signaling. Am J Pathol. 2012;180:186-98
33. van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H. et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959-63
34. Truong TT, Huynh K, Nakatsu MN, Deng SX. SSEA4 is a potential negative marker for the enrichment of human corneal epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:6315-20
35. Cheung SKC, Chuang PK, Huang HW, Hwang-Verslues WW, Cho CHH, Yang WB. et al. Stage-specific embryonic antigen-3 (SSEA-3) and β3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc Natl Acad Sci U S A. 2016;113:960-5
36. Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent “+4” cell markers. EMBO J. 2012;31:3079-91
37. Yao T, Chen Q, Zhang B, Zhou H, Lin Z. The expression of ALDH1 in cervical carcinoma. Med Sci Monit. 2011;17:HY21-6
38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8
39. Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998;13:3114-20
40. Raba G, Laudanski P, Kanczuga-Koda L. Immunohistochemical findings in primary fallopian tube cancer. Case report. Eur J Gynaecol Oncol. 2010;31:120-2
41. Shao R, Feng Y, Zou S, Weijdegård B, Wu G, Brännström M. et al. The role of estrogen in the pathophysiology of tubal ectopic pregnancy. Am J Transl Res. 2012;4:269-78
42. Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K. et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208:285-94
43. van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15-7
44. Liu W, Li H, Hong SH, Piszczek GP, Chen W, Rodgers GP. Olfactomedin 4 deletion induces colon adenocarcinoma in ApcMin/+ mice. Oncogene. 2016;35:5237-47
45. Liu RH, Yang MH, Xiang H, Bao LM, Yang HA, Yue LW. et al. Depletion of OLFM4 gene inhibits cell growth and increases sensitization to hydrogen peroxide and tumor necrosis factor-alpha induced-apoptosis in gastric cancer cells. J Biomed Sci. 2012;19:38
46. Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ. et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016;17:2312-25
47. Babb R, Chandrasekaran D, Carvalho Moreno Neves V, Sharpe PT. Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/β-catenin signaling in response to tooth damage. Sci Rep. 2017;7:3102
48. Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017;50:285-98
49. Ruscito I, Darb-Esfahani S, Kulbe H, Bellati F, Zizzari IG, Rahimi Koshkaki H. et al. The prognostic impact of cancer stem-like cell biomarker aldehyde dehydrogenase-1 (ALDH1) in ovarian cancer: A meta-analysis. Gynecol Oncol. 2018;150:151-7
50. Li Y, Chen T, Zhu J, Zhang H, Jiang H, Sun H. High ALDH activity defines ovarian cancer stem-like cells with enhanced invasiveness and EMT progress which are responsible for tumor invasion. Biochem Biophys Res Commun. 2018;495:1081-8
51. Borrás M, Hardy L, Lempereur F, El Khissiin AH, Legros N, Gol-Winkler R. et al. Estradiol-induced Down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol. 1994;48:325-36
52. Shao R, Weijdegård B, Ljungström K, Friberg A, Zhu C, Wang X. et al. Nuclear progesterone receptor A and B isoforms in mouse fallopian tube and uterus: implications for expression, regulation, and cellular function. Am J Physiol Endocrinol Metab. 2006;291:E59-72
Author contact
![]() Corresponding author: Dah-Ching Ding, M.D, Ph.D., Department of Obstetrics and Gynecology, Hualien Tzu Chi General Hospital, 707, Sec. 3, Chung-Yang Rd., Hualien 970, Taiwan. Tel: 886-3-8561825; Fax: 886-3-8577161; Email: dah1003com.tw
Corresponding author: Dah-Ching Ding, M.D, Ph.D., Department of Obstetrics and Gynecology, Hualien Tzu Chi General Hospital, 707, Sec. 3, Chung-Yang Rd., Hualien 970, Taiwan. Tel: 886-3-8561825; Fax: 886-3-8577161; Email: dah1003com.tw

 Global reach, higher impact
Global reach, higher impact