Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(14):3239-3245. doi:10.7150/jca.30102 This issue Cite
Research Paper
High numbers of CD163+ tumor-associated macrophages correlate with poor prognosis in multiple myeloma patients receiving bortezomib-based regimens
1. Department of Hematological Oncology, Sun Yat-sen University Cancer Center;State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China
2. Department of Pathology, Sun Yat-sen University Cancer Center;State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China
3. Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China
*The first two authors contributed equally to this work.
Received 2018-9-21; Accepted 2019-4-13; Published 2019-6-2
Abstract
The prognostic significance of tumor-associated macrophages (TAMs) in multiple myeloma (MM) in the era of novel drugs remains unclear. CD163 expression was detected by immunohistochemistry to determine the number of TAMs in 198 MM patients receiving bortezomib-based regimens and the data were used to evaluate its relevance with clinical characteristics, treatment response, and prognosis. Patients with high levels of infiltrated CD163+ TAMs (>55/HPF) at diagnosis tended to have more adverse clinical characteristics. Patients with high CD163+ TAM content (>55/HPF) at diagnosis had worse progression-free survival (PFS) (P<0.001) and overall survival (OS) (P<0.001),and achieved lower complete remission (CR)/near-CR rate (P<0.001), than patients with low CD163+ TAM levels. Multivariate analysis revealed that CD163+ TAM content was an independent adverse prognostic factor for PFS and OS. Our data indicated that CD163+ TAM content at diagnosis is a powerful predictor of prognosis for MM in the era of novel drugs, and this discovery offers new insight into potential therapeutic strategies.
Keywords: Tumor-associated macrophages, multiple myeloma, CD163, Prognosis
Introduction
Multiple myeloma (MM) is a fatal haematological malignancy that mainly affects elderly people, and is characterized by the proliferation of malignant plasma cells in the bone marrow, resulting in osteolytic lesions, anemia, renal impairment and hypercalcemia [1]. Clinically, the major clinical prognostic system is applied in MM: Revised International Staging System (R-ISS), which divides patients into three groups based on serum albumin, β2-microglobulin, lactate dehydrogenase levels, and cytogenetics [2]. The interaction between MM cells and the different cell components of the tumor microenvironment play a significant role in mediating drug resistance and maintaining minimal residual disease, ultimately influencing the clinical behavior and prognosis [3, 4]. Therefore, the R-ISS cannot provide complete prognostic information because of a lack of tumor-microenvironment information.
Tumor-associated macrophages (TAMs), which are important constituents of the inflammatory infiltrate in various malignancies, are M2-type macrophages, and are characterized with enhanced CD163 expression [5, 6]. Recently, Chen et al. explored the prognostic value of TAM in a cohort of patients and found that the pretreatment of CD163+ macrophage count in tumor tissue can serve as a valuable biomarker of prognostic factors [7]. However, only 30 patients (12.5%) received bortezomib-based or lenalidomide-based therapy and nearly a quarter of the patients received VAD or MP regimens, which are now considered inappropriate for MM patients. In recent years, the outcome of MM has remarkably improved due to the introduction of bortezomib in frontline regimens [8]. Thus, in the era of novel agents, whether TAM content can retain their prognostic value or not remains to be investigated.
In this retrospective study, we explored the correlation between CD163+ macrophage count in tumor tissues and the clinical features (such as response rate and survival) in MM patients treated with upfront bortezomib-based therapy.
Methods
Eligibility criteria
This retrospective study included 198 patients with symptomatic MM, who were treated in Sun Yat-Sen University Cancer Center from July 2009 to December 2017. These patients were identified using the hospital discharge registry system and their electronic medical records. This study was approved by the institutional review board and ethics committees of the Sun Yat-Sen University Cancer Center. All patients signed written informed consent for tissue samples and medical information. This study was conducted in agreement with the guidelines of the Declaration of Helsinki. The inclusion criteria for this study were as follows:(1) newly diagnosed with symptomatic MM based on the diagnostic criteria of the World Health Organization; (2) had either measurable monoclonal protein (M protein) in blood or urine; (3) previously untreated; (4) no previous or concomitant tumour; (5) available pathology specimens acquired before the initial treatment; (6) complete clinical information and long-term follow-up data were available. All patients were staged according to the International Staging System (ISS) staging.
Treatment and response evaluation
All patients received bortezomib-based regimens such as PAD (bortezomib, doxorubicin, and dexamethasone) [9], VCD (bortezomib, cyclophosphamide, and dexamethasone), [10] and VTD (bortezomib, thalidomide, and dexamethasone)[11]. Patients underwent at least four cycles of treatment, followed by autologous stem cell transplantation (if eligible) or maintenance with bortezomib. Treatment responses were evaluated after each cycle according to the International Myeloma Working Group criteria [12].
Immunohistochemistry (IHC)
Representative formalin-fixed paraffin-embedded tissues obtained from bone marrow biopsy were submitted for IHC to identify and quantify TAMs in MM. Immunohistochemical staining of CD163 was performed on selected cases by using a CD163 antibody (clone KP1, sc-20060; Santa Cruz) incubated at a 1:50 dilution. Four-micrometer-thick sections embedded with bone marrow paraffin were cut, placed on slides, deparaffinized in xylene, and hydrated in a graded alcohol series. The antigen retrieval procedure was performed by immersing the slides in 10-Mm sodium citrate buffer (pH 8.0) , and then heating the slides at 100 °C for 10 min. IHC was performed using a modified avidinbiotin peroxidase complex amplification and detection system.
For gathering the counts of CD163+ TAMs, three slides from each sample and five representative regions (the plasma cell-rich area, approximately 1 mm×1 mm) from each slide were inspected at low-power magnification (×100) under a light microscope (Olympus, BX51, Japan). Afterward, the number of CD163+ TAMs was assessed at high-power magnification (×400) from these fields per case. Only CD163+ cells that displayed macrophagic morphology were counted. The mean number per high-power field was calculated. Quantification was performed in duplicate by two different pathologists, and the data were then averaged and defined as the CD163+ TAM content of each case. Cases that varied significantly between the observers were re-evaluated to arrive at a consensus.
Statistical analysis
The CD163+ TAM counts were presented as median (min, max), whereas categorical data were expressed as n (percentages). Differences between the two groups were tested using the Mann-Whitney U test. The receiver operating characteristics (ROC) curve analysis was employed to determine the cutoff value for CD163+ TAMs in the prediction of survival. Kaplan-Meier method was used to calculate the probability of survival, and survival curves were tested using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Two-sided P values<0.05 were considered statistically significant. Statistical analysis was performed using the SPSS software (SPSS Standard version 19.0, SPSS Inc., Chicago, IL, USA).
Correlation of TAMs with clinical characteristics in MM patients.
| Characteristics | No | Median TAMs count (/hpf), (range) | P value |
|---|---|---|---|
| No. of cases | 198 | ||
| Age | 0.752 | ||
| ≤ 65 | 116 | 39 (5-126) | |
| >65 | 82 | 40 (5-160) | |
| Gender | 0.822 | ||
| male | 140 | 40 (5-160) | |
| female | 58 | 38(8-126) | |
| ECOG PS | 0.010 | ||
| 0-1 | 114 | 38 (5-126) | |
| ≥ 2 | 84 | 47 (5-160) | |
| ISS stage | 0.122 | ||
| I | 54 | 38 (5-86) | |
| II | 92 | 44 (5-126) | |
| III | 52 | 40 (8-160) | |
| Type of myeloma | 0.564 | ||
| IgG | 92 | 39(5-117) | |
| IgA | 64 | 43 (5-160) | |
| Light chain | 42 | 41 (5-126) | |
| Serum LDH | <0.001 | ||
| ≤ULN | 156 | 38 (5-160) | |
| >ULN | 42 | 56 (25-126) | |
| Genetic abnormalities | 0.519 | ||
| yesa | 88 | 42 (5-160) | |
| no | 110 | 38 (5-126) | |
| Response after treatment | <0.001 | ||
| CR/nCR | 76 | 31 (5-126) | |
| No CR/nCR | 122 | 57(20-160) |
a Patients with abnormalities of 13q14, 1q21, 14q32 and 17p13
Abbreviations: ECOG, Eastern Cooperative Oncology Group;ISS, international staging system;PS, performance status; LDH, lactate dehydrogenase; CR/nCR,Complete Remission/Near-complete Remission.
(A) Representation of low TAMs content in immunostained specimens by the LSAB method(×200) (B) Representation of high TAMs content in immunostained specimens by the LSAB method (×200)
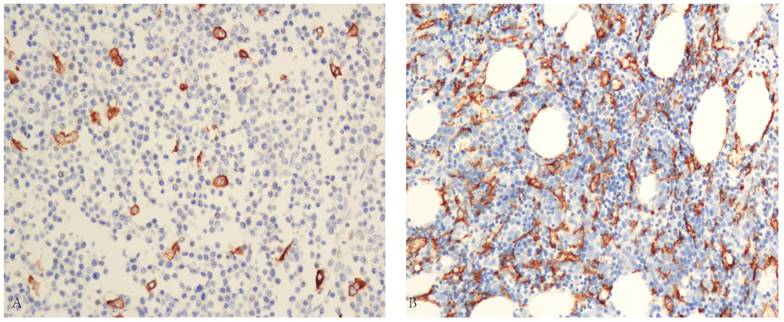
Results
Patient characteristics and correlation with CD163+ TAM content
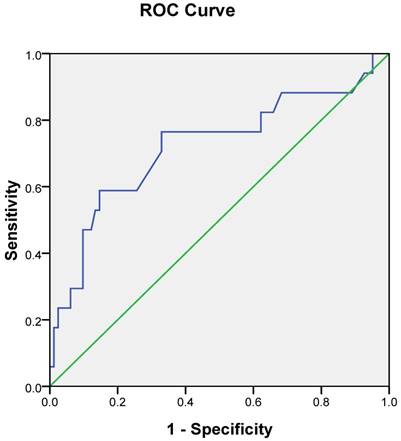
In total, 198 patients (140 male, 58 female; median age 59 years) met the inclusion criteria. Their clinical characteristics are listed in Table 1. More than half (58.6%) of the patients were <65 years old. A total of 114 patients (57.6%) showed favorable performance status (ECOG PS 0-1). Good balances of distribution were found in ISS stage with stages I (27.3%), II (46.5%), and III (26.3%). The types of monoclonal protein were as follows: IgG for 92 patients, IgA for 64 patients, and light chain disease for 42 patients. Elevated LDH levels were observed for 42 cases (21.2%). A total of 88 patients (44.4%) presented genetic abnormalities. CD163 expression was detected in the cytomembrane of the macrophages in all 198 cases. Myeloma cells were not stained with the CD163-antibody. Most cases showed CD163+ TAMs infiltrating massively in the tumor tissue (Fig. 1). The mean number of CD163+ TAM content of the whole group was 47.2/high-power field (HPF) with a median of 40/HPF (range, 5-160/HPF). Table 1 shows that the CD163+ TAM content was significantly higher in patients with poor PS, elevated LDH levels, or no complete remission (CR) after treatment. However, no significant correlation was found between CD163+ TAM and age, gender, type of myeloma, and genetic abnormalities. To identify an optimal cutoff point for the survival outcomes, the ROC curve analysis was selected. The most discriminative cutoff levels of CD163+ TAM content was 55/HPF with an area under the curve value of 0.721 (Fig. 2). Based on the ROC analysis result, we used the CD163+ TAM content >55/HPF as the cutoff value in the present study. Based on this cutoff value, 120 patients (60.6 %) were categorized into the low-TAM group (≤55/HPF), and 78 patients (39.4 %) were categorized into the high-TAM group (>55/HPF).
Treatment modalities and response
For the primary treatment modalities, 64 cases (32.3%) received chemotherapy followed by autologous stem cell transplantation (ASCT), and 134 cases (67.7%) received chemotherapy alone. The treatment details and responses are listed in Table 2. No significant difference was found in the treatment modalities between the low-TAM and high-TAM group (P=0.757). After the initial treatment involving ASCT, 76 of the 198 treated patients (38.4%) achieved CR/nCR. The CR/nCR rate in the low-TAM group was significantly higher than that in the high-TAM group (55.0% vs. 12.8%, P<0.001). The overall response rate in the low-TAM group was significantly higher than that in the high-TAM group (85.0% vs 56.4%, P<0.001).
ROC curve analysis for the optimal cut-off point of TAMs content. The most discriminative cut-off value of TAMs content was 55/hpf with an AUC value of 0.721. The sensitivity and specificity were 70.6% and 67.1%, respectively.
Primary treatment and response in patients with multiple myeloma.
| Treatment | Low-TAM group, n (%) | High-TAM group, n (%) | P value |
|---|---|---|---|
| Treatment modalities | 0.757 | ||
| CT followed by ASCT | 40 (33.3) | 24 (30.8) | |
| CT alone | 80 (66.7) | 54 (69.2) | |
| Treatment response | |||
| ORR (≥PR) | 102 (85.0) | 44 (56.4) | <0.001 |
| CR/nCR | 66 (55.0) | 10 (12.8) | <0.001 |
| VGPR | 16 (13.3) | 16 (20.5) | 0.236 |
| PR | 20 (16.7) | 18 (23.1) | 0.274 |
Abbreviations: CT, Chemotherapy; ASCT, Autologous Stem Cell Transplantation; CR/nCR, Complete Remission/Near-complete Remission; VGPR, Very Good Partial Response; ORR, Overall Response Rate; PR, Partial Response
Survival and prognostic factors
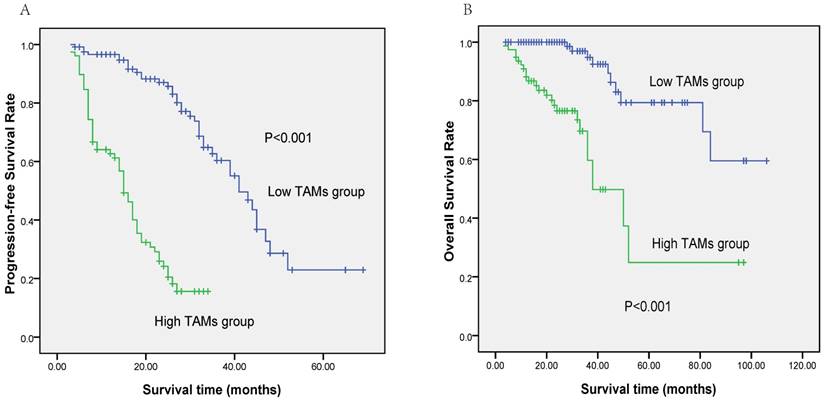
At a median follow-up of 28 months (3-106), the 3-year PFS and OS rates for all 198 patients were 41.3 (95%CI 32.3-50.3%) and 82.0% (95%CI 74.6-89.4%), respectively. Patients in the low-TAM group had significantly better survival than those in the high-TAM group (3-year PFS rate: 60.3% vs 15.6%, P<0.001, Figure 3A; 3-year OS rate: 94.8% vs 59.7%, P<0.001, Figure 3B). In the patients who received chemotherapy alone (n=134, 67.7%), high level of TAM content was associated with shorter PFS and OS (P<0.001 and P<0.001, respectively) and was also related to inferior PFS and OS (P<0.001 and P=0.018, respectively) in the patients who received chemotherapy followed by ASCT (n=64, 32.3%). Table 3 displays the results of the univariate and multivariate analyses of the potential predictors of PFS and OS. Multivariate analysis by using forward conditional Cox regression model revealed that TAMs content>55/HPF at diagnosis and certain cytogenetic abnormalities were two adverse factors for PFS and OS.
Discussion
Macrophages are the main components of the tumor microenvironments and are found in different types of cancer. Furthermore, TAMs have a phenotype very that is similar to that of M2-type macrophages, and actively suppress adaptive immunity and promote tumor initiation, growth, and angiogenesis [13-15]. The role of TAMs on influencing the biological behavior of tumor has been extensively studied in hematologic malignancies, including classic Hodgkin's lymphoma (CHL), non-Hodgkin lymphoma (NHL), and multiple myeloma[16-18]. Chen et al. evaluated the content of CD163+ TAMs in 240 cases of MM by IHC, and concluded that a high level of diametrically polarized TAMs is a novel independent poor prognostic factor [7]. Notably, most patients were not treated with bortezomib-based regimens in this study. A lot of progress has been made in MM treatment with the extensive use of novel drugs. New drugs such as bortezomib may overcome some prognostic factors established in the conventional chemotherapy period. However, few studies have looked into the prognostic significance of TAMs in the tumor microenvironment of MM in the era of new drugs.
In this study, we found that CD163+ TAM content was correlated with clinical characteristics, such as poor ECOG PS, elevated serum LDH, and no CR after bortezomib-based chemotherapy, indicating that TAMs might promote the development and progression of myeloma. According to the Cox regression model that included the levels of CD163+ TAM content, ISS stage, elevated serum LDH, fluorescence in situ hybridization (FISH), and ECOG PS score (>2), it was concluded that CD163+ TAM content and FISH abnormalities were independent prognostic factors for both PFS and OS.
Survival outcome of patients based on the TAMs content.(A) Progression-free survival (PFS) of patients according to TAMs content (≤55/hpf vs. >55/hpf). (B) Overall survival (OS) of patients according to TAMs content (≤55/hpf vs. >55/hpf).
Univariate and multivariate analysis of factors associated with Progression-Free Survival and Overall Survival of all patients.
| Parameters | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| P value | HR (95%CI) | P value | P value | HR (95%CI) | P value | |
| Age≥65 years | 0.272 | 0.682 | ||||
| Gender, male | 0.431 | 0.141 | ||||
| ECOG PS score (>2) | 0.043 | 0.177 | ||||
| ISS Stage | 0.026 | 0.042 | ||||
| LDH>ULN | 0.042 | 0.049 | ||||
| FISHa | 0.001 | 2.911 (1.894-4.473) | <0.001 | <0.001 | 6.820(2.781-16.725) | <0.001 |
| TAMs content>55/hpf | <0.001 | 8.301 (5.019-13.727) | <0.001 | <0.001 | 8.370(3.677-19.054) | <0.001 |
Abbreviations: OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence intervals; ECOG PS, Eastern Cooperative Oncology Group performance status;ISS, International Staging System; LDH, lactate dehydrogenase; FISH, interphase fluorescence in situ hybridization;
aPatients with abnormalities of 13q14, 1q21, 14q32 and 17p13 compared with no FISH abnormalities.
In the present study, the expression of CD163+ TAMs in MM patients who received bortezomib-based regimens was studied. On the basis of the ROC curve analyses, 55/HPF was an optimal cutoff value for distinguishing between different outcomes. Patients with low level of CD163+ TAM content (≤55/HPF) tended to have higher CR/nCR rates (55.0 %) than those with high level of CD163+ TAM content (>55/HPF) (12.8%) (P<0.001). This finding suggested that TAMs may be involved in the development of MM resistance to bortezomib-based chemotherapy, although its specific mechanism remains unclear. Zheng et al. found that TAMs can directly come in contact with myeloma cells and attenuate the activation of caspase-dependent apoptotic signaling, and therefore protect these cells from spontaneous and melphalan-induced apoptosis, which implies a crucial role of TAMs in the survival and chemotherapy-resistant mechanism of the myeloma cell [4]. The 3-year PFS and OS rate in the low-TAM group was longer than those in the high-TAM group (PFS, 60.3% vs. 15.6%, P<0.001; OS, 94.8% vs. 59.7%, P<0.001). Multivariate analysis including ISS stage, LDH level, and FISH in the molecular cytogenetics also indicated that CD163+ TAM content was an independent prognostic factor for PFS and OS. These results are similar to those of some previous studies [7, 18]. Anderson et al. found that macrophage-derived soluble CD163 was also a prognostic marker in patients with multiple myeloma, implying that TAM, as one of the important components in tumor microenvironment, may influence the clinical course and long-term survival of patients [19]. A common limitation was found among all other studies. First, the data on cytogenetic and FISH markers were not adequate to be included in the analysis. Second, the majority of the patients was treated with conventional chemotherapy regimens in all other studies. In other words, we first reported that the CD163+ macrophage count in tumor tissue was an independent adverse prognostic factor regardless of the present prognosis evaluation system in the era of novel drug.
Infiltrating macrophages exhibited the M1/M2 polarization phenotype, as a result of responding to the products of malignant cells and adapting to a range of activation states [20, 21]. M1-type macrophages, which are stimulated by interferon-γ and toll-like receptor ligands M1 macrophages, can secrete diverse cytokines and iNOS and participate in killing pathogens and tumor cells in Th1 immune response [22]. By contrast, M2-type macrophages, which are activated by interleukin-4, can secrete interleukin-6 (IL-6) and interleukin-10 (IL-10) and have the tendency to promote tumor growth [23]. CD68 is a general marker of macrophages and CD163 expression was more specific to M2 macrophages than CD68. In this study, we evaluated the number of CD163+ macrophages in tumor tissue that might mainly contain M2 macrophages, which constitute the predominant source of IL-10. In addition, we have demonstrated that increased serum levels of interleukin-10 correlate with negative prognostic factors in multiple myeloma in another study [24].Xu et al. have reported that M2-type macrophages can enhance the invasive activity of tumor cells and promote cancer stem cell-like properties through the secretion of IL-6 and IL-8 by paracrine secretion [25]. Additional experimental researches focusing on the functions of tumor-associated macrophages are needed to illuminate the mechanism of inducing drug resistance and promoting the development of myeloma.
In conclusion, our study demonstrated the close relationship of CD163+ TAM content with several clinical features of MM, including poor ECOG PS and elevated LDH levels. The high level of CD163+ TAM content is still an independent poor prognostic factor for myeloma in the era of novel drug. These results imply the role of TAMs in the pathogenesis of MM and provide a new insight into potential therapeutic strategies to further improve their therapeutic effect. Further prospective study should be performed with larger sample sizes to validate the prognostic value of TAMs in MM patients receiving bortezomib-based regimens.
Acknowledgements
We thank the patients and their families and all the investigators, including the physicians, nurses, and laboratory technicians in this study.
Funding
Our work was supported by the following funds: PhD Start-up Fund of Natural Science Foundation of Guangdong Province (contract/grant number: 2017A030310193); Young Teachers' Cultivation Project of Sun Yat-Sen University (No.16ykpy30); Science and Technology Projects of Guangdong Province (contract/grant numbers: 2014A020212577) and National Natural Science Foundation of China (contract/grant number: 81700148).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Smith D, Yong K. Multiple myeloma. BMJ (Clinical research ed). 2013;346:f3863
2. Palumbo A, Avet-Loiseau H, Oliva S. et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(26):2863-9
3. Panchabhai S, Kelemen K, Ahmann G. et al. Tumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myeloma. Leukemia. 2016;30(4):951-4
4. Zheng Y, Cai Z, Wang S. et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114(17):3625-8
5. Allavena P, Sica A, Solinas G. et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical reviews in oncology/hematology. 2008;66(1):1-9
6. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer research. 2006;66(2):605-12
7. Chen X, Chen J, Zhang W. et al. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget. 2017;8(68):112685-96
8. Kouroukis TC, Baldassarre FG, Haynes AE. et al. Bortezomib in multiple myeloma: systematic review and clinical considerations. Current oncology (Toronto, Ont). 2014;21(4):e573-603
9. Sonneveld P, Schmidt-Wolf IG, van der Holt B. et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(24):2946-55
10. Mai EK, Bertsch U, Durig J. et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721-9
11. Cavo M, Tacchetti P, Patriarca F. et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075-85
12. Durie BG, Harousseau JL, Miguel JS. et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467-73
13. Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunology letters. 2009;123(2):97-102
14. Fukuda K, Kobayashi A, Watabe K. The role of tumor-associated macrophage in tumor progression. Frontiers in bioscience (Scholar edition). 2012;4:787-98
15. Wu Y, Zheng L. Dynamic education of macrophages in different areas of human tumors. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2012;5(3):195-201
16. Steidl C, Lee T, Shah SP. et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362(10):875-85
17. Dave SS, Wright G, Tan B. et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. The New England journal of medicine. 2004;351(21):2159-69
18. Suyani E, Sucak GT, Akyurek N. et al. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Annals of hematology. 2013;92(5):669-77
19. Andersen MN, Abildgaard N, Maniecki MB. et al. Monocyte/macrophage-derived soluble CD163: a novel biomarker in multiple myeloma. European journal of haematology. 2014;93(1):41-7
20. Giraldo NA, Becht E, Remark R. et al. The immune contexture of primary and metastatic human tumours. Current opinion in immunology. 2014;27:8-15
21. Fridman WH, Pages F, Sautes-Fridman C. et al. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12(4):298-306
22. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11(11):723-37
23. Murray PJ, Allen JE, Biswas SK. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14-20
24. Wang H, Wang L, Chi PD. et al. High level of interleukin-10 in serum predicts poor prognosis in multiple myeloma. British journal of cancer. 2016;114(4):463-8
25. Xu H, Lai W, Zhang Y. et al. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC cancer. 2014;14:330
Author contact
![]() Corresponding authors: Hailin Tang, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. E-mail: tanghlorg.cn; Phone No.:+86-20-87342438 or Shu-xia Lin, Department of Pathology, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. E-mail: lisuzdcom; Phone No.:+86-20-87342462
Corresponding authors: Hailin Tang, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. E-mail: tanghlorg.cn; Phone No.:+86-20-87342438 or Shu-xia Lin, Department of Pathology, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China. E-mail: lisuzdcom; Phone No.:+86-20-87342462

 Global reach, higher impact
Global reach, higher impact