Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(14):3246-3252. doi:10.7150/jca.30046 This issue Cite
Research Paper
A Nomogram for Prediction of Overall Survival in Patients with Node-negative Gallbladder Cancer
1. Department of General Surgery, Sir Run-Run Shaw Hospital, Zhejiang University, Hangzhou 310016, China
2. Key Laboratory of Endoscopic Technique Research of Zhejiang Province, Sir Run-Run Shaw Hospital, Zhejiang University, Hangzhou 310016, China
3. Engineering Research Center of Cognitive Healthcare of Zhejiang Province, 310003, China
Received 2018-9-18; Accepted 2019-4-12; Published 2019-6-2
Abstract
Background & Aims: According to the stage of tumor, it's hard suitable to predict the prognosis for gallbladder cancer, especially for node-negative gallbladder cancer. Therefore, we aimed to create a nomogram based on demographic and clinicopathologic characteristics to estimate individualized potential impacts on postoperative overall survival.
Methods: 789 patients with node-negative gallbladder cancer were selected from the Surveillance, Epidemiology, and End Results and randomly divided into training and internal validation group. Univariate and multivariate survival analysis were used to identify prognostic factors. The nomogram was constructed using Cox proportional hazards models. We evaluated the performance of the nomogram with Harrell's concordance index and calibration curve. The nomogram was externally validated in 115 patients with node-negative gallbladder cancer from the Sir Run Run Shaw hospital.
Results: The nomogram for overall survival was built on the basis of five independent factors, such as age, sex, histology, T-stage, and number of examined lymph nodes. The C-index of nomogram for overall survival in the internal and external validation group was up to 0.724 and 0.716, respectively. Both of those calibration curves showed good agreement between predicted and observed outcomes in the 1-, 3-, 5-year overall survival. Compared to the 7th edition AJCC stage, the nomogram had a better difference in predicting overall survival, even could further classify patients into four risk subgroups in each stage.
Conclusion: This nomogram can be used as a decision model to predict the outcomes of postoperative overall survival for node-negative gallbladder cancer, and may give useful guidance to clinicians for next treatment.
Keywords: gallbladder cancer, nomogram, node-negative, overall survival
Introduction
Gallbladder cancer (GBC) is one of the most common and aggressive biliary tract malignancies and has a low annual incidence [1], poor prognosis [1-3] and high mortality. Surgery is recommended as the first line therapy for patients with resectable gallbladder cancer [4], but 5-year survival rate is rather low, with approximately 5%. Lymph node (LN) status plays an important role in prognosis [5-7], i.e. positive LN status indicates a poor prognosis. Several different LNs scoring systems, such as tumor-node-metastasis (TNM), lymph node ratio (LNR) and log odds of positive LN (LODDS), can be used to evaluate the prognosis of GBC, but there are rare of scoring systems for LN-negative patients.
The incidental gallbladder cancer (IGBC) is the most common form of gallbladder cancer diagnosed today [8], and many patients present without lymphatic metastasis. Interestingly, the 5-year survival rate of T1N0 patients is only up to 30% compared with a 3% survival rate in T1N1 patients. Positive LNs mean a worse prognosis, but LN status cannot be a prognostic factor for node-negative gallbladder cancer patients. Therefore, various covariates may have an influence on overall survival (OS) of node-negative patients. Fan [9] et al reported that the more number of examined lymph nodes in patients with node-negative gallbladder cancer, the better outcomes of postoperative overall survival. Min [10] et al showed that tumor characteristics, such as tumor differentiation, can predict long-term outcomes after surgery in patients with GBC. Except that, the patients' characteristics also have effects on prognosis. Unfortunately, due to the absence of large, prospective, randomized, clinical trial data, it is difficult to know which patients with lymph node-negative GBC will have better outcomes of postoperative overall survival and who need additional treatment or intensive follow-up.
In the study, we created a nomogram to help clinicians and patients to estimate individualized outcomes of postoperative overall survival and make individual plans for next therapy.
Flowchart showing selecting of SEER database for training and internal validation group.
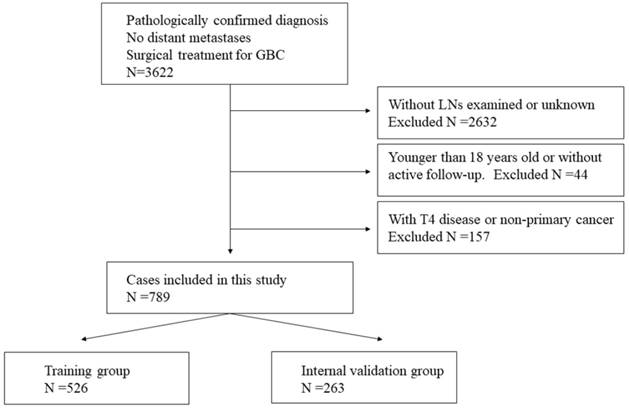
Materials and Methods
Study Population
An initial query of SEER database (2004-2014) identified 3622 patients who underwent surgical treatment for GBC with negative LNs status and migration stage. Exclusion criteria were as follows: patients who were younger than 18 years old, with T4 disease, without primary gallbladder tumor, no LN was examined, without active follow-up. And the final study cohort included 789 patients. The complete patient selection flowchart was shown in Figure 1. The standardized patients' data, including patient demographics, tumor morphology, staging, treatment details, follow-up and so on, were collected. To develop the prognostic nomogram and validate it based on a random split-sample approach, using a computerized module, the 789 patients were randomly divided into training group (n=526) and internal validation group (n=263) in a two-to-one ratio. Moreover, according to the selection criteria above, 115 patients from Sir Run Run Shaw hospital (2007-2013) were selected as external validation group.
Statistical Analysis
The primary end point of interest in this study was overall survival. Observed variables were age, sex, race, grade, histology, tumor size, pathologic T category, surgery type, No. of examined LNs and so on. In the training part, OS curves for different variable values were estimated using the Kaplan-Meier and compared using the log-rank test. Variables which showed significance in the univariate analysis (P< .05) were entered into multivariate regression survival analysis. Statistical analyses to identify prognostic factors were performed using the SPSS 24.0 (SPSS, Chicago, IL). According to the above statistical analyses, a nomogram, where we fitted an automated backward variable selection with respect to the Cox proportional hazards model, was performed using R software packages (http://www.r-project.org). In the validation and calibration part, the nomogram was subjected to 268 patients for internal validation of the primary training group, and external validation with 115 patients from Sir Run Run Shaw hospital. We evaluated the performance of the model with Harrell's concordance index (C-index) and calibration curves (1-, 3-, 5-year OS) and compared it with the 7th AJCC stage.
Results
Clinicopathologic Characteristics of Patients
Demographic and clinicopathologic characteristics of training and validation group were shown in Table 1. A total of 789 patients were included as primary training group and internal validation group in the study from SEER database. There were 381 patients (48.3%) older than 70 years, and 537 of patients (68.1%) were female. Pathological examination showed GBC which consisted of 72.9% adenocarcinoma (NOS), 9.5% papillary adenocarcinoma and 8.7% adenocarcinoma with subgroup type, captured 91.1 percent of histological type. The most proportion of patients' grade and T category of tumors were moderate and T2, respectively. Surgical details indicated that 19.0% patients underwent radical surgery with liver, while 100% patients were performed with lymphadenectomy. The median survival time was 71.8 months (95% IC, 67.1 to 76.5). 115 patients with node-negative gallbladder cancer were taken as external validation group. Most patients were female (75.7%) as similar to training group, but the percent of patients who were younger than 60 years was up to 40.9%. The similar results of pathological examination and surgical details were shown in external validation group. The median survival time was 79.3 months (95% IC, 71.9 to 86.7).
Patients and tumor characteristics of the SEER database and SRRSH database.
| SEER database (N=789) | SRRSH database (N=115) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OS (months) | OS (months) | ||||||||
| Characteristic | No. | % | Median | 95%CI | No. | % | Median | 95%CI | |
| Age, years | |||||||||
| <60 | 187 | 23.7 | 96.2 | 87.0 - 105.5 | 47 | 40.9 | 85.6 | 76.3 - 94.8 | |
| 60-70 | 221 | 28.0 | 74.7 | 65.9 - 83.6 | 36 | 31.3 | 69.4 | 56.2 - 82.5 | |
| >70 | 381 | 48.3 | 59.5 | 53.1 - 65.9 | 32 | 27.8 | 59.7 | 50.0 - 71.4 | |
| Sex | |||||||||
| Female | 537 | 68.1 | 76.5 | 70.9 - 82.2 | 87 | 75.7 | 80.6 | 72.4 - 88.9 | |
| Male | 252 | 31.9 | 60.1 | 52.0 - 68.2 | 28 | 24.3 | 55.4 | 45.2 - 65.6 | |
| Race | |||||||||
| White | 580 | 73.5 | 69.2 | 63.8 - 74.6 | NA | NA | NA | NA | |
| Black | 101 | 12.8 | 76.8 | 63.2 - 90.4 | NA | NA | NA | NA | |
| Other | 108 | 13.7 | 82.1 | 69.0 - 95.1 | 115 | 100 | 79.3 | 71.9 - 86.7 | |
| Histology | |||||||||
| Adenocarcinoma, NOS | 575 | 72.9 | 67.1 | 61.7 - 72.6 | 86 | 74.8 | 76.6 | 67.6 - 85.6 | |
| Papillary adenocarcinoma | 75 | 09.5 | 96.3 | 83.8 - 108.9 | 13 | 11.3 | 61.4 | 54.6 - 68.2 | |
| Adenocarcinoma with other type | 69 | 08.7 | 79.4 | 65.9 - 92.9 | 8 | 07.0 | NA | NA | |
| Others | 70 | 08.9 | 61.5 | 46.5 - 76.6 | 8 | 07.0 | NA | NA | |
| Grade | |||||||||
| Well | 154 | 19.5 | 77.3 | 66.6 - 88.1 | 21 | 18.3 | 79.1 | 62.5 - 95.6 | |
| Moderate | 408 | 51.7 | 74.7 | 68.2 - 81.2 | 67 | 58.3 | 67.0 | 60.4 - 73.6 | |
| Poor | 227 | 28.8 | 63.3 | 54.6 - 72.0 | 27 | 23.5 | 64.8 | 49.2 - 80.5 | |
| Tumor size | |||||||||
| ≤10 mm | 134 | 17.0 | 74.3 | 63.1 - 85.6 | 22 | 19.1 | 63.4 | 50.5 - 76.3 | |
| 10-30 mm | 371 | 47.0 | 74.8 | 68.1 - 81.4 | 53 | 46.1 | 75.9 | 67.0 - 84.8 | |
| 30-50 mm | 167 | 21.2 | 73.3 | 63.1 - 83.6 | 27 | 23.5 | 65.5 | 52.8 - 78.2 | |
| >50 mm | 117 | 14.8 | 54.2 | 43.1 - 65.2 | 13 | 11.3 | 67.3 | 42.7 - 91.9 | |
| T category | |||||||||
| T1a | 48 | 06.1 | 83.2 | 64.4 - 102.2 | 12 | 10.4 | 86.7 | 79.4 - 94.1 | |
| T1b | 110 | 13.9 | 80.8 | 67.3 - 91.9 | 14 | 12.2 | 60.8 | 49.0 - 72.4 | |
| T2 | 437 | 55.4 | 79.6 | 74.5 - 87.1 | 73 | 63.5 | 47.7 | 26.4 - 69.0 | |
| T3 | 194 | 24.6 | 43.2 | 35.2 - 51.2 | 16 | 13.9 | 38.4 | 28.4 - 48.5 | |
| Radical surgery with liver | |||||||||
| No | 639 | 81.0 | 48.7 | 45.2 - 52.1 | 96 | 83.5 | 73.2 | 64.3 - 82.1 | |
| Yes | 150 | 19.0 | 52.6 | 44.8 - 60.4 | 19 | 16.5 | 58.1 | 43.6 - 72.6 | |
| No. of examined LNs | |||||||||
| 1-2 | 323 | 40.9 | 60.4 | 53.3 - 67.6 | 55 | 47.8 | 71.7 | 60.0 - 83.4 | |
| 3-5 | 201 | 25.5 | 76.6 | 67.3 - 85.8 | 23 | 20.0 | 69.0 | 57.5 - 80.5 | |
| 6-9 | 196 | 24.8 | 82.7 | 73.1 - 92.3 | 29 | 25.2 | 83.7 | 75.3 - 92.1 | |
| ≥10 | 69 | 08.7 | 77.6 | 63.4 - 91.9 | 8 | 07.0 | 42.0 | 31.3 - 52.7 | |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results; SRRSH, Sir Run Run Shaw hospital; OS, overall survival; LN, lymph node; NA, not available.
Independent Prognostic Factors
The results of univariable and multivariable analyses with Cox regression model in training group were summarized in Table 2. According to the results of univariable analysis, there were significant differences in survival among the seven risk factors, such as age (P< .001), sex (P= .001), histology (P< .001), grade (P= .005), tumor size (P= .001), T category (P< .001), and No. of examined LNs (P< .001). However, no difference had been found in the type of surgery (P = .459) and race (P= .127). All significant factors were entered into the multivariable analysis with Cox regression model. Age (P< .001), sex (P= .001), histology (P= .016), T category (P< .001), No. of examined LNs (P= .004) were selected as independent prognostic factors for building the nomogram, because of those significances at P< 0.05.
A Nomogram for 1-, 3-, 5-year OS
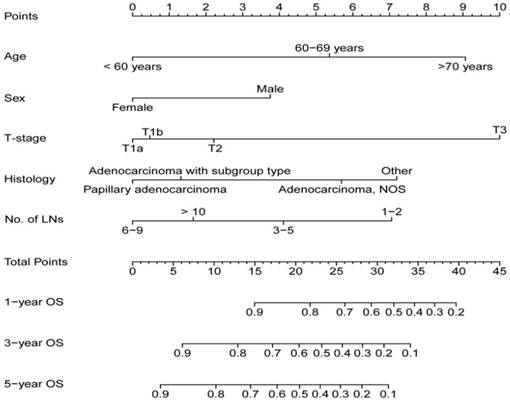
A nomogram for 1-, 3-, 5-year OS was established on the basis of five significant factors (Figure 2). In the nomogram, the contribution to the overall survival trend from the T category was the maximum, and the one from the sex affected the minimum. Histology, age and No. of examined LNs also had made moderate contributions for OS. Interestingly, the more No. of examined LNs didn't mean the longer OS. For example, there was a 75 years old female patient with adenocarcinoma (T1aN0) and 8 LNs examined after surgery. According to the nomogram, her total points was about 15 points and the percent of 1-, 3-, 5-year OS was approximately 90%, 76%, 70%, respectively. However, if the patient (T1aN0) with 10 LNs examined only differed from the No. of examined LNs, the percent of 1-, 3-, 5-year OS was lower than those of previous.
Univariable and Multivariable Analysis with Cox Regression in Training Group.
| Multivariable Analysis | ||||
|---|---|---|---|---|
| Variable | Univariable Analysis P | HR | 95%CI | P |
| Age, years | < .001 | < .001 | ||
| <60 | Reference | |||
| 60-70 | 1.935 | 1.298 - 2.884 | .001 | |
| >70 | 2.906 | 2.021 - 4.178 | < .001 | |
| Sex | .001 | .001 | ||
| Male | Reference | |||
| Female | 1.493 | 1.177 - 1.895 | .001 | |
| Histology | < .001 | .016 | ||
| Adenocarcinoma, NOS | Reference | |||
| Papillary adenocarcinoma | 0.518 | 0.312 - 0.859 | .011 | |
| Adenocarcinoma with other type | 0.576 | 0.576 - 0.349 | .031 | |
| Others | 1.011 | 0.675 - 1.514 | .959 | |
| Grade | .005 | .501 | ||
| Well | Reference | |||
| Moderate | 0.918 | 0.657 - 1.283 | .617 | |
| Poor | 1.079 | 0.749 - 1.552 | .684 | |
| Tumor size | .001 | .051 | ||
| ≤10 mm | Reference | |||
| 10-30 mm | 0.906 | 0.642 - 1.279 | .575 | |
| 30-50 mm | 0.998 | 0.673 - 1.479 | .991 | |
| >50 mm | 1.444 | 0.950 - 2.196 | .086 | |
| T category | < .001 | < .001 | ||
| T1a | Reference | |||
| T1b | 1.013 | 0.552 - 1.813 | .967 | |
| T2 | 1.291 | 0.674 - 2.471 | .464 | |
| T3 | 2.821 | 1.523 - 5.224 | .001 | |
| No. of examined LNS | < .001 | .004 | ||
| 1-2 | Reference | |||
| 3-5 | 0.719 | 0.535 - 0.965 | .028 | |
| 6-9 | 0.269 | 0.120 - 0.605 | .002 | |
| ≥10 | 0.286 | 0.119 - 0.686 | .005 | |
| Race | .127 | |||
| Radical surgery (with liver) | .359 | |||
Abbreviation: HR, Hazard Ratio
Performance and Validation of the Nomogram
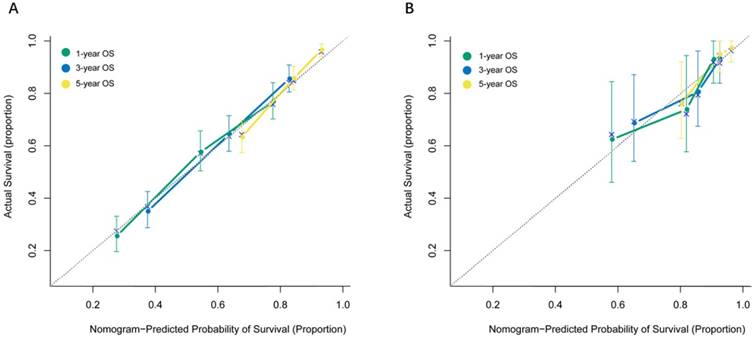
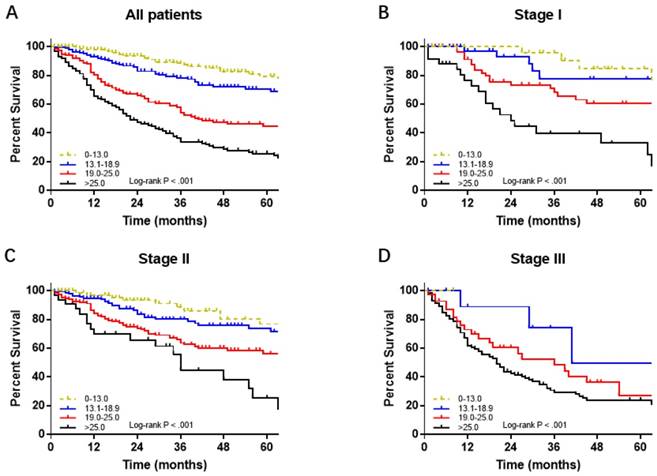
The performance of the nomogram was validated by discrimination and calibration curves. Discrimination, as measured by the bootstrap corrected C-index, was 0.724 (95% IC, 0.709 to 0.739) in internal validation and 0.716 (95% IC, 0.659 to 0.773) in external validation. The calibration plots for probability of 1-, 3-, 5-year OS showed good agreement among nomogram predicted and actual survival in both internal and external group (Figure 3). Compared to the 7th AJCC stage group, discrimination showed greater in both the internal (C-index, 0.724 vs. 0.619, P< 0.01) and external validation (C-index, 0.716 vs. 0.640, P< 0.01). Moreover, stratification into prognostic factors subgroups, which were divided into four risk subgroups (0-13.0, 13.1-18.9, 19.0-25.0, >25.0) according to total Points, showed distinction between Kaplan-Meier curves for survival time within each TNM stage (Figure 4).
Nomogram for estimating the 1-,3-, 5- year OS for node-negative GBC patients.
Internal and external calibration curves demonstrating how survival predictions from the model compare to the actual observed survival (A: Internal calibration curves for 1-,3-, 5- year OS, B: External calibration curves for 1-,3-, 5- year OS)
Survival curves of four risk subgroups in each 7thed AJCC Stage Group.
Discussion
Nomogram has been increasingly popular and important in personalized cancer prediction [11], in which clinicians optimize the patients' therapeutic recommendations according to their specific and individual information. As one of the cancer prediction models, it has been used in various cancers, such as lung [12-14], breast [15-17], pancreas [18, 19] and prostate cancer [20-22] et al. Although the 7th AJCC stage system is widely applied for GBC to estimate OS, it cannot be an ideal model for node-negative GBC in prediction of OS. For node-negative GBC patients, only T-stage can be used to evaluate the survival time, because the values of N-stage and M-stage are constantly as 0. Therefore, we developed the nomogram based on patients, tumor characteristic and surgical details to estimate the OS of operable GBC patients.
Just as cancer prediction models usually consist of many variables, five prognostic factors were included in the nomogram according to the results of univariable and multivariable analyses. T-stage was one of the most important factors. Interestingly, there were some differences from previous decision-making systems. For example, general regularity in GBC is that the more No. of LNs examined, the better prognosis. Inadequate removal of LNs may result in the misclassification of LN-positive patients as N0, and the N-stage has been referred as one of the strongest prognostic factors for gallbladder cancer patients [5]. Patients with LN metastasis will have a shorter survival time and 30-40% increased risk of death, compared to patients without LN metastasis [23, 24]. Surgery remains the only effective and potentially recommended treatment for patients with resectable and node-negative GBC [4]. The early LN metastasis is a characteristic of gallbladder adenocarcinoma [25]. Some studies suggested that extended regional lymphadenectomy could improve survival compared with standard regional lymphadenectomy. For complete resection, extended surgical procedures, such as extensive lymphadenectomy and major hepatectomy, even common bile duct (CBD) resection or pancreatoduodenectomy, are often required [26]. However, the more No. of examined LNs is accompanied by higher risk of complications, because the removal of too many LNs may cause the side effects such as lymphatic leakage. Thus, for balancing the risk against the reward for each subgroup, the most appropriate subgroup of No. of examined LNs is not more than 10, but 6 to 9 in the nomogram for the node-negative GBC patients. Age also had a great influence on survival time in the present study as expected. Generally, elder patients possess a poorer tolerance of stress and a damaged compensatory mechanism, but younger patients usually show more aggressive of the biological behavior of malignant tumors. We found that the younger patients with node-negative GBC, the longer survival time. Wang [27] et al showed the similar results in gallbladder cancer. The outcomes of OS also depended on sex and histology. Interestingly, the percent of the female was higher than the male in the patients with gallbladder cancer, while the female had a better result of OS.
Validation of the nomogram is as important as development. For validating the nomogram, we chose one thirds of patients from SEER database at random as internal validation and 115 patients from SRRSH database as external validation. The calibration curves presented excellent agreement between predicted and observed outcomes in the 1-, 3-, 5-year OS in SEER database group. In addition, good agreement is also shown in SRRSH database group. At the same time, the nomogram showed greater discrimination in predicting OS, as the value of C-index was up to 0.724 and 0.716 in SEER and SRRSH database group, respectively. Moreover, there was sufficient preponderance over the 7th AJCC stage group in estimating 1-, 3-, 5-year OS. More importantly, the nomogram could further classify patients into four risk subgroups in each stage of node-negative GBC patients. Identifying subgroups of patients might have a positive influence on the future treatment. It can give physicians some guides and help them select patients who need additional treatment or intensive follow-up. Although the nomogram is better than the 7th AJCC stage group, it still has some limitations which need to be considered in the present study. As a population-based database, SEER provides us with the largest series of gallbladder cancer patients available, but some of the known survival predictors, are nearly all missing in the SEER data, such as some tumor markers and molecular factors. This study was performed using SEER database and, therefore, is limited to predictive factors available in this database. In addition, the data for external validation of nomogram is not from large, prospective, randomized and multicenter clinical trials. Other prognostic factors, which are in the molecular and gene fields, will be collected and used to improve this nomogram in the future study.
In conclusion, we developed and validated a decision model on basis of SEER and SRRSH database to predict the outcomes of postoperative overall survival for node-negative gallbladder cancer patients. Using this nomogram, clinicians may be able to obtain useful guidance to select who need additional treatment or intensive follow-up, which helps make individual therapies for these patients.
Abbreviations
SEER: Surveillance, Epidemiology, and End Results; GBC: Gallbladder Cancer; OS: Overall Survival; LN: Lymph Node; SRRSH: Sir Run Run Shaw Hospital; TNM: Tumor‐Node‐Metastasis; LNR: Lymph Node Ratio; LODDS: Log Odds of Positive Lymph Node.
Acknowledgements
This work was funded by Key Research and Development Plan Projects of Zhejiang Province (2017C01018) and Opening Fund of Engineering Research Center of Cognitive Healthcare of Zhejiang Province (2018KFJJ09).
Author Contributions
Mingyu Chen performed the research, Bin Zhang and Long Pan collected and analyzed the data, Mingyu Chen and Jiasheng Cao designed the research study and wrote the paper, and Xiujun Cai contributed to the design of the study. All authors read and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S. et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20:146-59
2. Yee K, Sheppard BC, Domreis J, Blanke CD. Cancers of the gallbladder and biliary ducts. Oncology (Williston Park). 2002;16:939-46 49; discussion 49-50, 52-3, 56-7
3. Ferretti S, Gafa L. Upper gastrointestinal tract cancers: oesophagus, stomach, liver, gallbladder and biliary ducts, pancreas. Epidemiol Prev. 2004;28:34-42
4. Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M. et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41-54
5. Tsukada K, Kurosaki I, Uchida K, Shirai Y, Oohashi Y, Yokoyama N. et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661-7
6. Fong Y, Wagman L, Gonen M, Crawford J, Reed W, Swanson R. et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006;243:767-71 discussion 71-4
7. Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16:221-32
8. Pitt SC, Jin LX, Hall BL, Strasberg SM, Pitt HA. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg. 2014;260:128-33
9. Fan DX, Xu RW, Li YC, Zhao BQ, Sun MY. Impact of the number of examined lymph nodes on outcomes in patients with lymph node-negative gallbladder carcinoma. World J Gastroenterol. 2018;24:2886-92
10. Min JH, Kang TW, Cha DI, Kim SH, Shin KS, Lee JE. et al. Apparent diffusion coefficient as a potential marker for tumour differentiation, staging and long-term clinical outcomes in gallbladder cancer. Eur Radiol. 2018
11. Freedman AN, Seminara D, Gail MH, Hartge P, Colditz GA, Ballard-Barbash R. et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97:715-23
12. Desseroit MC, Visvikis D, Tixier F, Majdoub M, Perdrisot R, Guillevin R. et al. Development of a nomogram combining clinical staging with (18)F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur J Nucl Med Mol Imaging. 2016;43:1477-85
13. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D. et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861-9
14. Zeng Q, Xue N, Dai D, Xing S, He X, Li S. et al. A Nomogram based on Inflammatory Factors C-Reactive Protein and Fibrinogen to Predict the Prognostic Value in Patients with Resected Non-Small Cell Lung Cancer. J Cancer. 2017;8:744-53
15. Dihge L, Bendahl PO, Ryden L. Nomograms for preoperative prediction of axillary nodal status in breast cancer. Br J Surg. 2017;104:1494-505
16. Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, Budel LM, Contant CM, van der Pool AE. Validation of Six Nomograms for Predicting Non-sentinel Lymph Node Metastases in a Dutch Breast Cancer Population. Ann Surg Oncol. 2016;23:477-81
17. Tsoutsou PG, Jeanneret Sozzi W, Matzinger O, Ozsahin M. Nomograms predicting locoregional recurrence in the subtype era of breast cancer. J Clin Oncol. 2013;31:647-8
18. Hijioka S, Shimizu Y, Mizuno N, Hara K, Imaoka H, Mekky MA. et al. Can long-term follow-up strategies be determined using a nomogram-based prediction model of malignancy among intraductal papillary mucinous neoplasms of the pancreas? Pancreas. 2014;43:367-72
19. Jang JY, Park T, Lee S, Kim Y, Lee SY, Kim SW. et al. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg. 2017;266:1062-8
20. Brockman JA, Alanee S, Vickers AJ, Scardino PT, Wood DP, Kibel AS. et al. Nomogram Predicting Prostate Cancer-specific Mortality for Men with Biochemical Recurrence After Radical Prostatectomy. Eur Urol. 2015;67:1160-7
21. Hirasawa Y, Nakashima J, Sugihara T, Takizawa I, Gondo T, Nakagami Y. et al. Development of a Nomogram for Predicting Severe Neutropenia Associated With Docetaxel-Based Chemotherapy in Patients With Castration-Resistant Prostate Cancer. Clin Genitourin Cancer. 2017;15:176-81
22. Lughezzani G, Lazzeri M, Haese A, McNicholas T, de la Taille A, Buffi NM. et al. Multicenter European external validation of a prostate health index-based nomogram for predicting prostate cancer at extended biopsy. Eur Urol. 2014;66:906-12
23. Liu GJ, Li XH, Chen YX, Sun HD, Zhao GM, Hu SY. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. World J Gastroenterol. 2013;19:5150-8
24. Scheingraber S, Justinger C, Stremovskaia T, Weinrich M, Igna D, Schilling MK. The standardized surgical approach improves outcome of gallbladder cancer. World J Surg Oncol. 2007;5:55
25. Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989-1995. Cancer. 1998;83:2618-28
26. Birnbaum DJ, Vigano L, Ferrero A, Langella S, Russolillo N, Capussotti L. Locally advanced gallbladder cancer: which patients benefit from resection? Eur J Surg Oncol. 2014;40:1008-15
27. Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD. et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol. 2011;29:4627-32
Author contact
![]() Corresponding author: Dr. Xiujun Cai, Department of General Surgery, Sir Run-Run Shaw Hospital, Key Laboratory of Endoscopic Technique Research of Zhejiang Province, and Engineering Research Center of Cognitive Healthcare of Zhejiang Province, Zhejiang University, No.3 East Qingchun Road, Hangzhou 310016, China. E-mail: srrsh_cxjedu.cn; Tel: 86 571 86006617; Fax: 86 571 86044817
Corresponding author: Dr. Xiujun Cai, Department of General Surgery, Sir Run-Run Shaw Hospital, Key Laboratory of Endoscopic Technique Research of Zhejiang Province, and Engineering Research Center of Cognitive Healthcare of Zhejiang Province, Zhejiang University, No.3 East Qingchun Road, Hangzhou 310016, China. E-mail: srrsh_cxjedu.cn; Tel: 86 571 86006617; Fax: 86 571 86044817

 Global reach, higher impact
Global reach, higher impact