Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(14):3284-3290. doi:10.7150/jca.29979 This issue Cite
Research Paper
Risk Factors Associated with Precancerous Lesions of Esophageal Squamous Cell Carcinoma: a Screening Study in a High Risk Chinese Population
1. Department of Science and Education, Shandong Cancer Hospital Affiliated to Shandong University, Jinan, Shandong, China.
2. Department of Oncology, School of Medicine, Shandong University, Jinan, Shandong, China.
3. Department of Biostatistics, School of Public Health, Shandong University, Jinan, Shandong, China.
4. Outpatient Department, Shandong Hospital of Traditional Chinese Medicine, Jinan, Shandong, China.
5. Cancer Screening Center, Feicheng Hospital, Jinan, Shandong, China.
6. Department of Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Jinan, Shandong, China.
Received 2018-9-16; Accepted 2019-4-4; Published 2019-6-2
Abstract
Background: Esophageal squamous cell carcinoma (ESCC) has been having a high mortality rate in China. Most patients are diagnosed in advanced stages, leading to the poor prognosis and low 5-year survival rate. Detection of precancerous lesions or early cancers is the key to improving this situation. Although previous studies have identified some risk factors for ESCC, they rarely paid attention to the premalignant esophageal lesions. We thus initiated a population-based screening study aiming to assess risk factors associated with esophageal precancerous lesions (EPLs) in a high risk Chinese population.
Methods: From September 2013 to July 2015, we screened residents aged 40-69 years from 53 randomly selected communities in Feicheng, China (n = 5076). Each participant went through questionnaire interview, physical examination, endoscopy and biopsy. Using logistic regression, we compared participants with EPLs to that with normal esophageal mucosa for finding potential risk factors of EPLs.
Results: A total of 570 participants were diagnosed with EPLs. We observed no association between EPLs and tobacco smoking or alcohol consumption in unadjusted or adjusted model. In the adjusted model, the OR (95% CI) was 1.84 (1.18-2.89) for people of drinking shallow-well water comparing to people who was drinking tap-water. In a comparison of participants with good oral health, the ESD/ESCC ORs (95% CI) for those with very poor or poor oral health, were 1.78 (1.28-2.49) and 1.58 (1.16-2.15) respectively. However, no statistical significance was observed after adjustment. Moreover, cereal straw heating (OR= 1.74, 95% CI: 0.90-3.36, P=0.099) may lead to increased risk of EPLs.
Conclusion: In Feicheng population, tobacco smoking or alcohol consumption may not be risk factors of EPLs. Low-quality drinking water raised the EPLs risk. Bad house heating materials, such as cereal straw, may lead to high EPLs risk.
Keywords: esophageal squamous dysplasia, population-based, esophageal precancerous lesions, drinking water, house heating method, oral health
Introduction
The incidence rate of esophageal cancer (EC) is ranked eighth in the most common cancer-related diseases worldwide (1). There were 455,800 new EC cases and 400,200 deaths caused by EC around the world in 2012 (2). Although some cancers, such as breast cancer and prostate cancer, have higher incidence rates than EC, they have lower mortality rates, which reflects lower fatality rates compared to EC.
There is also a remarkable difference in EC incidence rates among different geographical regions. The highest incidence rates were found in China, Iran, South America, and South Africa, which is often referred to as the “esophageal cancer belt” (2). As one of the countries in this belt, China bears a heavy disease burden with respect to EC (3), with Feicheng having one of the highest rates of incidence. Whereas the incidence of esophageal adenocarcinoma has been rising slowly and steadily over the past four decades, squamous cell carcinoma (ESCC) is still the most prevalent subtype in China (4, 5). It is among the five leading causes of cancer death in both men and women (6).
For ESCC patients, the cancer stage at first diagnosis is a decisive factor for prognosis (7). Because the early symptoms of ESCC are not obvious enough to attract the patient's attention, individuals are often in the advanced stages of cancer when they go to hospital for examination. Thus the age-specific 5-year survival rate for adults with EC is 10.9% in Europe (8) and ~10-15% in China (9-11). Moreover, without active and appropriate treatments, patients who have been diagnosed as having EPLs may continue to develop into an advanced stage (12). Currently, the factors causing high rates of ESCC in Feicheng remain obscure. Here we used a population-based design study, a powerful and critical method, to identify the possible risk factors for EPLs/ESCC.
Materials and Methods
Setting
This study was launched by the Shandong Cancer Hospital, Shandong Cancer Control and Prevention office, and Department of Biostatistics of Shandong University. From September 2013 to July 2015, we recruited residents aged 40-69 years from 53 randomly selected communities in Feicheng, China. Ethical approval from the medical ethics committee of the Shandong Cancer Hospital was obtained. The reference coding is SDTHEC.
Study design
Feicheng is located in the central part of Shandong Province, with a total of 605 communities. We randomly selected 53 communities in Feicheng. The work of recruitment was assisted by Feicheng Disease Control Center and Community Health Centers. The purpose and process of this research were thoroughly explained to each eligible participant. A signed informed consent was mandatory before recruitment.
Inclusion criteria: 1) Being a permanent resident in Feicheng. 2) Aged 40-69 years.
Exclusion criteria: 1) Refusing to participate in the study. 2) Working outside Feicheng more than six months a year. 3) Having endoscopy contraindications. 4) Having an iodine allergy or hyperthyroidism. 5) Having EC history (13).
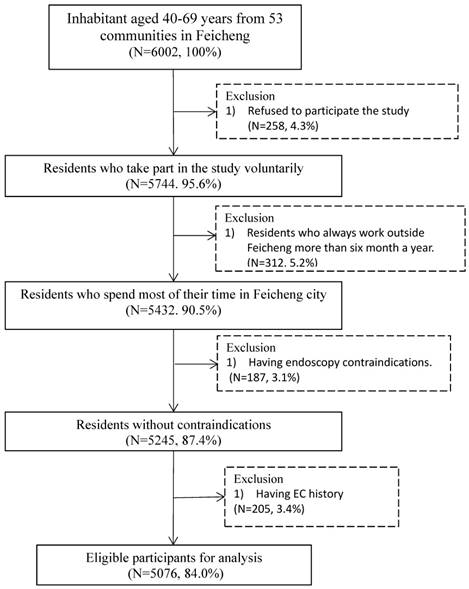
There are 5076 participants in the final analysis. Figure 1 summarizes the inclusion and exclusion process of this study.
Questionnaire interview was conducted at Community Health Centers by trained investigators using a structured questionnaire. Detailed demographic characteristics, habits, lifestyle information as well as health and disease information was collected. Participants went through a simple physical examination and then endoscopic test at the Cancer Screening Center of Feicheng Hospital. During the endoscopy, physicians used Lugol's iodine staining method to find suspicious tissues, which were biopsied and analyzed.
In order of severity, esophageal mucosa status mainly includes normal esophageal mucosa, minor mucosa changes, esophagitis, esophageal squamous simple hyperplasia (ESSH), and esophageal squamous dysplasia (ESD) (14). ESD is classified as mild, moderate, or severe. Mild and moderate ESD together falls under low-grade ESD. Severe ESD, including squamous cell carcinoma in situ, is considered as the high-grade ESD (15). Mild ESD is the earliest form of EPLs. The progression, from chronic esophagitis, by way of ESSH, to ESD, is known as a common route to the ESCC (12, 14, 16). In our study, we grouped the participants into 3 groups: 'normal', 'minor changes/esophagitis/ESSH', and 'ESD/ESCC'. For the participants with multiple diagnosis records in one biopsy, we only care about the most severe one.
Statistical analysis
All the variables in this study are categorical. So we calculated frequencies and percentages for each variable. We described the difference in demographic characteristics, habits, lifestyle, and health information between 3 groups by χ2-test (Table 2). To assess the association between ESD/ESCC and the factors of interest, we used multinomial logistic regression model to estimate the unadjusted odds ratios (ORs) with 95% confidence intervals (CIs). Further adjusting for sex and age, adjusted ORs (95% CIs) were also showed in Table 3. The group of 'normal' was defined as the reference throughout the whole analysis.
Flow chart of inclusion and exclusion for the screening study in Feicheng
Results
As shown in Table 1, a total of 570 EPLs cases and 56 ESCC cases were diagnosed. EPLs cases included 406 cases of mild ESD, 105 cases of moderate ESD, and 59 cases of severe ESD (or squamous cell carcinoma in situ). Cases of ESCC were dominated by early-stage cancer, containing 30 cases of intramucosal carcinoma and 26 cases of invasive carcinoma.
Diagnosis results among the participants
| Type | Number (%) |
|---|---|
| Normal esophagus | 3554 (70.02) |
| Minor changes, esophagitis and ESSH | 896 (17.65) |
| ESD | 570 (11.23) |
| Esophageal squamous cell carcinoma | 56 (1.10) |
Table 2 presents the demographic characteristics, as well as distribution of habits, lifestyle and health information. Among the participants, 51.64% were men, 90.84% were farmers. Overall, study population had a relatively low education level, most of individuals didn't or just completed the 9-year compulsory education. And participants who were diagnosed with ESD/ESCC were more likely older people. Tobacco smoking and alcohol consumption were both significantly different between different pathological groups. Participants who mostly drank tap-water in the past 10 years were less in the group of 'ESD/ESCC' compared with another two groups. In terms of heating method in winter, cereal straw and coal heating were more common in the group of 'ESD/ESCC', electric heating was more common in 'norma' group. Meanwhile, 21.25% participants in the group of 'ESD/ESCC' had a very poor oral health, whereas 9.11% in good oral health.
Table 3 further examined associations between variables of interest and EPLs risk. There was no association observed between EPLs risk and tobacco smoking or alcohol consumption in both unadjusted and adjusted model. As compared with tap-water drinking people, the crude ORs (95% CIs) were 2.10 (1.01-4.37) for lake/pond water drinking people, 2.19 (1.40-3.42) for shallow-well water drinking people, and 1.57 (0.95-2.60) for deep-well water drinking people. And the adjusted OR (95% CI) was 1.84 (1.18-2.89) for people of drinking shallow-well water comparing to people who has been drinking tap-water. In a comparison of participants with good oral health to those with very poor or poor oral health, the ESD/ESCC ORs (95% CIs) were 1.78 (1.28-2.49) and 1.58 (1.16-2.15) respectively. However, the risks were not statistically significant after adjustment. Moreover, electric heating (OR=0.68, 95% CI: 0.34-1.36) was associated with lower EPLs risk. Cereal straw heating (OR= 1.87, 95% CI: 0.98-3.59) and coal heating (OR= 1.62, 95% CI: 0.92-2.84) both led to increased risk of EPLs. In adjusted model, the OR (95% CI) was 1.74 (0.90-3.36) for participants who have been taking cereal straw heating method.
Characteristics of 5076 participants from a high risk Chinese population
| Factor | Normal (n=3554) | Minor changes/esophagitis/ESSH (n=896) | ESD/ ESCC (n=626) | χ2 | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 1879(52.87) | 399(44.53) | 343(54.79) | 22.775 | 0.000 |
| Female | 1675(47.13) | 497(55.47) | 283(45.21) | ||
| Age | |||||
| 40-49 | 656(18.46) | 116(12.95) | 41(6.55) | 121.905 | 0.000 |
| 50-59 | 1336(37.59) | 323(36.05) | 185(29.55) | ||
| 60-69 | 1385(38.97) | 406(45.31) | 333(53.19) | ||
| ≥70 | 177(4.98) | 51(5.69) | 67(10.70) | ||
| Occupation | |||||
| Farmer | 3197(89.95) | 825(92.08) | 589(94.09) | 12.933 | 0.002 |
| Non-farmer | 357(10.05) | 71(7.92) | 37(5.91) | ||
| Education | |||||
| No schooling | 439(12.35) | 145(16.18) | 124(19.81) | 36.012 | 0.000 |
| Primary school (1-6 years) | 1071(30.14) | 268(29.91) | 202(32.27) | ||
| Junior high school (7-9 years) | 1582(44.51) | 381(42.52) | 230(36.74) | ||
| ≥High school (≥10 years) | 462(13.00) | 102(11.38) | 70(11.18) | ||
| Tobacco smoking | |||||
| Non-smoker | 2223(62.55) | 620(69.20) | 391(62.46) | 17.221 | 0.002 |
| Current smoker | 917(25.8) | 195(21.76) | 150(23.96) | ||
| Former smoker | 414(11.65) | 81(9.04) | 85(13.58) | ||
| Alcohol consumption | |||||
| Non-drinker | 2188(61.56) | 577(64.41) | 380(60.70) | 11.280 | 0.024 |
| Current drinker | 1077(30.30) | 263(29.35) | 179(28.59) | ||
| Former drinker | 287(8.08) | 55(6.14) | 67(10.71) | ||
| Tea consumption | |||||
| Never | 696(19.58) | 184(20.54) | 110(17.57) | 3.247 | 0.517 |
| Occasionally | 266(7.48) | 68(7.59) | 41(6.55) | ||
| Regularly | 2592(72.93) | 644(71.88) | 475(75.88) | ||
| Source of drinking water | |||||
| Lake/pond water | 71(1.99) | 11(1.23) | 13(2.08) | 25.010 | 0.000 |
| Shallow-well water | 2736(76.98) | 705(78.68) | 523(83.55) | ||
| Deep-well water | 495(13.93) | 104(11.61) | 68(10.86) | ||
| Tap water | 252(7.10) | 76(8.48) | 22(3.51) | ||
| Meal temperature | |||||
| Burning hot | 53(1.49) | 16(1.79) | 11(1.76) | 2.200 | 0.900 |
| Hot | 1261(35.48) | 320(35.71) | 238(38.02) | ||
| Warm | 1954(54.98) | 489(54.58) | 330(52.72) | ||
| Cold | 286(8.05) | 71(7.92) | 47(7.51) | ||
| Exercise | |||||
| No exercise | 1739(48.93) | 420(46.88) | 308(49.20) | 3.930 | 0.686 |
| Seldom of exercise | 1629(45.84) | 438(48.88) | 288(46.01) | ||
| Moderate of exercise | 154(4.33) | 31(3.46) | 26(4.15) | ||
| Frequent of exercise | 32(0.90) | 7(0.78) | 4(0.64) | ||
| House heating method | |||||
| Cereal straw heating | 186(5.23) | 48(5.36) | 40(6.39) | 23.172 | 0.001 |
| Electric heating | 307(8.64) | 63(7.03) | 24(3.83) | ||
| Coal heating | 2705(76.11) | 699(78.01) | 503(80.35) | ||
| Natural gas heating | 122(3.43) | 37(4.13) | 14(2.24) | ||
| Oral health | |||||
| Very poor | 589(16.57) | 137(15.29) | 133(21.25) | 28.643 | 0.000 |
| Poor | 1241(34.92) | 361(40.29) | 249(39.78) | ||
| Moderate | 1274(35.85) | 297(33.15) | 187(29.87) | ||
| Good | 450(12.66) | 101(11.27) | 57(9.11) | ||
| Gastroesophageal reflux disease | |||||
| No | 2383(67.05) | 616(68.75) | 444(70.93) | 4.086 | 0.130 |
| Yes | 1171(32.95) | 280(31.25) | 182(29.07) | ||
| Diabetes | |||||
| No | 3397(95.58) | 847(94.53) | 608(97.12) | 5.876 | 0.053 |
| Yes | 157(4.42) | 49(5.47) | 18(2.88) | ||
| Family history of cancer | |||||
| No | 2856(80.36) | 696(77.68) | 480(76.68) | 6.468 | 0.039 |
| Yes | 698(19.64) | 200(22.32) | 146(23.32) | ||
Odds ratios and 95% confidence intervals for the association between investigated variables and high EPLs/ESCC risk
| Normal & ESD/ESCC | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |
|---|---|---|---|---|---|
| Gender | Male | 1.08(0.91-1.28) | 0.374 | 1.32(0.99-1.76) | 0.063 |
| Female | Reference | ||||
| Age | 40-49 | 0.17(0.11-0.25) | 0.000 | 0.17(0.11-0.28) | 0.000 |
| 50-59 | 0.37(0.27-0.50) | 0.000 | 0.41(0.29-0.58) | 0.000 | |
| 60-69 | 0.64(0.47-0.86) | 0.004 | 0.62(0.45-0.86) | 0.004 | |
| ≥70 | Reference | ||||
| Occupation | Farmer | 1.78(1.25-2.52) | 0.001 | 1.57(1.10-2.25) | 0.013 |
| Non-farmer | Reference | ||||
| Education | No schooling | 1.86(1.35-2.57) | 0.000 | 1.57(1.09-2.24) | 0.013 |
| Primary school | 1.24(0.93-1.67) | 0.143 | 1.12(0.82-1.52) | 0.485 | |
| Junior high school | 0.96(0.72-1.28) | 0.778 | 1.06(0.79-1.41) | 0.716 | |
| ≥High school | Reference | ||||
| Tobacco smoking | Former smoker | 1.17(0.90-1.51) | 0.238 | 0.88(0.64-1.19) | 0.398 |
| Current smoker | 0.93(0.76-1.14) | 0.484 | 0.84(0.65-1.09) | 0.200 | |
| Non-smoker | Reference | ||||
| Alcohol Consumption | Former drinker | 1.04(0.61-1.79) | 0.744 | 1.05(0.75-1.45) | 0.790 |
| Current drinker | 0.96(0.79-1.16) | 0.654 | 0.94(0.74-1.19) | 0.599 | |
| Non-drinker | Reference | ||||
| Source of drinking water | Lake/pond water | 2.10(1.01-4.37) | 0.048 | 1.71(0.81-3.58) | 0.157 |
| Shallow-well water | 2.19(1.40-3.42) | 0.001 | 1.84(1.18-2.89) | 0.008 | |
| Deep-well water | 1.57(0.95-2.60) | 0.078 | 1.38(0.82-2.29) | 0.217 | |
| Tap water | Reference | ||||
| Meal temperature | Burning hot | 1.26(0.62-2.59) | 0.524 | 1.40(0.68-2.91) | 0.363 |
| Hot | 1.15(0.82-1.61) | 0.422 | 1.18(0.84-1.67) | 0.331 | |
| Warm | 1.03(0.74-1.43) | 0.871 | 1.07(0.77-1.49) | 0.690 | |
| Cold | Reference | ||||
| House heating method | Cereal straw heating | 1.87(0.98-3.59) | 0.058 | 1.74(0.90-3.36) | 0.099 |
| Electric heating | 0.68(0.34-1.36) | 0.277 | 0.79((0.39-1.59) | 0.514 | |
| Coal heating | 1.62(0.92-2.84) | 0.092 | 1.53(0.87-2.96) | 0.144 | |
| Natural gas heating | Reference | ||||
| Oral heath | Very poor | 1.78(1.28-2.49) | 0.001 | 1.20(0.85-1.70) | 0.295 |
| Poor | 1.58(1.16-2.15) | 0.003 | 1.20(0.88-1.65) | 0.253 | |
| Moderate | 1.16(0.85-1.59) | 0.360 | 1.03(0.75-1.42) | 0.865 | |
| Good | Reference | ||||
| Family history of cancer | Yes | 1.24(1.02-1.52) | 0.035 | 1.30(1.05-1.59) | 0.014 |
| No | Reference | ||||
Discussion
We conducted a population-based screening study in Feicheng, China, aiming to figure out the risk factors associated with EPLs. We found that drinking low-quality water might lead to an increased risk of having EPLs. Previous studies indicated that ESCC is the result of a combination of multiple risk factors, containing environmental and biological factors (17, 18). Drinking water is one of the basic necessities of life, as well as a potential way of exposure to risk factors. Different from pond water or well water, tap water had been purified and disinfected before delivering to users. Purification and disinfection can significantly reduce the concentrations of most carcinogens. Several studies suggested that high level of nitrate in drinking water would increase the risk of ESCC, possibly through the formation of N-nitroso compounds (19-21). So we should conduct a further study to find out the specific carcinogens in drinking water, which have been increasing the incidence of EPLs/ESCC in Feicheng.
Another main finding of our study was that house heating method may influence the odds of having EPLs/ESCC. Cereal straw heating is associated with the raised risks of having EPLs. Meanwhile, electric heating might be associated with reduced risk of having EPLs/ESCC. Some studies found that indoor air pollution caused by using stoves or fireplaces is associated with the higher risk of having lung cancer and breast cancer (22-24). Only one study suggested that using heating stoves without ventilator installed in their home may lead to increased risk of ESD (25). Our research examined in details the use of different materials for house heating in this area, and showed that cereal straw heating is a risk factor for EPLs. Our primary hypothesis is that bad heating materials can cause loads of pollutants, such as polycyclic aromatic hydrocarbons and sulfur dioxide, which may contaminate air, water and food. Biomass fuel is widely used in daily heating in Feicheng population, thus significantly increasing the chance of people contacting carcinogens or mutagens.
The third interesting finding was that we observed no significant association between tobacco smoking and the risk of EPLs/ESCC. Tobacco smoking was identified as a strong determinant of ESCC in many western countries (5, 26, 27). And a previous national-wide case-control study (28) that was conducted in China also indicated the same association. However, this is not the case in Feicheng. Our study even suggested a mild inverse association between tobacco consumption and EPLs/ESCC in the adjusted model. This inverse association occurred both in current smoker and former smoker, but without statistically significant. With respect to alcohol consumption, unlike many studies (27, 29), we didn't observe any statistically significant increased/decreased EPLs/ESCC risks among participants who were current drinkers or former drinkers. This is possibly due to the smokers/drinkers in this area consuming relatively small daily amounts of tobacco/alcohol, and indicates the fact that there must be the other risk factors dominating the incidence of EPLs/ESCC in this population.
Furthermore, several studies reported poor oral health as a risk factor for ESCC (30, 31). People that lacked regular oral hygiene or had too much tooth loss/decay are more susceptible to ESCC (32-34). Only two studies reported the association between poor oral health and the increased risk of ESD but with small cases (25, 35). In unadjusted model, our finding is consistent with these studies. However, after adjusting for sex and age, the increased EPLs/ESCC risk was not statistically significant. Elder people (>60 years old) in poor oral health group accounted for 53.70%. Meanwhile this proportion in moderate oral health group and good oral health group were 38.45% and 27.80% respectively, which may be the main cause of above mentioned results.
To the best of our knowledge, this is the largest population-based screening study to date to investigate the EPLs - both for sample size and number of cases found. The study has several strengths. First of all, due to the population-based research design and the high participation rate (84%), subjects in our study would be more likely representative of the general population in Feicheng. Secondly, by conducting the interview prior to endoscopic test for each participant, we are able to reduce the possible changes in their habits, lifestyle, and behavior after being diagnosed with the disease. Third, we collected comprehensive questionnaire data. And all subjects received the gold standard test (endoscopy with Lugol's iodine staining and biopsy) for EPLs, which allowed us to examine the numerous risk factors accurately.
Meanwhile, we should be cautious about the results interpretation due to limitations of the study. The first concern is recall bias, which is caused by the inaccuracy or incompleteness of the remembering information by study participants, and which is a common issue in cross-sectional study. Our well-trained investigators had tried their best to help every participant to fill out questionnaire forms. During data collection, study objectives and hypothesis were blinded to all the investigators and participants. Another potential problem must to be considered is sampling error. Generally, physicians would pick out one through three skeptical blocks of tissue for biopsy in one time endoscopy, the risk of false negative results might exist. What's more, our study lacked detailed information on environmental exposure. Although our findings indicated that low quality drinking water and bad house heating methods are risk factors for having EPLs, we are not able to explain the mechanisms underlying these associations. Future plans of our research include a follow-up study and an environmental investigation to confirm the observed associations in this study.
In conclusion, EPLs is brought on by a confluence of risk factors, which vary between different populations though. We should not perform identical strategy to prevent this disease, especially in an area with extremely high incidence rate. In Feicheng population, people who consume tobacco or alcohol may not be more vulnerable to EPLs/ESCC. Low-quality drinking water might raise the risk of having EPLs. Bad house heating methods associated with increased EPLs risk which should be held in high regard.
Abbreviations
ESCC: esophageal squamous cell carcinoma; ESD: esophageal squamous dysplasia; EPLs: esophageal precancerous lesions; EC: esophageal cancer; ESSH: esophageal squamous simple hyperplasia.
Acknowledgements
We greatly appreciate those individuals who took part in our study. Without their dedication and cooperation, our study could not have begun. In addition, our study has been supported by many research groups and organizations. We are very grateful for all the supports giving by investigators, clinicians and pathologists from Shandong Cancer Hospital, Shandong Cancer Control and Prevention office, Department of Biostatistics of Shandong University, Cancer Center of Feicheng Hospital, Centers of Disease Control, and Community Health Centers.
Funding
This work was supported by National Natural Science Foundation of China (81573246), Key Research & Development Plan of Shandong Province (2017GSF18101) and National Key Research & Development Program of China (2016YFC1302800, 2016YFC0901400).
Competing Interests
The authors have declared that no competing interest exists.
References
1. IARC. World Cancer Report 2014. Geneva, Switzerland: WHO Press. 2014
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
3. Sun X, Zhao D, Liu Y, Liu Y, Yuan Z, Wang J, Xue F. The long-term spatial-temporal trends and burden of esophageal cancer in one high-risk area: A population-registered study in Feicheng, China. PLoS One. 2017;12:e0173211
4. Martin-Richard M, Diaz Beveridge R, Arrazubi V, Alsina M, Galan Guzman M, Custodio AB, Gomez C, Munoz FL, Pazo R, Rivera F. SEOM Clinical Guideline for the diagnosis and treatment of esophageal cancer (2016). Clin Transl Oncol. 2016;18:1179-1186
5. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. The Lancet. 2013;381:400-412
6. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115-132
7. Bus P, Lemmens VE, van Oijen MG, Creemers G-J, Nieuwenhuijzen GA, van Baal JW, Siersema PD. Prognostic factors for medium- and long-term survival of esophageal cancer patients in the Netherlands. Journal of Surgical Oncology. 2014;109:465-471
8. Anderson LA, Tavilla A, Brenner H, Luttmann S, Navarro C, Gavin AT, Holleczek B, Johnston BT, Cook MB, Bannon F, Sant M, Group E-W. Survival for oesophageal, stomach and small intestine cancers in Europe 1999-2007: Results from EUROCARE-5. Eur J Cancer. 2015;51:2144-2157
9. Crane SJ, Locke GR 3rd, Harmsen WS, Zinsmeister AR, Romero Y, Talley NJ. Survival trends in patients with gastric and esophageal adenocarcinomas: a population-based study. Mayo Clin Proc. 2008;83:1087-1094
10. Peyre CG, Hagen JA, DeMeester SR, Van Lanschot JJ, Holscher A, Law S, Ruol A, Ancona E, Griffin SM, Altorki NK, Rice TW, Wong J, Lerut T, DeMeester TR. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248:979-985
11. Rodriguez-Camacho E, Pita-Fernandez S, Pertega-Diaz S, Lopez-Calvino B, Seoane-Pillado T. Clinical-pathological characteristics and prognosis of a cohort of oesophageal cancer patients: a competing risks survival analysis. J Epidemiol. 2015;25:231-238
12. Appelman HD, Matejcic M, Parker MI, Riddell RH, Salemme M, Swanson PE, Villanacci V. Progression of esophageal dysplasia to cancer. Ann N Y Acad Sci. 2014;1325:96-107
13. Dantoft TM, Ebstrup JF, Linneberg A, Skovbjerg S, Madsen AL, Mehlsen J, Brinth L, Eplov LF, Carstensen TW, Schroder A, Fink PK, Mortensen EL, Hansen T, Pedersen O, Jorgensen T. Cohort description: The Danish study of Functional Disorders. Clin Epidemiol. 2017;9:127-139
14. Liu X, Zhang M, Ying S, Zhang C, Lin R, Zheng J, Zhang G, Tian D, Guo Y, Du C, Chen Y, Chen S, Su X, Ji J, Deng W, Li X, Qiu S, Yan R, Xu Z, Wang Y, Guo Y, Cui J, Zhuang S, Yu H, Zheng Q, Marom M, Sheng S, Zhang G, Hu S, Li R, Su M. Genetic Alterations in Esophageal Tissues From Squamous Dysplasia to Carcinoma. Gastroenterology. 2017;153:166-177
15. Shimizu M, Ban S, Odze RD. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin North Am. 2007;36:797-811 v-vi
16. Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550-563
17. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387
18. Al-Haddad S, El-Zimaity H, Hafezi-Bakhtiari S, Rajendra S, Streutker CJ, Vajpeyi R, Wang B. Infection and esophageal cancer. Ann N Y Acad Sci. 2014;1325:187-196
19. Cao W, Han J, Yuan Y, Xu Z, Yang S, He W. Drinking water: a risk factor for high incidence of esophageal cancer in Anyang, China. Environ Geochem Health. 2016;38:773-782
20. Golozar A, Etemadi A, Kamangar F, Fazeltabar Malekshah A, Islami F, Nasrollahzadeh D, Abedi-Ardekani B, Khoshnia M, Pourshams A, Semnani S, Marjani HA, Shakeri R, Sotoudeh M, Brennan P, Taylor P, Boffetta P, Abnet C, Dawsey S, Malekzadeh R. Food preparation methods, drinking water source, and esophageal squamous cell carcinoma in the high-risk area of Golestan, Northeast Iran. Eur J Cancer Prev. 2016;25:123-129
21. Keshavarzi B, Moore F, Najmeddin A, Rahmani F, Malekzadeh A. Quality of drinking water and high incidence rate of esophageal cancer in Golestan province of Iran: a probable link. Environ Geochem Health. 2012;34:15-26
22. Alexandra W ST, Steven S, Jan B, Susan S, Irina M, Kathleen M, Jiyoung A, Pavel J, Regina S, Marilie G. Indoor air pollution exposure from use of indoor stoves and fireplaces in association with breastcancer: a case-control study. Environmental Health. 2014;13:108
23. Galeone C, Pelucchi C, La Vecchia C, Negri E, Bosetti C, Hu J. Indoor air pollution from solid fuel use, chronic lung diseases and lung cancer in Harbin, Northeast China. Eur J Cancer Prev. 2008;17:473-478
24. Mu L, Liu L, Niu R, Zhao B, Shi J, Li Y, Swanson M, Scheider W, Su J, Chang SC, Yu S, Zhang ZF. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control. 2013;24:439-450
25. Wei WQ, Abnet CC, Lu N, Roth MJ, Wang GQ, Dye BA, Dong ZW, Taylor PR, Albert P, Qiao YL, Dawsey SM. Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut. 2005;54:759-763
26. Yates M, Cheong E, Luben R, Igali L, Fitzgerald R, Khaw KT, Hart A. Body mass index, smoking, and alcohol and risks of Barrett's esophagus and esophageal adenocarcinoma: a UK prospective cohort study. Dig Dis Sci. 2014;59:1552-1559
27. Ishiguro S, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Tsugane S, Group JS. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study). Cancer Lett. 2009;275:240-246
28. Jiang JM, Zeng XJ, Chen JS, Ping z, Li JY, Zhang KL, Wu YP, Liu BQ. Smoking and mortality from esophageal cancer in China: a large case-control study of 19,734 male esophageal cancer deaths and 104,846 living spouse controls. Int J Cancer. 2006;119:1427-1432
29. Lee CH, Wu DC, Lee JM, Wu IC, Goan YG, Kao EL, Huang HL, Chan TF, Chou SH, Chou YP, Lee CY, Chen PS, Ho CK, He J, Wu MT. Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur J Cancer. 2007;43:1188-1199
30. Dar NA, Islami F, Bhat GA, Shah IA, Makhdoomi MA, Iqbal B, Rafiq R, Lone MM, Abnet CC, Boffetta P. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer. 2013;109:1367-1372
31. Abnet CC, Kamangar F, Islami F, Nasrollahzadeh D, Brennan P, Aghcheli K, Merat S, Pourshams A, Marjani HA, Ebadati A, Sotoudeh M, Boffetta P, Malekzadeh R, Dawsey SM. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3062-3068
32. Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34:467-474
33. Guha N, Boffetta P, Wunsch Filho V, Eluf Neto J, Shangina O, Zaridze D, Curado MP, Koifman S, Matos E, Menezes A, Szeszenia-Dabrowska N, Fernandez L, Mates D, Daudt AW, Lissowska J, Dikshit R, Brennan P. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166:1159-1173
34. Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222-1227
35. Alireza S Fk, Saman F, Farrokh S, Christian A, Sanford D. Poor Oral Health as a Risk Factor for Esophageal Squamous Dysplasia in Northeastern Iran. ANTICANCER RESEARCH. 2005;25:543-546
Author contact
![]() Corresponding authors: Jialin Wang, Shandong cancer hospital affiliated to Shandong University, Jinan 250117, Shandong, China. Tel: +86 15553112317. E-mail: wangjialin6681com. Jinming Yu, Shandong cancer hospital affiliated to Shandong University, Jinan 250117, Shandong, China. Tel: +86 13806406293. E-mail: sdyujinmingcom.
Corresponding authors: Jialin Wang, Shandong cancer hospital affiliated to Shandong University, Jinan 250117, Shandong, China. Tel: +86 15553112317. E-mail: wangjialin6681com. Jinming Yu, Shandong cancer hospital affiliated to Shandong University, Jinan 250117, Shandong, China. Tel: +86 13806406293. E-mail: sdyujinmingcom.

 Global reach, higher impact
Global reach, higher impact