Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(16):3657-3664. doi:10.7150/jca.32716 This issue Cite
Research Paper
Pre-treatment Serum Lactate Dehydrogenase Predicts Distant Metastasis and Poor Survival in Nasopharyngeal Carcinoma
Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, Wuhan 430030, People's Republic of China.
Received 2019-1-1; Accepted 2019-5-16; Published 2019-6-9
Abstract
Background: Pre-treatment serum lactate dehydrogenase (LDH) has emerged as prognostic factor for many cancers. In this study, we evaluated the value of LDH in predicting distant metastasis and poor survival for patients with nasopharyngeal carcinoma (NPC).
Methods: Clinical data from 172 non-metastatic NPC patients were retrospectively collected and serum LDH levels were routinely measured before treatment. The independent-samples t test was used to calculate differences between serum LDH levels from the various patient groups. Receiver-operating characteristic (ROC) curve analysis was performed to select the optimal cutoff points. The Kaplan-Meier method and log-rank test were adopted to calculate and compare the distant metastasis free survival (DMFS) and overall survival (OS) rates. The Cox proportional hazards model was used to carry out univariate and multivariate analyses.
Results: NPC patients progressed with distant metastasis often have higher pre-treatment serum LDH levels than those did not develop distant metastasis (mean LDH level was 237.1U/L and 108.8U/L, respectively, p=0.001). Elevated LDH level was identified as an independent prognostic factor for poor DMFS (hazard ratio (HR), 8.31; 95% confidence interval (CI), 2.44-28.32; p=0.001) and OS (HR, 4.45; 95% CI, 1.77-11.21; p=0.002). Moreover, subgroup analyses revealed significant associations between serum LDH level and worse survival in advanced stage patients.
Conclusions: Pre-treatment serum LDH level can predict distant metastasis and associate with the poor survival in patients with NPC.
Keywords: nasopharyngeal carcinoma, lactate dehydrogenase, distant metastasis, survival.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck cancer in Asia, especially in the southern provinces of China [1]. Due to its anatomical location and radiosensitivity, radiotherapy is the primary therapeutic approach for this disease [2]. In the time of conventional radiotherapy (CRT), locoregional recurrence and distant metastasis contributed equally to the treatment failures of NPC [3]. In recent years, intensity-modulated radiotherapy (IMRT) which can deliver higher dose to the tumor and lower dose to the surrounding normal tissues had been widely used for NPC treatment. The technical advantages of IMRT achieved excellent local and regional controls [4, 5], making distant metastasis became the main cause of treatment failures among NPC patients. Therefore, identifying NPC patients with high risk of distant metastasis and subsequent poor prognosis before treatment is necessary and helpful for better treatment decision.
Nowadays, tumor-node-metastasis (TNM) staging system is the most broadly adopted tool for treatment decision and outcome prediction in NPC patients. However, NPC patients with the same TNM stage often undergo different clinical courses [6, 7]. The possible interpretation may be that TNM staging system classifies the extent of disease chiefly on anatomical information and does not take biological heterogeneity into consideration [8]. Thus, identifying biomarkers associated with prognosis may complement the TNM staging system in the prognostication of NPC. Recently, more and more molecular biomarkers were identified as prognostic factors for NPC, including Epstein-Barr virus DNA loads, microRNA signature and epidermal growth factor receptor overexpression [9-11]. However, some issues need to be identified before these biomarkers are applied to routine clinical practice, such as the high cost and the large inter-laboratory variability [12]. Therefore, screening of some inexpensive, objective and easily detected markers to predict the outcome of NPC patients is urgently needed.
It is now generally accepted that the metabolism of cancer cells differs from that of normal cells. Cancer cells preferentially metabolize glucose by glycolysis to generate energy even in the presence of adequate oxygen [13]. In those metabolic enzymes involved in glycolysis, lactate dehydrogenase (LDH) is the most important one as it can convert pyruvate to lactate at the end of glycolysis [14]. LDH has been identified to possess a prognostic role in several kinds of cancers [15-17]. The international prognostic index (IPI) is a common clinical tool used to predict outcomes in patients with non-Hodgkin's lymphoma and it contains five risk factors including serum LDH [18]. In addition, serum LDH level has been adopted in TNM staging system for the prognostication of melanoma [19]. Thus, serum LDH which is easily detected in clinical practices may allow widespread clinical use and contribute to prognosis estimation.
The prognostic value of serum LDH level in NPC patients has been investigated in several studies [20-25]. However, varied results are reported and some studies are based on survival data from patients who received CRT [20, 23]. In addition, most of them came from high endemic areas, including Guangdong [21, 24], Guangxi [23] and Fujian [25] provinces. Moreover, the predictive value of serum LDH for distant metastasis in NPC patients is still not well defined. Therefore, non-metastatic NPC patients from middle incidence area treated with IMRT were collected in this study. The pre-treatment serum LDH level and its role in predicting distant metastasis and poor survival were evaluated.
Materials and Methods
Patient population
All patients included in this study were treated at Cancer Center, Tongji Hospital between January 2012 and December 2013. This study was carried out with the approval of our ethical committee. Patient's clinical features were obtained from the medical records and the inclusion criteria were: (1) histologically confirmed NPC; (2) no previous malignancy diseases; (3) no evidence of distant metastasis; (4) no previous treatment for NPC; (5) with complete record of pre-treatment serum LDH level; (6) absence of other serious diseases which could affect the level of serum LDH (acute or chronic hepatitis, liver cirrhosis, congestive heart failure, pulmonary infarction, kidney disease, and so on); (7) with complete follow-up data.
Treatment and follow-up
Full routine workup was completed before the initiation of treatment, including endoscopic examination of the nasopharynx, physical examination, magnetic resonance imaging scan of the nasopharynx and neck, chest computed tomography, abdominal ultrasonography, whole body bone scanning and hematologic testing. Serum LDH levels were measured using the Roche Cobas 8000 system (Roche, Indianapolis, IN, USA). The seventh edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) staging system was used for stage classification. All patients in this study were treated with IMRT with or without chemotherapy. According to our institutional guidelines, patients with stage I disease generally did not receive chemotherapy while patients with stage II-IV disease received concurrent chemoradiotherapy and/or neoadjuvant or adjuvant chemotherapy. The total planned dose was 68 to 70 Gy to the nasopharyngeal region, and 60 to 66 Gy to the involved cervical node. The regimens of neoadjuvant chemotherapy included TP regimen (docetaxel 75 mg/m2 intravenous on day 1 plus cisplatin 25 mg/m2 intravenous on days 1-3) and TPF regimen (docetaxel 75 mg/m2 intravenous on day 1, cisplatin 25 mg/m2 intravenous on days 1-3 plus 5-fluorouracil 750 mg/m2/d continuously intravenous on days 1-5). These chemotherapy regimens were repeated every 3 weeks for 2-3 cycles. Concurrent chemoradiotherapy was performed during the period of radiotherapy (cisplatin 25 mg/m2 on day 1, repeat every week). For patients received adjuvant chemotherapy, the TP or TPF regimens (the same as neoadjuvant chemotherapy) were repeated every 3 weeks for 2-4 cycles. After the completion of treatment, all patients were asked to return to the hospital for examination every 3 months during the first year, every 6 months for the next 4 years, and then annually. The duration of follow-up was calculated from the day of treatment to the day of death or November 2018.
Statistical analysis
The independent-samples t test was used to calculate differences between serum LDH levels from the various patient groups. Receiver-operating characteristic (ROC) curve analysis was adopted to determine the most appropriate cut-off points of LDH for survival. The primary endpoint of this study was distant metastasis-free survival (DMFS) and the secondary endpoint was overall survival (OS). DMFS was defined as the time from treatment to the first observation of distant metastasis. OS was measured as the first date of treatment to the date of death. Patients still alive or without progression were censored at the date of the last contact. The Kaplan-Meier method and log-rank test were adopted to calculate and compare the DMFS and OS rates. Univariate and multivariate analyses were carried out using the Cox proportional hazards model. All statistical tests were two-sided, and p<0.05 was considered statistically significant. Statistical analyses were performed using MedCalc statistical software version 11.0 (MedCalc Software, Mariakerke, Belgium) and the Statistical Package for Social Sciences version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Characteristics of the 172 NPC patients who met all of the criteria were shown in Table 1. These patients' median age of diagnosis was 45 years (range, 20-74 years), and 60 cases were 50 years or older. 124 were male and 48 were female, with a sex ratio of 2.6:1. In terms of histological types, there were 17, 78 and 77 patients were with the type of squamous cell carcinoma, non-keratinizing carcinoma and undifferentiated carcinoma. The number of cancer patients at stage I, II, III and IV disease were 6, 36, 78 and 52, respectively. Chemotherapy was administered to 166 patients. Concurrent chemotherapy was delivered to 28 patients, neoadjuvant-concurrent chemotherapy to 132 patients, and neoadjuvant-concurrent-adjuvant chemotherapy to 6 patients. With a median follow-up time of 54 months (range, 6-82 months), 36 patients (20.9%) developed distant metastasis and 22 patients (12.8%) were dead after treatment. Among them, 11 patients progressed with distant metastasis and 5 patients died with early stage (I/II), and 25 patients developed distant metastasis and 17 patients died with advanced stage (III/IV).
Clinical characteristics of the patient population (n=172).
| Characteristics | Number of patients | Percentage (%) | ||
|---|---|---|---|---|
| Gender | ||||
| Male | 124 | 72.1 | ||
| Female | 48 | 27.9 | ||
| Age at diagnosis (years) | ||||
| ≥50 | 60 | 34.9 | ||
| <50 | 112 | 65.1 | ||
| Histological subtype | ||||
| Squamous cell carcinoma | 17 | 9.9 | ||
| Non-keratinizing carcinoma | 78 | 45.3 | ||
| Undifferentiated carcinoma | 77 | 44.8 | ||
| Tumor stage | ||||
| T1 | 26 | 15.1 | ||
| T2 | 68 | 39.5 | ||
| T3 | 50 | 29.1 | ||
| T4 | 28 | 16.3 | ||
| Node stage | ||||
| N0 | 8 | 4.7 | ||
| N1 | 56 | 32.5 | ||
| N2 | 79 | 45.9 | ||
| N3 | 29 | 16.9 | ||
| UICC/AJCC stage | ||||
| I | 6 | 3.5 | ||
| II | 36 | 20.9 | ||
| III | 78 | 45.4 | ||
| IV | 52 | 30.2 | ||
| Survival | ||||
| Metastasis | 36 | 20.9 | ||
| Death | 22 | 12.8 |
Abbreviations: UICC/AJCC = Union for International Cancer Control/American Joint Committee on Cancer.
Serum lactate dehydrogenase (LDH) levels and metastatic patterns in patients with nasopharyngeal carcinoma (n=172).
| Metastatic patterns | Number | Baseline serum LDH levels (U/L) | |||
|---|---|---|---|---|---|
| Median | Range | Mean ± SD | |||
| Total cases | 172 | 173.5 | 108-817 | 192.6 ± 71.3 | |
| Distant metastasis | 36 | 196.5 | 134-817 | 237.1 ± 129.2 | |
| Bone | 15 | 176 | 134-484 | 207.9 ± 88.4 | |
| Lung | 13 | 210 | 154-817 | 274.9 ± 183.1 | |
| Liver | 8 | 199 | 145-352 | 230.6 ± 75.9 | |
| Non-distant metastasis | 136 | 172 | 108-318 | 108.8 ± 38.5 | |
Abbreviations: LDH = lactate dehydrogenase; U/L = unit/liter; SD = standard deviation.
Independent samples t test
At the start of this study, the role of pre-treatment serum LDH level in predicting distant metastasis was analyzed by independent-samples t test. We divided NPC patients into two groups, including those developed distant metastasis and those did not progress with distant metastasis. As showed in Table 2, patients who experienced post-treatment distant metastasis were found to have higher baseline serum LDH levels before treatment (mean LDH levels were 237.1U/L and 108.8U/L, respectively, p=0.001). Then, we analyzed the associations between serum LDH level and metastatic sites. The mean LDH levels for bone, lung and liver metastasis were 207.9U/L, 274.9U/L and 230.6U/L, respectively. No significant relevance was observed between them (bone versus lung, p=0.218; bone versus liver, p=0.545; lung versus liver, p=0.877).
Receiver-operating characteristic (ROC) curves for lactate dehydrogenase (LDH) based on distance metastasis free survival (DMFS) and overall survival (OS).
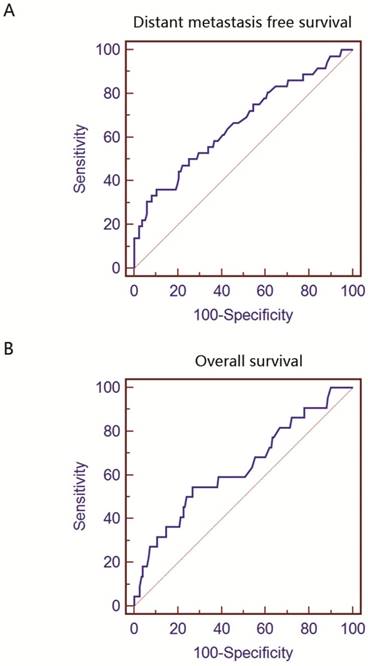
ROC analysis
Using DMFS as an end point, the cut-off value for LDH provided by ROC analysis was 229U/L. As shown in Figure 1A, the area under the curve (AUC) for DMFS was 0.662 (p=0.003). The ROC curve for OS was presented in Figure 1B. The optimal cut-off level and AUC was 199U/L and 0.634 (p=0.005), respectively. These values calculated by ROC analysis were adopted in subsequent survival analysis and used to stratify patients into different groups.
Univariate and multivariate analysis
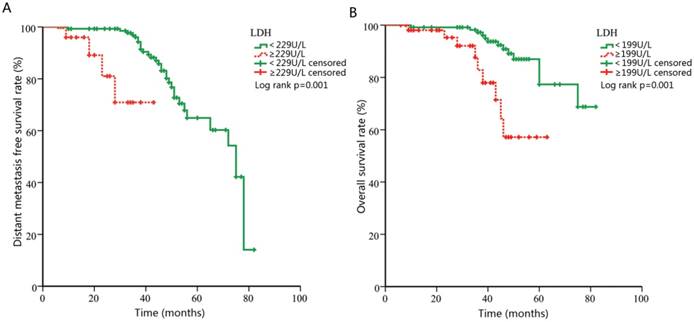
Univariate analyses were performed using gender, age, histology, clinical stage, and LDH level as possible variables. It identified age ≥50 (p=0.002), UICC/AJCC stage (p=0.002) and LDH ≥229U/L (p=0.001, Figure 2A) as significant predictors of DMFS. Multivariate analysis confirmed that LDH ≥229U/L was independent risk factor for DMFS ((hazard ratio (HR), 8.31; 95% confidence interval (CI), 2.44-28.32; p=0.001), Table 3). Univariate and multivariate analysis were also performed in OS analysis. As showed in Table 4, univariate analysis revealed that age ≥50 (p=0.001), UICC/AJCC stage (p=0.016) and LDH ≥199U/L (p=0.001, Figure 2B) were statistically significantly associated with OS. Multivariate analysis showed that LDH ≥199U/L (HR, 4.45; 95% CI, 1.77-11.21; p=0.002) was independent prognostic predictor for patients' mortality.
Univariate and multivariate analysis of clinicopathological parameters for the prediction of distant metastasis-free survival in patients with nasopharyngeal carcinoma (n=172).
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Gender | |||||
| Male | 1 (Referent) | ||||
| Female | 0.75 (0.35-1.62) | 0.471 | |||
| Age at diagnosis (years) | |||||
| <50 | 1 (Referent) | 1 (Referent) | |||
| ≥50 | 2.87 (1.45-5.67) | 0.002 | 2.75 (1.36-5.55) | 0.005 | |
| Histological subtype | |||||
| Squamous cell carcinoma | 1 (Referent) | ||||
| Non-keratinizing carcinoma | 0.85 (0.19-3.69) | 0.826 | |||
| Undifferentiated carcinoma | 0.62 (0.30-1.28) | 0.191 | |||
| UICC/AJCC stage | |||||
| I-II | 1 (Referent) | 1 (Referent) | |||
| III-IV | 4.78 (1.75-13.07) | 0.002 | 5.76 (2.10-15.74) | 0.001 | |
| Serum LDH (U/L) | |||||
| <229 | 1 (Referent) | 1 (Referent) | |||
| ≥229 | 7.92 (2.36-26.57) | 0.001 | 8.31 (2.44-28.32) | 0.001 | |
Abbreviations: HR = hazard ratio; CI = confidence interval; UICC/AJCC = Union for International Cancer Control/American Joint Committee on Cancer; LDH = lactate dehydrogenase; U/L = unit/liter.
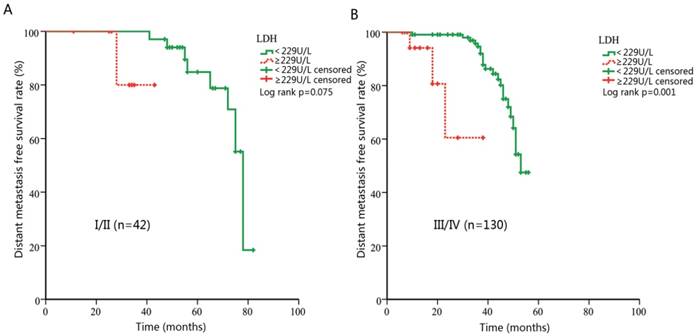
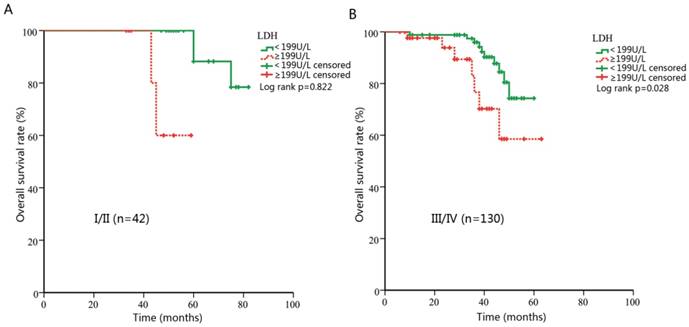
Subgroup analysis stratified by clinical stage
Theoretically, LDH levels in patients with advanced stages are more likely to be influenced by hypoxic conditions in tumor cells. Pre-treatment LDH might be more predictive in advanced cancers. Thus, we further performed a subgroup analysis and evaluated the prognostic effect of LDH in NPC patients with different clinical stages. For early stage subgroup, there was a tendency towards shorter DMFS for patients with higher LDH, however, not statistically significant (p=0.075, Figure 3A). When patients with advanced stage were analyzed, significant associations were found between DMFS and LDH level (HR, 10.47; 95% CI, 2.52-43.45; p=0.001, Figure 3B). Meanwhile, no statistical significance was observed for OS analysis in the early-stage subgroup (p=0.822, Figure 4A). However, in patients with advanced NPC, those with higher pretreatment serum LDH had significantly shorter OS (HR, 2.99; 95% CI, 1.12-7.94; p=0.028, Figure 4B).
Univariate and multivariate analysis of clinicopathological parameters for the prediction of overall survival in patients with nasopharyngeal carcinoma (n=172).
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Gender | |||||
| Male | 1 (Referent) | ||||
| Female | 0.50 (0.17-1.49) | 0.213 | |||
| Age at diagnosis (years) | |||||
| <50 | 1 (Referent) | 1 (Referent) | |||
| ≥50 | 4.85 (2.02-11.66) | 0.001 | 5.81 (2.33-14.53) | 0.001 | |
| Histological subtype | |||||
| Squamous cell carcinoma | 1 (Referent) | ||||
| Non-keratinizing carcinoma | 1.18 (0.26-5.36) | 0.827 | |||
| Undifferentiated carcinoma | 0.77 (0.31-1.88) | 0.560 | |||
| UICC/AJCC stage | |||||
| I-II | 1 (Referent) | 1 (Referent) | |||
| III-IV | 4.37 (1.32-14.49) | 0.016 | 3.67 (1.10-12.25) | 0.034 | |
| Serum LDH (U/L) | |||||
| <199 | 1 (Referent) | 1 (Referent) | |||
| ≥199 | 4.24 (1.74-10.31) | 0.001 | 4.45 (1.77-11.21) | 0.002 | |
Abbreviations: HR = hazard ratio; CI = confidence interval; UICC/AJCC = Union for International Cancer Control/American Joint Committee on Cancer; LDH = lactate dehydrogenase; U/L = unit/liter.
Kaplan-Meier curves for distance metastasis free survival (DMFS) and overall survival (OS) according to pretreatment lactate dehydrogenase (LDH).
Kaplan-Meier curves for distance metastasis free survival (DMFS) according to pre-treatment lactate dehydrogenase (LDH) in patients with different clinical stages. (A) DMFS stratified by LDH in patients with early stage. (B) DMFS stratified by LDH in patients with advanced stage.
Kaplan-Meier curves for overall survival (OS) according to pre-treatment lactate dehydrogenase (LDH) in patients with different clinical stages. (A) OS stratified by LDH in patients with early stage. (B) OS stratified by LDH in patients with advanced stage.
Discussion
Nowadays, radiotherapy is the primary treatment strategy for NPC. With the application of IMRT in NPC management, local recurrence substantially decreases and distant metastasis becomes the main cause of treatment failure. Besides, TNM system is not always satisfactory to guide treatment planning and survival prediction for NPC patients [26]. Therefore, identifying some biomarkers to predict distant metastasis and future survival in NPC patients is urgently needed. In this study, we provided the evidence that routinely available pre-treatment LDH level could act as an independent prognostic factor for patients with NPC.
A number of studies have identified the prognostic value of serum LDH level in NPC patients. However, most patients in these studies came from high endemic areas. This may generate a high risk of selection bias and affect the true conclusions. Besides, the results of previous studies were inconsistent in regards to the prognostic impact of LDH in NPC [27]. Therefore, new researches from middle- or low-incidence areas are urgently needed to increase the reliability of serum LDH level as a prognostic factor in NPC. Our study is the first study conducted in central China with large patient population. We found that patients with higher pretreatment LDH level were more likely to progress with distant metastasis. What's more, our results revealed that serum LDH is an independently predictive factor for both DMFS and OS. In our subgroup analyses, significant associations of serum LDH with DMFS and OS were observed among patients with advanced stage. For the early stage group, there was a tendency toward shorter DMFS and OS in patients with higher LDH level even if the statistical tests were not significant. These results confirmed that pre-treatment serum LDH could be reliable prognostic factor for patients with NPC. Patients with NPC with early clinical stage tend to survive longer without progression and the number of outcome (progression or death) is usually small. In our study, only 11 cases had progression and 5 patients died among the 42 patients with early-stage disease. The small number of outcome might attribute this nonsignificant association in the early stage. In conclusion, our study added more new evidences and reduced the selection bias to make LDH a reliable biomarker in predicting distant metastasis and poor survival in NPC.
NPC is a highly invasive and metastatic tumor, with approximately 75% of patients present with regional lymph node metastasis and 10% present with distant metastasis at the time of diagnosis [28, 29]. Our study found that serum LDH level at the time of diagnosis had a significant impact on the rates of distant metastasis. Patients who experienced post-treatment distant metastasis were found to have markedly elevated baseline serum LDH levels. Although the exact mechanism associated with this observation remains unknown, a possible reason is the presence of organs micro-metastases. Some patients without obvious clinical evidence of metastases at diagnosis may already have subclinical micro-metastases that cannot be detected by regular examinations [30]. Therefore, baseline serum LDH level may have been elevated owing to organs injury and enzyme leakage in patients with micro-metastasis. As distant metastasis is now the main failure pattern for cases of NPC, detecting occult metastases could optimize staging and treatment strategies. Our results provided the evidence that LDH might help to identify patients at high risk of metastasis.
Although the prognostic value of LDH has been studied extensively, the underlying mechanism linking LDH to poor survival remains unknown. It has been hypothesized that serum LDH level may reflect the extent of hypoxia in tumor cells, since it catalyzes the transformation of pyruvate to lactate in hypoxia conditions. Tumor cells are often starved of oxygen due to its rapid proliferation. In producing energy, cancer cells can use anaerobic glycolysis which enables it to be independent of oxygen. This phenomenon is known as the Warburg effect and is one of the predominant metabolic processes that occur during malignant transformation [31]. As the key kinase of this process, LDH ensures the efficiency of this process and can be detected in the serum. Besides, elevated serum LDH level has been suggested to be a marker of immune suppression in cancer patients [32]. Ding et al found that LDH allows tumor cells to suppress and evade the immune system by altering the tumor microenvironment. Taken together, serum LDH level may reflect the hypoxia in tumor cells and immune suppression in patients which lead to poor prognosis. What's more, LDH is emerging as one of the most interesting molecular targets for the development of glycolytic inhibitors to possibly use in cancer therapy [33]. Further deeper investigations towards LDH may promote its clinical utility in cancers.
Our study provided a valuable biomarker for clinicians to plan treatment strategies for NPC patients, but our study has some limitations. First, our samples were obtained from a single center. A larger sample size from other institutes to validate our results is warranted. Second, other hematologic markers associated with poor survival were not adopted in our analysis, such as the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio [34]. Third, this is a retrospective study. Given these limitations, future large randomized trials are needed to improve and update our results.
Conclusion
In summary, pre-treatment serum LDH level was found to be a predictive factor for distant metastasis and poor survival in NPC patients treated with IMRT. As LDH can be measured easily and inexpensively, it may be adopted in widespread clinical use and contribute to prognosis estimation. However, considering the retrospective nature of this study, further large-scaled prospective trials are still warranted to verify our results.
Abbreviations
AJCC: American Joint Committee on Cancer; AUC: area under the curve; CI: confidence interval; CRT: conventional radiotherapy; DMFS: distant metastasis free survival; HR: hazard ratio; IMRT: intensity-modulated radiotherapy; IPI: international prognostic index; L: liter; LDH: lactate dehydrogenase; NPC: nasopharyngeal carcinoma; OS: overall survival; ROC: receiver operating characteristics; SD: standard deviation; TNM: tumor-node-metastasis; U: unit; UICC: Union for International Cancer Control.
Acknowledgements
This study is supported by the Foundation of Tongji Hospital (No. 2018A08).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chan AT. Nasopharyngeal carcinoma. Ann Oncol. 2010;21(Suppl 7):vii308-12
2. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-24
3. Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM. et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161-8
4. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q. et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286-93
5. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ. et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661-8
6. Xu J, Wan XB, Huang XF, Chan KC, Hong MH, Wang LH. et al. Serologic antienzyme rate of Epstein-Barr virus DNase-specific neutralizing antibody segregates TNM classification in nasopharyngeal carcinoma. J Clin Oncol. 2010;28:5202-9
7. Wang HY, Sun BY, Zhu ZH, Chang ET, To KF, Hwang JS. et al. Eight-signature classifier for prediction of nasopharyngeal carcinoma survival. J Clin Oncol. 2011;29:4516-25
8. Lee AWM, Ng WT, Chan LK, Chan OSH, Hung WM, Chan CC. et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012;48:1007-13
9. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS. et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461-70
10. Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei RR. et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13:633-41
11. Sun W, Long G, Wang J, Mei Q, Liu D, Hu G. Prognostic role of epidermal growth factor receptor in nasopharyngeal carcinoma: a meta-analysis. Head Neck. 2014;36:1508-16
12. Le QT, Zhang Q, Cao H, Cheng AJ, Pinsky BA, Hong RL. et al. An international collaboration to harmonize the quantitative plasma Epstein-Barr virus DNA assay for future biomarker-guided trials in nasopharyngeal carcinoma. Clin Cancer Res. 2013;19:2208-15
13. Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703-7
14. Gallo M, Sapio L, Spina A, Naviglio D, Calogero A, Naviglio S. Lactic dehydrogenase and cancer: an overview. Front Biosci (Landmark Ed). 2015;20:1234-49
15. Chen B, Dai D, Tang H, Chen X, Ai X, Huang X. et al. Pre-treatment serum alkaline phosphatase and lactate dehydrogenase as prognostic factors in triple negative breast cancer. J Cancer. 2016;7:2309-16
16. Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J. et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2018 doi: 10.1007/s10120-018-0897-8
17. Chen ZH, Qiu MZ, Wu XY, Wu QN, Lu JH, Zeng ZL. et al. Elevated baseline serum lactate dehydrogenase indicates a poor prognosis in primary duodenum adenocarcinoma patients. J Cancer. 2018;9:512-20
18. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987-94
19. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR. et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-206
20. Wan XB, Wei L, Li H, Dong M, Lin Q, Ma XK. et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer. 2013;49:2356-64
21. Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y. et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2012;82:e359-65
22. Oei RW, Ye L, Kong F, Du C, Zhai R, Xu T. et al. Pre-treatment serum lactate dehydrogenase is predictive of survival in patients with nasopharyngeal carcinoma undergoing intensity-modulated radiotherapy. J Cancer. 2018;9:54-63
23. Wei Z, Zeng X, Xu J, Duan X, Xie Y. Prognostic value of pretreatment serum levels of lactate dehydrogenase in nonmetastatic nasopharyngeal carcinoma: single-site analysis of 601 patients in a highly endemic area. Onco Targets Ther. 2014;7:739-49
24. Zhou GQ, Ren XY, Mao YP, Chen L, Sun Y, Liu LZ. et al. Prognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Sci Rep. 2016;6:22326
25. Chen Z, Guo Q, Lu T, Lin S, Zong J, Zhan S. et al. Pretreatment serum lactate dehydrogenase level as an independent prognostic factor of nasopharyngeal carcinoma in the intensity-modulated radiation therapy era. Med Sci Monit. 2017;23:437-45
26. Sun R, Qiu HZ, Mai HQ, Zhang Q, Hong MH, Li YX. et al. Prognostic value and differences of the sixth and seventh editions of the UICC/AJCC staging systems in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2013;139:307-14
27. Zhang M, Wei S, Su L, Lv W, Hong J. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population: A meta-analysis. Medicine (Baltimore). 2016;95:e4494
28. Wei WI, Mok VW. The management of neck metastases in nasopharyngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2007;15:99-102
29. Huang CJ, Leung SW, Lian SL, Wang CJ, Fang FM, Ho YH. Patterns of distant metastases in nasopharyngeal carcinoma. Kaohsiung J Med Sci. 1996;12:229-34
30. Lin JC, Chen KY, Wang WY, Jan JS, Liang WM, Wei YH. Evaluation of cytokeratin-19 mRNA as a tumor marker in the peripheral blood of nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Cancer. 2002;97:548-53
31. Biswas S, Lunec J, Bartlett K. Non-glucose metabolism in cancer cells-is it all in the fat? Cancer Metastasis Rev. 2012;31:689-98
32. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-63
33. Di Stefano G, Manerba M, Di Ianni L, Fiume L. Lactate dehydrogenase inhibition: exploring possible applications beyond cancer treatment. Future Med Chem. 2016;8:713-25
34. Sun W, Zhang L, Luo M, Hu G, Mei Q, Liu D. et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck. 2016;38(Suppl 1):E1332-40
Author contact
![]() Corresponding author: Dr. Wei Sun, Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, Wuhan 430030, China. Phone: +86-27-83663517; E-mail: sunweitjhedu.cn.
Corresponding author: Dr. Wei Sun, Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, Wuhan 430030, China. Phone: +86-27-83663517; E-mail: sunweitjhedu.cn.

 Global reach, higher impact
Global reach, higher impact