Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(18):4208-4216. doi:10.7150/jca.33457 This issue Cite
Research Paper
The Role of Upregulated DDX11 as A Potential Prognostic and Diagnostic Biomarker in Lung Adenocarcinoma
1. Precision Medicine Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
2. Key Laboratory of Clinical Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
*These authors contributed equally to this work.
Received 2019-1-23; Accepted 2019-5-19; Published 2019-7-10
Abstract
Background: Lung adenocarcinoma (ADC) is the main cause of cancer-related mortality in lung cancer patients. DEAD/DEAH box helicase 11 (DDX11) was previously shown to be dysregulated and to exert oncogenic activity in cancer. However, the diagnostic value and clinical significance of DDX11 in ADC remain unknown.
Methods: A total of 513 ADC and 59 normal tissue samples were obtained from The Cancer Genome Atlas (TCGA) database, and the mRNA expression level of DDX11 in ADC was evaluated. Additionally, a meta-analysis of 7 ADC cohorts from the Gene Expression Omnibus (GEO) database was conducted to validate the DDX11 expression pattern. Moreover, receiver operating characteristic (ROC) curve analysis was used to identify the diagnostic power of DDX11 in ADC. A tissue microarray (TMA) comprising 86 ADC specimens and their adjacent normal specimens was applied to indicate DDX11 protein expression status. In addition, Kaplan-Meier and Cox regression analyses were conducted to validate the prognostic value of DDX11 in ADC. Finally, the molecular mechanism of DDX11 action in ADC was predicted by gene set enrichment analysis (GSEA).
Results: DDX11 was upregulated in ADC tissues and was associated with worse overall survival (OS). ROC curves of DDX11 showed high values for diagnosis. Additionally, DDX11 expression has remarkable correlations with DNA replication and the cell cycle G1-S phase pathway. Consistently, it was associated with cell cycle genes, such as CCNA2, CCNB1, CCNC, CCND1, CCNE1, CDK2, CDK4 and CDK6. Moreover, high CCNA2, CCNB1, CCNE1 and CDK6 expression in ADC patients predicted worse OS and progression-free survival (PFS).
Conclusion: DDX11 was significantly upregulated and predicted poor prognosis in ADC. This gene might serve as a potential novel prognostic and diagnostic biomarker for ADC.
Keywords: DDX11, lung adenocarcinoma, diagnosis, proliferation, cell cycle
Introduction
Lung cancer is one of the main causes of cancer-associated mortality worldwide. This disease is subdivided into non-small cell lung cancer (NSCLC) and small cell lung cancer, with NSCLC accounting for approximately 80-85% of all lung cancers [1, 2]. Additionally, lung adenocarcinoma (ADC) is the most frequent subtype of NSCLC, accounting for approximately 40% of these cancers [3]. Although great advances have been made in the diagnosis and treatment of ADC, such as chemotherapy, immunotherapy and targeted therapy [4, 5], the 5-year overall survival (OS) rate of ADC patients with advanced stage is approximately 50%-70% due to the malignant features of high metastasis and recurrence [6, 7]. Therefore, powerful diagnostic and therapeutic strategies are still urgently needed to improve the prognosis of ADC patients.
The DDX11 (alias ChlR1) gene is located on human chromosome 12p11 and encodes an orthologue of the yeast gene Chl1, which is a member of the DEAD/DEAH box family of ATP-dependent helicases [8, 9]. DDX11 plays a significant role in the cohesion of chromosome arms and centromeres. Mitotic failure occurs due to replicated chromosomes failing to segregate after prometaphase arrest when DDX11 is depleted [10, 11]. Previous studies have proven that DDX11 biallelic mutations cause Warsaw breakage syndrome [12-14]. Additionally, a previous study suggested that the DDX11 expression level is high in melanomas and plays a key role in cancer progression [15]. However, until now, there have been no relevant studies focused on the expression and function of DDX11 in ADC.
The findings of our study indicated that the DDX11 expression level was significantly higher in ADC tissues than in adjacent normal tissues. Then, we observed that high DDX11 expression was associated with poor prognosis. Further, receiver operating characteristic (ROC) curve and meta-analysis showed a reliable diagnostic value for DDX11 in ADC patients. The expression of DDX11 was crucially correlated with the cell cycle G1-S phase and the DNA replication pathway. In summary, our results indicated that DDX11 might be a promising diagnostic and prognostic biomarker for ADC.
Materials and Methods
TCGA data source
The data for 513 ADC and 59 normal tissue samples from The Cancer Genome Atlas (TCGA, https://tcga-data.nci.nih.gov/tcga/) database were downloaded for gene expression analyses and survival analyses. A total of 500 of the 513 ADC patients with follow-up survival time information were divided into higher and lower DDX11 expression groups by using X-tile, a recently developed tool for the evaluation of biological relevance between a biomarker and the patient outcome, and the discovery of population cut-points based on marker expression [16]. The survival analysis was conducted using the Kaplan-Meier method and a log-rank test. The raw data were analysed by BRB-array tools as previously reporte [17, 18].
GEO data source
Seven ADC datasets accompanied with scientific publications (GSE27262, GSE30219, GSE31210, GSE33532, GSE30219, GSE7670, and GSE10072) were gathered through the GEO database (http://www.ncbi.nlm.nih.gov/geo/, Gene Expression Omnibus). Then, we used meta-analysis to evaluate the diagnostic value of DDX11. The characteristics of the datasets, such as Cohort ID, RNAseq platforms, number of samples (tumour and non-tumour samples), publication year and country, are presented in Table S1.
Tissue samples
A microarray of 86 ADC tumour and adjacent normal tissue samples, which was constructed utilizing a core diameter of 1.5 mm, was obtained from a commercial tissue microarray analysis (TMA) company (Shanghai OutdoBiotech, China). All experiments were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
GSEA
GSEA was used to confirm the distribution of the individual genes of TCGA ADC datasets. The expression profiles of 513 samples from TCGA database were divided into two groups according to gene expression. GSEA v2.0 was then performed to verify whether the gene sets from the MSigDB database v4.0 are positively related to the expression of DDX11. The statistical significance threshold was set at P < 0.05.
Statistics for the meta-analysis
A meta-analysis was carried out to examine the pooled diagnostic power of DDX11 with the data from the GEO database using Stata software. We assessed heterogeneity among studies using I2 statistics. When I2 >50%, significant heterogeneity would be considered, and a random model would be performed for the meta-analysis. The subgroup analysis was carried out according to the following factors: region, sample size and publication year. Begg's test and Egger's test were used to determine the bias of the publications.
Immunohistochemistry (IHC)
IHC was performed as previously reported [19, 20]. Briefly, 5 μm thick TMA sections were deparaffinized and then treated with hydrogen peroxide to quench endogenous peroxidase activity. Subsequently, the sections were incubated overnight with a rabbit anti-human DDX11 antibody (1:200, Abcam, USA) at 4℃. Then, the immunoreactive cells were detected by Signal Stain® DAB (CST, USA) and counterstained with Haematoxylin QS (Vector Laboratories). Two experienced pathologists, who were blinded to the clinicopathological data, separately evaluated the immunostaining samples, and the samples were scored according to the proportion of positive cells as follows: 1, <25%; 2, 25%-50%; 3, 51%-75%; and 4, 76%-100%. The staining intensity was scored as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; 3+, strong staining; and 4+, and intense staining. The multiply of the two sub scores (range 0-16) was classified low expression (range 0-8) and high expression (range 8-16), respectively for statistical analysis.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (version 7.0, GraphPad Software, Inc., La Jolla, CA, USA) and SPSS software (version 23.0, SPSS Inc., Chicago, IL). A chi-squared test was used to examine the correlation between DDX11 expression levels and the clinicopathological parameters. Kaplan-Meier curves were utilized to analyse the OS of ADC patients. Cox regression analysis of univariate and multivariate variables was used to indicate the relationship between the different variables and survival. ROC curves were utilized to examine the pooled diagnostic power of DDX11 in ADC. Heatmaps were used to show the patterns of mRNA expression according to tumour-node-metastasis (TNM) and histological type. Pearson's correlation was performed to ascertain the linear correlation between 2 variables. P<0.05 is regarded as statistically significant. All data are presented as the means ± SD. All experiments were carried out at least three times.
Results
DDX11 mRNA is upregulated and correlated with poor prognosis in ADC
To investigate the expression of DDX11 in cancers, TCGA data analysis was conducted to identify DDX11 mRNA expression levels. The results demonstrated that DDX11 mRNA was highly expressed in numerous tumour samples compared with its expression in non-tumour tissues (Figure 1A). Consistently, DDX11 is upregulated in tumour tissues of ADC (Figure 1B). Moreover, survival analysis revealed that the high expression of DDX11 could predict poorer overall survival (OS) (Figure 1C). Taken together, these results indicate that DDX11 might be a novel prognostic biomarker for ADC patients.
DDX11 mRNA was overexpressed in ADC tissues and negatively correlated with survival in TCGA cohort. Notes: (A and B) DDX11 mRNA expression in non-tumour tissues and ADC tumour tissues. (C) Kaplan-Meier estimation of the OS of ADC patients stratified by DDX11 expression. Abbreviations: DDX11: DEAD/DEAH box helicase 11; OS: overall survival; ADC: adenocarcinoma.
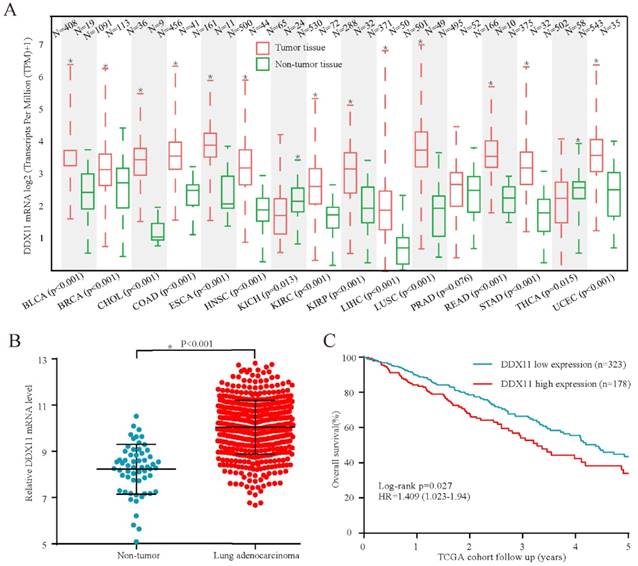
High expression of DDX11 protein was negatively correlated with survival. Notes: (A) Representative images of DDX11 staining in ADC tissues. (B) Increased expression of DDX11 in ADC tissues (P=0.0081). (C) Representative DDX11 staining in ADC and non-tumour tissues. (D) Kaplan-Meier analysis showing the correlation between DDX11 expression levels and the OS of 86 ADC patients. Abbreviations: DDX11: DEAD/DEAH box helicase 11; ADC: lung adenocarcinoma; IHC: immunohistochemistry; OS: overall survival.
The relationship between DDX11 expression and the clinicopathological features of lung adenocarcinoma patients
| Clinicopathological features | No. of cases (%) | DDX11 expression level | P value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Age (years) | ≤40 | 41 | 15 | 26 | 0.458 |
| >40 | 45 | 20 | 25 | ||
| Gender | Female | 40 | 18 | 22 | 0.448 |
| Male | 46 | 17 | 29 | ||
| TNM | Ⅰ and Ⅱ | 40 | 22 | 18 | 0.013* |
| Ⅲ and Ⅳ | 31 | 8 | 23 | ||
| NA | 15 | 5 | 10 | ||
| Histological type | Mixed Subtype | 57 | 24 | 23 | 0.264 |
| Other Subtype | 29 | 11 | 18 | ||
| Tumour size | ≤3 cm | 32 | 18 | 14 | 0.023* |
| >3 cm | 54 | 17 | 37 | ||
Notes: *P<0.05. **P<0.01.
Abbreviations: TNM: tumour-node-metastasis; NA: not available; HR: hazard ratio; CI: confidential interval; DDX11: DEAD/H-box helicase 11.
Upregulated DDX11 protein is associated with clinicopathological characteristics and the poor prognosis of ADC
Subsequently, considering the expression difference in mRNA, we performed immunohistochemistry to evaluate the DDX11 protein expression status in ADC. According to the staining intensity, DDX11 staining was scored from 1+ to 4+ (Figure 2A). A score of 1+ to 2+ was defined as low DDX11 expression, whereas a score of 3+ to 4+ was defined as high DDX11 expression. Consistent with the results of TCGA and GEO database analyses, the expression levels of DDX11 protein were significantly upregulated in ADC tissues (Figure 2B). Furthermore, DDX11 expression was significantly positively related to tumour size and the TNM stage of the patients (Table 1). Additionally, Kaplan-Meier analysis indicated that high expression of DDX11 was remarkably correlated with poor OS in ADC patients (P = 0.036, Figure 2D). Moreover, univariate and multivariate analyses demonstrated that, in addition to the TNM stage, DDX11 might be an independent prognostic factor for ADC patients (Table 2). In summary, these findings strongly suggested that DDX11 might serve as a prognostic biomarker in ADC.
A meta-analysis on the diagnostic value of DDX11 in ADC through the GEO database
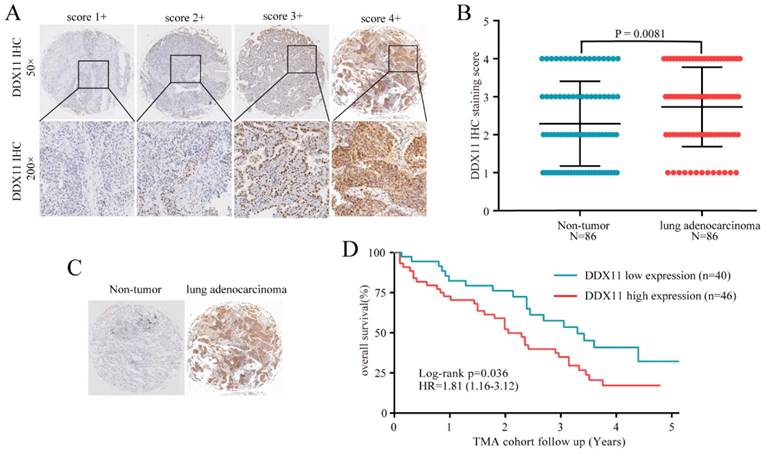
To further confirm the mRNA expression of DDX11 in ADC, a total of 7 microarrays in the GEO database were collected and extracted in the present study. As shown in the forest plot (Figure 3A), because of the significant heterogeneity among the micrograms (I2 value was 64.7%), a random effect model analysis was performed, showing that the expression of DDX11 was higher tumour tissue than in non-tumour tissue (pooled standard mean difference (SMD)=0.83, 95% CI=(0.51-1.14), P=0.009). The results are consistent in Figure 3C. Sensitivity analyses noted that there were no significant differences (Figure 3B and 3D). Begg's test (P =0.230) and Egger's test (P =0.288) showed no statistical significance. In total, there was no significant publication bias among these studies. In Figure 4, the ROC analysis revealed a significant diagnostic value in ADC. The results of the ROC analysis from TCGA database are shown in Figure 4A (area under the curve (AUC), 0.875; 95% CI, 0.0.836-0.914; P<0.001), and the corresponding specificity and sensibility were 0.793 and 0.831, respectively. The AUC was 0.882 (95% CI: 0.786-0.978. P < 0.001) in GSE27262, and the corresponding specificity and sensibility were 0.72 and 0.96, respectively. The specificity and sensibility were 71% and 90%, respectively. The AUC was 0.844 (95% confidence interval (CI): 0.770-0.918. P < 0.001) in GSE31210, and the corresponding specificity and sensibility were 0.79 and 0.71, respectively. The AUC of DDX11 expression was 0.790 (95% CI: 0.672-0.907, P = 0.001) in GSE30219. The AUC was 0.758 (95% CI: 0.668-0.849. P < 0.001) in GSE10072, and the corresponding specificity and sensibility were 0.72 and 0.76, respectively. The AUC was 0.724 (95% CI: 0.593-0.856. P = 0.004) in GSE7670, and the corresponding specificity and sensibility were 0.5 and 0.857, respectively. In summary, DDX11 could be a possible indicator to assist the diagnosis of ADC.
Univariate and multivariate analyses of the overall survival of lung adenocarcinoma patients
| Clinicopathological features | Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% (CI) | P value | HR | 95% (CI) | P value | ||
| Age (years) | ≤40 | 1.000 | 0.919-1.710 | 0.547 | |||
| >40 | 1.253 | ||||||
| Gender | Female | 1.000 | 0.825-1.536 | 0.457 | |||
| Male | 1.125 | ||||||
| TNM stage | Stage I - II | 1.000 | 1.577-3.076 | <0.001** | 1.000 | 1.546-3.021 | <0.001** |
| Stage III - IV | 3.591 | 2.161 | |||||
| Histological type | Mixed Subtype | 1.000 | 0.700-1.585 | 0.262 | |||
| Other Subtype | 1.053 | ||||||
| Tumour size | ≤3 cm | 1.000 | 1.692-3.181 | 0.017* | 1.000 | 1.246-1.729 | 0.175 |
| >3 cm | 2.458 | 1.685 | |||||
| DDX11 expression | Low | 1.000 | 1.783-2.983 | 0.024* | 1.000 | 1.536-2.966 | 0.036* |
| High | 2.334 | 2.021 | |||||
Notes: *P<0.05. **P<0.01.
Abbreviations: TNM: tumour-node-metastasis; HR: hazard ratio; CI: confidential interval; DDX11: DEAD/H-box helicase 11.
DDX11 expression was markedly increased in ADC tissues and showed high diagnostic value in the GEO dataset. Notes: (A) DDX11 expression in ADC and normal tissues. (B) Expression between ADC and normal tissues. (C) Forest plot evaluating differences in DDX11 expression between ADC and normal tissues. The high and low DDX11 expressing tissues were regarded as the experimental and control groups, respectively. (D) Sensitivity analysis of the hazard ratios was calculated by omitting each microarray in turn. Abbreviations: DDX11: DEAD/DEAH box helicase 11; GEO: Gene Expression Omnibus; ADC: adenocarcinoma; SMD: standard mean difference; CI: confidence interval.
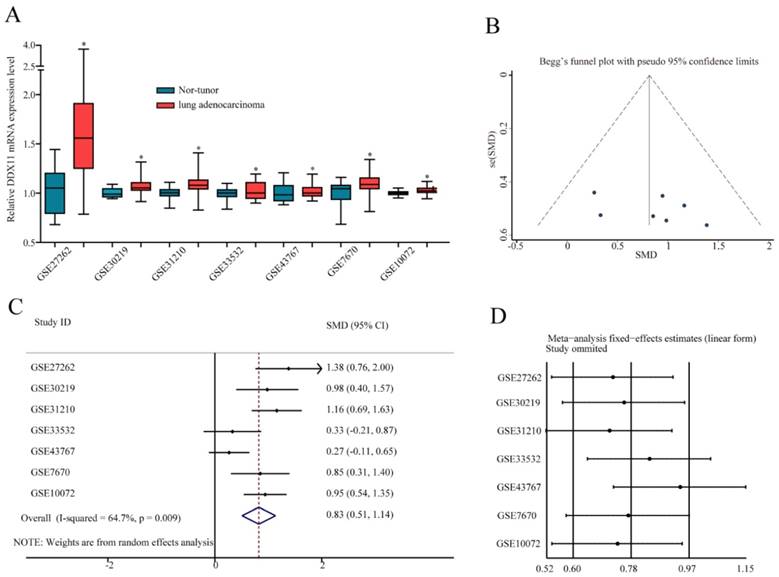
ROC curves for evaluating the diagnostic power of DDX11 in ADC. Notes: (A) TCGA cohort. (B) GSE27262. (C) GSE31210. (D) GSE30219. (E) GSE10072. (F) GSE7670. Abbreviations: AUC: area under the curve; DDX11: DEAD/DEAH box helicase 11: ADC: adenocarcinoma; ROC: receiver operating characteristic; TCGA: The Cancer Genome Atlas; CI: confidence interval.
The potential molecular mechanism mediated by DDX11 in ADC
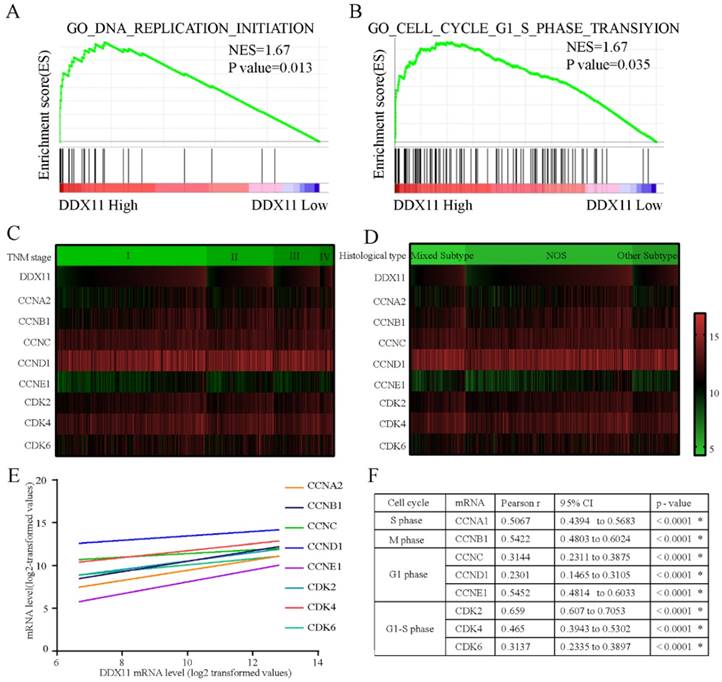
To explore the underlying mechanisms by which DDX11 is involved in ADC progression, we conducted a GSEA based on TCGA ADC cohort. The GSEA showed that DDX11 upregulation was associated with the activation of DNA replication and the cell cycle G1-S phase transition pathway (Figure 5A and 5B). Cell cycle-related gene expression patterns according to TNM stage and histological type were described in a heatmap plot of 505 ADC patients from TCGA database (Figure 5C and 5D). Additionally, we found a significant positive relationship between the DDX11 expression level and the genes involved in the cell cycle G1-S phase transition and DNA replication (Figure 5E and 5F), such as CCNA2 (P < 0.0001, R=0.5067), CCNB1 (P < 0.0001, R=0.5422), CCNC (P < 0.0001, R=0.3114), CCND1 (P < 0.0001, R=0.2301), CCNE1 (P < 0.0001, R=0.5452) and CDK2 (P < 0.0001, R=0.659), CDK4 (P < 0.0001, R=0.465), and CDK6 (P < 0.0001, R=0.3137). Moreover, ADC patients with high expression of CCNA2, CCNB1, CCND1, CCNE1, and CDK6 had a worse OS, and CCNA2, CCNB1, CCNE1 and CDK6 were associated with worse PFS. These findings showed that DDX11 likely contributed to the poor prognosis of ADC through cell proliferation.
Discussion
ADC accounts for almost 50% of lung cancers, and although the diagnostic and therapeutic techniques for ADC have made significant progress, the 5-year OS for ADC patients remains poor [21]. Therefore, it is of vital importance to elucidate the molecular mechanisms of ADC development and identify novel prognostic biomarkers and therapeutic targets for ADC. A few proteins, including HMGA1 [22], IDH1 [23], CEA and CYFRA [24], have been reported to be differentially expressed in ADC and associated with ADC progression.
DDX11, a DNA-dependent ATPase and helicase, is involved in the processing of the lagging strand during DNA replication and in the maintenance of the fork structure for the establishment of cohesion [25, 26]. Recent studies have shown an oncogenic function for DDX11 in a few cancers. For example, Bhattacharya et al. reported that high DDX11 expression was significantly related to poor prognosis in advanced melanomas. However, its functional role and clinical significance in ADC have never been reported. In this study, we consistently found high DDX11 expression in ADC tissues by TCGA, GEO database and Zhengzhou University (ZZU) ADC cohort analyses. DDX11 overexpression was significantly correlated with the OS rate. Furthermore, univariate and multivariate analyses indicated that DDX11 expression might be an independent prognostic element in ADC. These results showed that DDX11 could serve as a promising biomarker for prognostic prediction in ADC.
Molecular mechanism of DDX11 action in ADC. Notes: (A B) GSEA of the relationship between high DDX11 expression and genes associated with cell cycle G1_S_phase (GO _ cell _ cycle_G1_S_phase) and DNA replication (GO _ DNA_ replication). (C D) Heatmap of mRNA expression according to TNM and histological type. The genes assessed were CCNA2, CCNB1, CCNC, CCND1, CCNE1, CDK2, CDK4 and CDK6. (E F) Correlation of DDX11 mRNA expression with the other genes. Abbreviations: GSEA: gene set enrichment analysis; DDX11: DEAD/DEAH box helicase 11; NES: normalized enrichment score; CI: confidence interval; NOS: not otherwise specified; TNM: tumor node metastasis.
To illustrate the diagnostic power of DDX11 in ADC, we conducted a ROC curve analysis, and the results showed that the diagnostic value of ROC curves was satisfactory. To obtain convincing evidence of DDX11 diagnostic power, we identified the diagnostic power of DDX11 for ADC by a meta-analysis of previous studies downloaded from GEO ADC datasets. Therefore, DDX11 might be a reliable diagnostic marker for ADC.
We further investigated the underlying mechanism of DDX11 in promoting ADC tumorigenesis. Bioinformatic analysis indicated that high DDX11 expression was closely linked to DNA replication and the cell cycle G1-S phase transition. Numerous studies have confirmed that the cell cycle is a complex and strictly controlled process [27] that is frequently dysregulated in tumorigenesis, including ADC [28, 29]. Furthermore, previous studies have reported that several proteins, such as FGF[30], ERBB3 [31] and MFN2 [32], may influence lung cancer progression through cell cycle pathways, Consistently, studies by Bhattacharya C et al. have demonstrated a key role for DDX11 in the proliferation and cell cycle progression of advanced melanoma. In addition, our present study found that DDX11 expression was positively associated with CCNA2, CCNB1, CCNC, CCND1, CCNE1, CDK2, CDK4 and CDK6, which are involved in the cell cycle and DNA replication [33-36]. These results suggested that DDX11 might play a significant role in regulating the cell cycle G1-S phase transition and DNA replication in ADC progression.
Conclusion
Our findings provide the first evidence that DDX11 is overexpressed in ADC and has a close correlation with cancer progression, and the performance of DDX11 in predicting a poor prognosis in ADC is also satisfactory. Taken together, these findings suggest that DDX11 might be a potential prognostic and diagnostic biomarker for patients with ADC.
Supplementary Material
Supplementary table.
Acknowledgements
This study was supported by funds from the National Natural Science Foundation of China (81702346) and the Medicine, Science and Technology research project of Henan Province (201602032). The funding body had no role in the design of the study, the collection, analysis, and interpretation of the data, or the writing of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Molina JR, Ping Y, Cassivi SD. et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-94
2. Goldstraw P, Ball D, Jett JR. et al. Non-small-cell lung cancer. Lancet. 2011;378:1727-40
3. Ridge CA, Mcerlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30:93-8
4. Domingues D, Turner A, Silva MD. et al. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6:1221-35
5. Mazza V, Cappuzzo F. Immunotherapy and lung cancer: from therapeutic cancer vaccination to novel approaches. J Thorac Dis. 2016;8:E1348-E1350
6. Sawabata N. Prognosis of lung cancer patients in Japan according to data from the Japanese Joint Committee of Lung Cancer Registry. Respir Investig. 2014;52:317-21
7. Goldstraw P, Rami-Porta R, Asamura H. et al. The IASLC Lung Cancer Staging Project: Proposals forRevision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39-51
8. Hirota Y, Lahti JM. Characterization of the enzymatic activity of hChlR1, a novel human DNA helicase. Nucleic Acids Res. 2000;28:917-24
9. Skibbens RV. Chl1p, a DNA helicase-like protein in budding yeast, functions in sister-chromatid cohesion. Genetics. 2004;166:33
10. Chung G, O'Neil NJ, Rose AM. CHL-1 provides an essential function affecting cell proliferation and chromosome stability in Caenorhabditis elegans. DNA Repair. 2011;10:1174-82
11. Datta A, Brosh RM. New Insights Into DNA Helicases as Druggable Targets for Cancer Therapy. Frontiers in Molecular Biosciences. 2018;5:59-68
12. Bharti SK, Khan I, Banerjee T. et al. Molecular functions and cellular roles of the ChlR1 (DDX11) helicase defective in the rare cohesinopathy Warsaw breakage syndrome. Cell Mol Life Sci. 2014;71:1-15
13. José-Mario CC, Sanjay Kumar B, Sommers JA. et al. Identification and biochemical characterization of a novel mutation in DDX11 causing Warsaw breakage syndrome. Hum Mutat. 2013;34:103-7
14. Pisani F, Napolitano E, Napolitano L. et al. Molecular and Cellular Functions of the Warsaw Breakage Syndrome DNA Helicase DDX11. Genes. 2018;9:564-584
15. Bhattacharya C, Wang X, Becker D. The DEAD/DEAH box helicase, DDX11, is essential for the survival of advanced melanomas. Mol Cancer. 2012;11:82-92
16. Camp RL, Marisa DF, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-9
17. Bao J, Yu Y, Chen J. et al. MiR-126 negatively regulates PLK-4 to impact the development of hepatocellular carcinoma via ATR/CHEK1 pathway. Cell Death Dis. 2018:9 1045-1055
18. Zhang J, He Y, Yu Y. et al. Upregulation of miR-374a promotes tumor metastasis and progression by downregulating LACTB and predicts unfavorable prognosis in breast cancer. Cancer Med. 2018;7:3351-62
19. He Y, Xue C, Yu Y. et al. CD44 is overexpressed and correlated with tumor progression in gallbladder cancer. Cancer Med. 2018;7:3351-3362
20. Xue C, He Y, Hu Q. et al. Downregulation of PiM1 regulates glycolysis and suppresses tumor progression in gallbladder cancer. Cancer Manag Res. 2018;10:5101-5112
21. R S, D N, A J. Cancer statistics, 2013. Ca-A Cancer Journal for Clinicians. 2015;60:277-300
22. Chen CA, Chang JT, Ho Y. et al. MiR-26 down-regulates TNF-α/NF-κB signalling and IL-6 expression by silencing HMGA1 and MALT1. Nucleic Acids Res. 2016;44:3772-87
23. Nan S, Zhaoli C, Fengwei T. et al. Isocitrate dehydrogenase 1 is a novel plasma biomarker for the diagnosis of non-small cell lung cancer. Clin Cancer Res. 2013;19:5136-45
24. Yoo Jin H, Jin H, Hye-Jeong L. et al. Analysis of tumor markers in the cytological fluid obtained from computed tomography-guided needle aspiration biopsy for the diagnosis of non-small cell lung cancer. J Thorac Oncol. 2011;6:1330-5
25. Akira I, Tongyuan L, Roby SK. et al. Loss of ChlR1 helicase in mouse causes lethality due to the accumulation of aneuploid cells generated by cohesion defects and placental malformation. Cell Cycle. 2007;6:1646-54
26. Maria S. Two steps forward, one step back: determining XPD helicase mechanism by single-molecule fluorescence and high-resolution optical tweezers. DNA Repair. 2014;20:58-70
27. Zhu Y, Gu J, Li Y. et al. MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett. 2018;412:59-69
28. Hill JW, Tansavatdi K, Lockett KL. et al. Validation of the cell cycle G(2) delay assay in assessing ionizing radiation sensitivity and breast cancer risk. Cancer Manag Res. 2009;1:39-48
29. Rizk S, Maalouf K, Baydoun E. 201 Kefir Induces Cell-Cycle Arrest and Apoptosis in HTLV-1 Negative Malignant T-Lymphocytes. Cancer Manag Res. 2011;11:39-47
30. Fischer H, Taylor N, Allerstorfer S. et al. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408-19
31. Gunamani S, Smith GT, Akira M. et al. Cell cycle activation in lung adenocarcinoma cells by the ErbB3/phosphatidylinositol 3-kinase/Akt pathway. Carcinogenesis. 2003;24:1581-92
32. Lou Y, Li R, Liu J. et al. Mitofusin-2 over-expresses and leads to dysregulation of cell cycle and cell invasion in lung adenocarcinoma. Medical Oncology. 2015;32:132-142
33. B P. The CCN family of genes: a brief history. Mol Pathol. 2001;54:103-4
34. Bonelli P, Tuccillo FM, Borrelli A. et al. CDK/CCN and CDKI alterations for cancer prognosis and therapeutic predictivity. Biomed Res Int. 2014;2014:361020-361031
35. Collins I, Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5:366-73
36. Pines J, Jackman M, Simpson K. Assays for CDK activity and DNA replication in the cell cycle. Curr Protoc Cell Biol. 2001 Chapter 8: Unit 8.2
Author contact
![]() Corresponding author: Yan Yu, Precision Medicine Center, The First Affiliated Hospital of Zhengzhou University, Henan, China. E-mails: fccyuyedu.cn
Corresponding author: Yan Yu, Precision Medicine Center, The First Affiliated Hospital of Zhengzhou University, Henan, China. E-mails: fccyuyedu.cn

 Global reach, higher impact
Global reach, higher impact