Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(18):4278-4285. doi:10.7150/jca.31859 This issue Cite
Research Paper
GnRHa for Ovarian Protection and the Association between AMH and Ovarian Function during Adjuvant Chemotherapy for Breast Cancer
1. Department of Breast Disease, Peking Union Medical College Hospital, Shuaifuyuan, Wangfujing, Beijing 100730, China
2. Department of Clinical laboratory, Peking Union Medical College Hospital, Shuaifuyuan, Wangfujing, Beijing 100730, China.
Received 2018-11-28; Accepted 2019-5-19; Published 2019-7-10
Abstract
Background: Chemotherapy impairs ovarian function in premenopausal breast cancer patients. Many breast cancer patients experience menopause earlier and therefore lose their reproductive abilities. The protective effect of gonadotropin-releasing hormone agonist (GnRha) upon the ovary is clearly apparent for hormone receptor (HR) negative patients, although the available data is not consistent for the patient body as a whole when considered regardless of HR status. It is also unknown whether levels of Anti-Mullerian Hormone (AMH) can reflect the influence of chemotherapy upon the ovary.
Methods: We randomly assigned 98 premenopausal breast cancer patients regardless HR-positive or -negative to receive either standard chemotherapy with GnRHa (GnRHa group) or standard chemotherapy without GnRHa (control group). Our primary end point was ovarian failure rate (OVF) at 1 year. In addition, we observed the change of AMH level during chemotherapy and the association between AMH and OVF.
Results: OVF was significantly lower (44.7%) in the GnRHa group than in the control group (80.6%; P=0.002). Median AMH levels were significantly higher before chemotherapy when compared to after 1/2cycles of chemotherapy, both in the GnRHa group (1.86ng/ml vs 0.12ng/ml; P=0.000) and in the control group (1.57ng/ml vs 0.10ng/ml; P=0.000). OVF was 91.3% in the AMH baseline level <1.1ng/ml group and 63.5% in the AMH baseline level >1.1ng/ml group (P=0.013).
Conclusion: Data showed that GnRHa may have a protective effect on young breast cancer patients regardless of HR during chemotherapy. AMH could reflect changes of OVF during chemotherapy and predict OVF after chemotherapy.
Keywords: Breast cancer, Chemotherapy, Ovarian function, GnRH agonist, AMH
Introduction
Approximately 6% of patients with breast cancer are less than 40 years old, and 1 in 200 people under 40 are likely to develop breast cancer[1]. The mean age of diagnosis for breast cancer patients in China is 45-55 years, which is far younger than Western women[2]. Young age has been shown to represent an independent predictor of poor prognosis in breast cancer[3-5] and most patients in this age group receive chemotherapy. These patients have a high risk of transient or permanent amenorrhea, and there is more long-term risk of premature ovarian failure in patients who continue to undergo or recover their menstrual cycle[6]. A previous study estimated that one year of chemotherapy will lead to 1.5 years of reproductive loss[7].
The onset of premature menopause depends on the age of the patient and the type of chemotherapy administered[8]. Premature ovarian failure has significant consequences, including infertility, sexual dysfunction and vasomotor symptoms[9]. Previous studies have shown that young survivors of breast cancer consider premature menopause, sexual dysfunction and infertility to represent the most distressing aspects of their cancer experience[10]. Due to concerns about infertility, 29% of breast cancer patients will request changes to the decisions made about their treatment [11] One previous study of breast cancer patients younger than 40 found that 68% of these patients discussed fertility problems with their doctors prior to the treatment commencing. Some of these patients refused chemotherapy or changed their chemotherapy regimen because of reproductive concerns, and 10% of patients took measures to protect their reproductive function[12]. Another study used a questionnaire to survey breast cancer patients younger than 35 years; 59% of patients expressed a desire to have more children and 8% of patients said they would not wish to receive chemotherapy because it could reduce their fertility[13]. Consequently, it is very important for young breast cancer patients to protect their ovarian function during chemotherapy. In addition, it is important for clinicians and patients to understand the state of ovarian function and to be able to predict the effect of chemotherapy on ovarian function. Thus far, no laboratory test has been able to accurately reflect ovarian function and predict ovarian function after chemotherapy.
Anti-Mullerian Hormone (AMH) is released from the granulosa cells of antral follicles and can be measured by serum concentrations, which are known to be proportional to the development of ovarian development. Consequently, AMH is considered to represent a marker of ovarian aging[14]. Levels of AMH provide an indirect indicator of the number of antral and pre-antral follicles in the ovary and are widely used in clinical practice[15]. Serum levels of AMH have also been shown to indirectly reflect the remaining primordial follicles of the ovarian reserve. Consequently, AMH can be used to predict reproductive longevity[16, 17] and has become a biochemical marker of ovarian reserve[17].
GnRHa can protect ovarian function during chemotherapy for hormone receptor (HR)-negative breast cancer patients[18]. However for HR-positive patients, the effect has not been clearly defined. This study aimed to examine whether GnRHa can protect ovarian function during chemotherapy, regardless of the state of the (HR), to investigate whether AMH levels can reflect changes of ovarian function during chemotherapy and finally, to investigate whether AMH levels can predict regular menstruation after chemotherapy.
Materials and Methods
Material
Detection of the levels of AMH, Follicle-Stimulating Hormone (FSH), Prolactin (PRL), Estradiol (E2), luteinizing hormone (LH), Testosterone (T) detection was conducted using an automated chemiluminescence immunoassay analyzer (Beckman Coulter UniCel DXI800, Brea, CA, USA) along with corresponding reagents, calibration materials and quality control materials.
Patients
Premenopausal women, aged 18 to 45 years, were eligible for enrollment if they had operable stage I to IIIA breast cancer, regardless of hormone receptor status, for which treatment with adjuvant anthracyclines-containing chemotherapy was planned. The use of trastuzumab was permitted in patients with tumors which over-expressed human epidermal growth factor receptor 2 (HER2). Estrogen receptor (ER) and Progesterone receptor (PR) were performed at a clinical laboratory in PUMCH hospital by routine methods. All patients provided written informed consent for participation. Eligible participants were administered with tamoxifen if their hormone receptor status was positive. Patients were excluded for the following reasons: stage IV breast cancer or with distant metastasis; presence of other malignancies over the last 5 years; undergoing chemotherapy or receiving GnRHa, fitted with an intrauterine device, taking ovulation-promoting drugs or oral contraceptives within the previous three months; definite diagnosis of polycystic ovarian syndrome, irregular menstruation, amenorrhea; pregnancy or lactation.
Study Design
In this study, patients were randomly assigned, in a 1:1 ratio, to standard adjuvant chemotherapy with the GnRH agonist goserelin (chemotherapy plus goserelin group) or to chemotherapy without goserelin (chemotherapy-alone group). The choice of the standard anthracyclines-containing chemotherapy regimen was left to the discretion of the clinical doctors. For patients randomly assigned to the chemotherapy plus goserelin group, we administered goserelin at a dose of 3.6 mg subcutaneously every 4 weeks from within 1 week of the initial chemotherapy dose and was continued to within 2 weeks of, or after, the final of chemotherapy.
Randomization was stratified according to age (<40 years vs. 40 to 45 years) and hormone receptor status (positive or negative). The primary objective was to investigate the rate of ovarian failure (OVF) which was defined as amenorrhea for the preceding 6 months. Patients who became pregnant were considered not to have had ovarian failure. Additional end points were disease free survival (DFS) and overall survival (OS). Events of overall survival included deaths due to any cause while events of disease free survival included breast cancer recurrence and metastasis. In a previous study[19], ovarian reserve decline was defined as when AMH levels were lower than 1.1ng/ml; consequently, in the present study, we separated our patients into two groups according to their AMH levels (<1.1ng/ml and >1.1ng/ml) and then investigated the association between OVF and AMH grouping.
Statistical Analysis
We originally aimed to recruit 240 eligible patients. We estimated that with this sample size, and using a two-group binomial design, our study would have more than 80% power to detect an absolute reduction of 15% points in the rate of ovarian failure, at a one-sided significance level of 0.025. Primary analysis was based on Chi-square tests and the Mann-Whitney test. In addition, we examined levels of AMH, FSH, E2, PRL and T before chemotherapy, 1/2 cycles after chemotherapy, 6 months after chemotherapy and 1 year after chemotherapy. We analyzed patient characteristics according to randomization group and stratification variables. We also observed the changes of AMH during and after chemotherapy using the Wilcoxon signed rank test and analyzed the relationship between clinical features including AMH and ovarian failure by Chi-square tests. Finally, exploratory Kaplan-Meier curves for disease free survival were calculated. According to the study-design specifications, a one-sided alpha level of 0.025 was used to indicate statistical significance for the primary end-point analysis of ovarian failure; for all other P values, a two-sided alpha level of 0.05 was used to indicate statistical significance. The cutoff date for all analyses was June 2017.
Results
Patients
We recruited and randomized a total of 98 patients between August 2015 and November 2016. One patient was not eligible and another patient was not evaluated up to the end point of this study. Consequently, our final analysis involved 96 patients (45 in the chemotherapy-alone group and 51 in the chemotherapy plus goserelin group). The median follow-up time was 15 months at the end of the analysis. Patient baseline characteristics and hormone levels are shown in Table 1 and Table 2. The median age of our patients was 39.0 years. All patients received anthracycline-based therapy and 79% of patients received therapy featuring either paclitaxel or docetaxel. The clinical characteristics of the two groups were well balanced. All hormone receptor positive patients received endocrine therapy, and the endocrine therapy drug was toremifen. There were no significant differences in the levels of AMH, FSH, LH, E2 or T between the two groups at baseline.
Baseline characteristics of patients, according to study group
| Characteristic | All eligible patients | ||
|---|---|---|---|
| Overall | Chemo alone | Chemo plus Goserelin | |
| No (%) | No (%) | No (%) | |
| Age | |||
| Median(range) | 39.0 | 40.0 | 37.0 |
| <40 yr | 53(55.2%) | 21(46.7%) | 32(62.7%) |
| >=40 yr | 43(44.8%) | 24(53.3%) | 19(37.3%) |
| T stage | |||
| T1 | 61(63.5%) | 34(75.6%) | 27(52.9%) |
| T2 | 30(31.3%) | 9(20.0%) | 21(41.2%) |
| T3 | 5(5.2%) | 2(4.4%) | 3(5.9%) |
| LN | |||
| LN- | 31(32.3%) | 15(33.3%) | 16(31.4%) |
| LN+ | 65(67.7%) | 30(66.7%) | 35(68.6%) |
| ER | |||
| ER- | 30(31.3%) | 12(26.7%) | 18(35.3%) |
| ER+ | 66(68.7%) | 33(73.3%) | 33(64.7%) |
| HER-2 status | |||
| HER-2- | 67(69.8%) | 31(68.9%) | 36(70.6%) |
| HER-2+ | 29(30.2%) | 14(31.1%) | 15(29.4%) |
Chemo denotes chemotherapy.
AMH, FSH, E2, LH, PRL, T level in different age groups before chemotherapy (Mann-Whitney test)
| All eligible patients | |||||||
|---|---|---|---|---|---|---|---|
| Overall | Chemo alone | Chemo plus Goserelin | P value | ||||
| Median level | No (%) | Median level | No (%) | Median level | No (%) | ||
| AMH ng/ml | 1.68 | 1.57 | 1.86 | 0.003 | |||
| <40 yr | 2.23 | 53(55.2) | 1.68 | 21(46.7) | 2.55 | 32(62.7) | 0.453 |
| >=40 yr | 1.23 | 43(44.8) | 1.33 | 24(53.3) | 0.87 | 19(37.3) | 0.002 |
| FSH mIU/ml | 6.30 | 6.52 | 5.97 | 0.119 | |||
| <40 yr | 5.91 | 53(55.2) | 6.70 | 21(46.7) | 4.98 | 32(62.7) | 0.785 |
| >=40 yr | 6.82 | 43(44.8) | 6.50 | 24(53.3) | 8.30 | 19(37.3) | 0.037 |
| E2 pg/ml | 50.25 | 56.00 | 43.00 | 0.271 | |||
| <40 yr | 48.80 | 53(55.2) | 34.00 | 21(46.7) | 53.50 | 32(62.7) | 0.101 |
| >=40 yr | 53.00 | 43(44.8) | 65.00 | 24(53.3) | 43.00 | 19(37.3) | 0.720 |
| LH mIU/ml | 5.07 | 4.63 | 5.59 | 0.218 | |||
| <40 yr | 4.90 | 53(55.2) | 4.60 | 21(46.7) | 5.14 | 32(62.7) | 0.609 |
| >=40 yr | 5.27 | 43(44.8) | 4.72 | 24(53.3) | 5.87 | 19(37.3) | 0.201 |
| PRL ng/ml | 19.61 | 20.07 | 17.92 | 0.067 | |||
| <40 yr | 23.04 | 53(55.2) | 33.74 | 21(46.7) | 21.19 | 32(62.7) | 0.069 |
| >=40 yr | 17.74 | 43(44.8) | 19.20 | 24(53.3) | 17.15 | 19(37.3) | 0.416 |
| T ng/ml | 0.44 | 0.44 | 0.42 | 0.455 | |||
| <40 yr | 0.44 | 53(55.2) | 0.44 | 21(46.7) | 0.51 | 32(62.7) | 0.716 |
| >=40 yr | 0.41 | 43(44.8) | 0.45 | 24(53.3) | 0.40 | 19(37.3) | 0.259 |
Chemo denotes chemotherapy.
AMH levels
There was a statistically significant relationship between patient age and AMH levels. Patients below and above the age of 40 were compared with regards to median AMH level (2.23ng/ml and 1.23ng/ml, respectively), median FSH level (5.91mIU/ml and 6.82mIU/ml, respectively), median E2 level (48.80pg/ml and 53.00pg/ml, respectively), median LH level (4.90mIU/ml and 5.27mIU/ml, respectively) and T (0.44ng/ml and 0.41ng/ml, respectively). Our analysis showed that the relationship between age and median AMH level was statistically significant (P=0.003; Table 2).
AMH level decreased significantly during chemotherapy in the chemotherapy plus goserelin group and in the chemotherapy-alone group. However, there was no significant difference, in terms of median AMH levels when compared before and 1 year after chemotherapy for either the chemotherapy plus goserelin group (P=0.141; Table 3) or in the chemotherapy-alone group (P=0.109; Table 3).
Change of AMH before and after chemotherapy (Wilcoxon Signed Ranks Test)
| Chemo plus Goserelin | Chemo alone | |||
|---|---|---|---|---|
| AMH | P value | AMH | P value | |
| Before chemo | 1.86 | 1.57 | ||
| 1/2 cycles after chemo | 0.12 | 0.000 | 0.10 | 0.000 |
| 6 months after chemo | 0.04 | 0.000 | 0.03 | 0.000 |
| 1 year after chemotherapy | 0.05 | 0.141 | 0.09 | 0.109 |
Chemo denotes chemotherapy.
There was no significant difference in median AMH levels between the two groups when compared before chemotherapy (P=0.561; Mann-Whitney test; Table 1); in the chemotherapy-alone group, median AMH level before chemotherapy was 1.57 ng/mL, as compared to 1.86 ng/mL in the chemotherapy plus goserelin group. In the chemotherapy plus goserelin group, the AMH level after 1/2 cycles of chemotherapy was 0.12 ng/mL, which fell to 0.04 ng/ml after 6 months; both of these levels were significantly lower than the median AMH level prior to chemotherapy (1.86 ng/mL; p<0.05; Table 3). In the chemotherapy plus goserelin group, the median AMH level after 1 year of chemotherapy was 0.05 ng/ml, which was lower than the level of 1.86 ng/mL before chemotherapy but was not statistically significant (p>0.05; Wilcoxon Signed Ranks Test; Table 3).
In the chemotherapy-alone group, the median AMH level after 1/2 cycles of chemotherapy was 0.10 ng/mL, and 6 months after chemotherapy had fallen to 0.03; these levels were both significantly lower than the pre-chemotherapy level of 1.57 ng/mL, lowered as compared to 1.57 ng/mL (p<0.05; Wilcoxon Signed Ranks Test; Table 3). In the chemotherapy-alone group, median AMH level after 1 year of chemotherapy was 0.09 ng/mL, which was lower than the level before chemotherapy (1.57 ng/mL) but was not statistically different (p>0.05; Wilcoxon Signed Ranks Test; Table 3).
Ovarian Dysfunction
Our study investigated four different factors which may be potentially associated with ovarian function recovery: age, baseline AMH, the administration of GnRHa and the addition of endocrine therapy (Table 4). Of the 96 patients, 74 valid information on their menstrual status was obtained. In 74 patients, 2 baseline blood samples were not detected valid information. In 74 patients, endocrine therapy was not available in one patient.
Association between OVF and age, AMH baseline level and chemotherapy treatment (Chi-square tests)
| Without OVF No (%) | With OVF No (%) | P value | |
|---|---|---|---|
| Overall | 0.002 | ||
| Age<40 | 22(55%) | 18(45%) | |
| Age>=40 | 6(17.6%) | 28(82.4%) | |
| Over all AMH | 0.018 | ||
| Baseline<1.1ng/ml | 4(17.4%) | 19(82.6%) | |
| Baseline>1.1 ng/ml | 24(49.0%) | 25(51.0%) | |
| Endocrine therapy | 0.174 | ||
| Without | 10(40%) | 15(60%) | |
| With | 11(22.9%) | 37(77.1%) | |
| GnRHa | 0.002 | ||
| Without | 7(19.4%) | 29(80.6%) | |
| With | 21(55.3%) | 17(44.7%) |
Chemo denotes chemotherapy.
In terms of age, our analysis showed that younger women (<40 years) had a significantly lower rate of OVF (ovarian function failure) than older women (>=40 years) after treatment (P=0.002; Chi-square test; Table 4). Other analysis showed that detectable levels of baseline AMH (≥1.1 ng/ml) were significantly associated with a lower rate of OVF (P=0.013; Chi-square test; Table 4) and that the administration of GnRHa was significantly associated with a lower rate of OVF (P=0.002; Chi-square test; Table 4). Furthermore, OVF tended to be higher in patients receiving to endocrine although this relationship was not significant (P=0.174; Chi-square test; Table 4).
OVF was evaluated at one year after treatment (Table 5) and analysis included patients with menstrual status data. After 1 year, relevant data were available for 74 patients (75.6% of the study population). OVF was evident in 29 out of the 36 patients (80.6%) in the chemotherapy-alone group and in 17 out of 38 patients (44.7%) in the chemotherapy plus goserelin group (P=0.002). In the HR+ group, data were available for 45 patients (68.1%). OVF was evident in 20 out of the 24 patients (83.3%) in the chemotherapy-alone group and in 10 out of the 21 (47.6%) patients in the chemotherapy plus goserelin group (P=0.025). In the HR- group, data were available for 25 patients (78.1%). OVF was evident in 7 out of 10 patients (70.0%) in the chemotherapy-alone group and in 5 out of the 15 patients (33.3%) in the chemotherapy plus goserelin group (P=0.111).
Association between GnRHa and OVF (Chi-square tests)
| Chemo alone | Chemo plus Goserelin | P value | |||
|---|---|---|---|---|---|
| Without OVF | With OVF | Without OVF | With OVF | ||
| Over all | 7(19.4%) | 29(80.6%) | 21(55.3%) | 17(44.7%) | 0.002 |
| HR- | 3(30%) | 7(70%) | 10(66.7%) | 5(33.3%) | 0.111 |
| HR+ | 4(16.0%) | 21(84.0%) | 11(47.8%) | 12(52.0%) | 0.029 |
Chemo denotes chemotherapy.
Disease Free Survival and Overall Survival
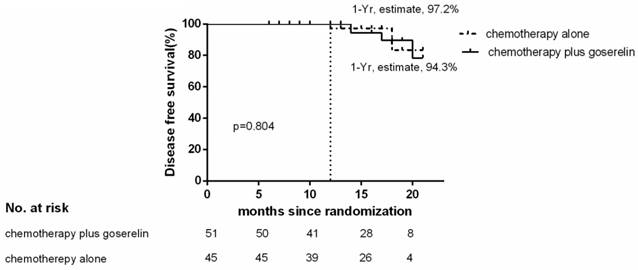
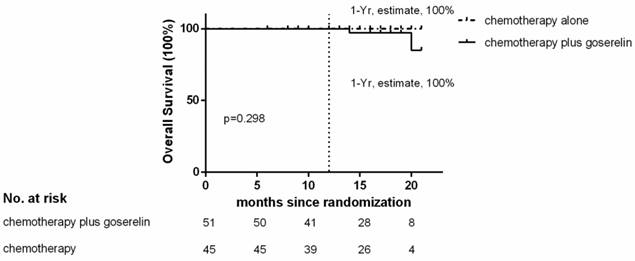
Of the 96 patients analyzed, only 3 patients in the chemotherapy group, and 4 patients in the chemotherapy plus goserelin group experienced recurrence or died. The 1-year Kaplan-Meier estimate of the rate of disease free survival was 97.2% in the chemotherapy-alone group and 94.3% in the chemotherapy plus goserelin group (Figure 1). None of the patients in the chemotherapy-alone group died, although 2 died in the chemotherapy plus goserelin group. The 1-year Kaplan-Meier estimate of the rate of overall survival was 100.0% in the chemotherapy-alone group and 100.0% in the goserelin group (Figure 2). All 98 randomized patients showed no significant difference in terms of DFS and OS when compared between the chemotherapy-alone group and the chemotherapy plus goserelin group (P=0.804, P=0.298).
Disease-free Survival. The 1‑ year estimate of disease ‑ free survival is Kaplan-Meier estimates. There were 3 relapses or deaths in the chemotherapy-alone group and 4 in the chemotherapy-plus-goserelin group.
Overall Survival. The 1‑ year estimate of overall survival is Kaplan-Meier estimates. There were 2 deaths in the chemotherapy‑plus‑goserelin group and 0 in the chemotherapy-alone group.
Discussion
The latest clinical trial concluded that GnRHa, combined with tamoxifen or aromatase inhibitors, could improve disease free survival for hormone receptor positive (HR+) premenopausal high-risk breast cancer patients[20]. However, there is a lack of research addressing whether GnRHa can protect ovarian function during chemotherapy in patients with breast cancer. There are only few reliable randomized controlled studies and existing clinical findings are limited and inconclusive[21]. Several studies have indicated that GaRHa has no protective effect upon the ovary during chemotherapy; in these studies, it was notable that menstrual recovery ratio or menstrual recovery time were used as end points[22-24]. However, the PROMISE-GIM6[25] study found that the proportion of patients with early menopause was significantly lower in a GnRHa group than in a control group. The POEMS[18] study further found that ovarian failure was significantly lower in the GnRHa group than in the control group. Consistent with these two earlier studies, our present study concluded that OVF was significantly lower in the chemotherapy plus goserelin group compared to the chemotherapy-alone group.
For obstetricians and gynecologists, the most appropriate ovarian reserve screening tests to use in practice are basal follicle-stimulating hormone (FSH) plus estradiol levels or AMH levels. In particular, AMH is useful for this test as it remains stable throughout menstrual cycles and it can therefore be tested at any point [26]. Our present study confirms and extends previous findings in that AMH declines rapidly [27, 28] and remains low during chemotherapy[27]. However, our present data are consistent with one previous study [29] in that AMH level was associated with age, and AMH levels in patients below 40 years were found to be higher than in patients over 40 years of age. Patients with higher baseline AMH are more likely to restore menstruation compared with lower AMH, which is consistent with previous study[30]. The reason may be that AMH is the most relevant hormone indicator of ovarian reserve capacity[27]. In addition, our study found that AMH levels decreased significantly during chemotherapy, and that there was no significant difference in AMH levels when compared between before chemotherapy and 1 year after chemotherapy. Furthermore, patients with higher AMH levels prior to chemotherapy had lower OVF when measured 1 year after chemotherapy. These results indicate that AMH can reflect the effect of chemotherapy upon ovarian function in real time and predict OVF after chemotherapy.
The current guidelines of the American Society of Clinical Oncology encourage patients of reproductive age to discuss fertility preservation if infertility is a potential risk of their proposed therapy[31]. More than one hundred babies have been born from frozen ovaries taken from cancer patients[32, 33], but embryo cryopreservation involves time, cost, the use of sperm from a partner or a donor and a cycle of ovarian stimulation, which may limit assisted reproduction options for many young women who are receiving chemotherapy. GnRHa treatment, combined with chemotherapy, may be a more convenient option and can be used in combination with other fertility preservation techniques. Although this treatment has potential side effects, including the loss of bone density, it is anticipated that this treatment regimen can encourage the long-term preservation of ovarian function. Furthermore, for those patients who are not interested in fertility preservation, GnRHa may help avoid unwanted menopausal and osteopenia.
There has been far less research on the effects of combination chemotherapy including GnRHa on breast cancer survival. The POEMS[18] study suggested that GnRHa combined chemotherapy can increase DFS and OS. However, our present study did not have positive results in terms of survival as in previous studies, possibly because of our short follow-up period.
Previous studies have concluded that GnRHa has an ovarian protective effect on HR-negative breast cancer patients during chemotherapy. Our study, despite our short follow-up period, showed that regardless of HR status, GnRHa has a protective effect on young breast cancer patients during chemotherapy. Our data provides further evidence for clinicians with regards to the use of GnRHa for HR+ breast cancer patients. In addition, AMH levels are significantly related to age and can reflect changes of ovarian function during chemotherapy and predict OVF after chemotherapy. AMH levels can provide doctors with additional information with which to draw up a strategy for fertility protection during chemotherapy.
In the future, we need more follow up time to prove that GnRHa can increase DFS for breast cancer patients and with more events, we can possibly further define the clinical use of GnRHa according to AMH levels during chemotherapy.
As a conclusion, Our study showed that GnRHa may have a protective effect on young breast cancer patients regardless of hormone receptor during chemotherapy. In addition, the level of AMH is significantly related to age, reflect changes of ovarian function during chemotherapy and predict ovarian failure after chemotherapy.
Acknowledgements
We would like to thank all of our patients for participating in this study and everyone who contributed towards the article who does not meet the criteria for authorship.
Funding
This work was supported by Beijing Science and Technology Commission 2016: Science and Technology Project (Optimization of breast cancer screening program for the right age women in Beijing). The role of the funding consists of collection, analysis, and interpretation of data.
Availability of data and materials
The datasets generated during and/or analyzed in the current study are not publically available but are available from the corresponding author on reasonable request.
Authors' contributions
ZY designed the study, conducted the literature search, analyzed the data and drafted the manuscript. LY and ZYD conducted the literature search and revised the manuscript. CXQ and WYJ tested and analyzed laboratory data. All authors read and approved the final manuscript. The tables and figures in the paper are original for this article and the authors have permission to use them.
Ethics approval and consent to participate
Peking Union Medical College Hospital ethics committee reviewed the study protocol and deemed the study exempt from full review. All patients in our study provided written informed consent for participation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Siegel R, Xu J. et al. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277-300
2. Fan L, Strasser-Weippl K, Li JJ. et al. Breast cancer in China. The Lancet Oncology. 2014;15(7):e279-89
3. Adami HO, Malker B, Holmberg L. et al. The relation between survival and age at diagnosis in breast cancer. The New England journal of medicine. 1986;315(9):559-63
4. de la Rochefordiere A, Asselain B, Campana F. et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341(8852):1039-43
5. Anders CK, Hsu DS, Broadwater G. et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(20):3324-30
6. Partridge A, Gelber S, Gelber RD. et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. European journal of cancer. 2007;43(11):1646-53
7. Gerber B, Dieterich M, Muller H. et al. Controversies in preservation of ovary function and fertility in patients with breast cancer. Breast cancer research and treatment. 2008;108(1):1-7
8. Petrek JA, Naughton MJ, Case LD. et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(7):1045-51
9. Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(5):753-8
10. Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psycho-oncology. 2004;13(5):295-308
11. Partridge AH, Gelber S, Peppercorn J. et al. Web-based survey of fertility issues in young women with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(20):4174-83
12. Ruddy KJ, Gelber SI, Tamimi RM. et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(11):1151-6
13. Senkus E, Gomez H, Dirix L. et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98. Psycho-oncology. 2014;23(2):173-82
14. Kevenaar ME, Meerasahib MF, Kramer P. et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147(7):3228-34
15. Hansen KR, Hodnett GM, Knowlton N. et al. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertility and sterility. 2011;95(1):170-5
16. Broer SL, Broekmans FJ, Laven JS. et al. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Human reproduction update. 2014;20(5):688-701
17. Dewailly D, Andersen CY, Balen A. et al. The physiology and clinical utility of anti-Mullerian hormone in women. Human reproduction update. 2014;20(3):370-85
18. Moore HC, Unger JM, Phillips KA. et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. The New England journal of medicine. 2015;372(10):923-32
19. Ferraretti AP, La Marca A, Fauser BC. et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human reproduction. 2011;26(7):1616-24
20. Francis PA, Regan MM, Fleming GF. et al. Adjuvant ovarian suppression in premenopausal breast cancer. The New England journal of medicine. 2015;372(5):436-46
21. Turner NH, Partridge A, Sanna G. et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertain. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(9):2224-35
22. Gerber B, von Minckwitz G, Stehle H. et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(17):2334-41
23. Munster PN, Moore AP, Ismail-Khan R. et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(5):533-8
24. Elgindy EA, El-Haieg DO, Khorshid OM. et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstetrics and gynecology. 2013;121(1):78-86
25. Del Mastro L, Boni L, Michelotti A. et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. Jama. 2011;306(3):269-76
26. Committee on Gynecologic P. Committee opinion no. 618: Ovarian reserve testing. Obstetrics and gynecology. 2015;125(1):268-73
27. Freour T, Barriere P, Masson D. Anti-mullerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. European journal of cancer. 2017;74:1-8
28. Bala J, Seth S, Dhankhar R. et al. Chemotherapy: Impact on Anti-Mullerian Hormone Levels in Breast Carcinoma. Journal of clinical and diagnostic research: JCDR. 2016;10(2):BC19-21
29. Acibucu F, Acibucu DO, Akkar OB. et al. Evaluation of Ovarian Reserve with AMH Level in Patients with Well-Differentiated Thyroid Cancer Receiving Radioactive Iodine Ablation Treatment. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2016;124(10):593-6
30. Silva C, Caramelo O, Almeida-Santos T. et al. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Human reproduction. 2016;31(12):2737-49
31. Loren AW, Mangu PB, Beck LN. et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(19):2500-10
32. Imbert R, Moffa F, Tsepelidis S. et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Human reproduction. 2014;29(9):1931-40
33. Van der Ven H, Liebenthron J, Beckmann M. et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Human reproduction. 2016;31(9):2031-41
Author contact
![]() Corresponding author: Sun Qiang, E-mail: bakenfishcom, FAX and TEL numbers: 0086-010-69152700
Corresponding author: Sun Qiang, E-mail: bakenfishcom, FAX and TEL numbers: 0086-010-69152700

 Global reach, higher impact
Global reach, higher impact