3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(20):4954-4960. doi:10.7150/jca.31544 This issue Cite
Research Paper
The voltage-gated sodium channel Nav1.7 associated with endometrial cancer
1. Department of Obstetrics and Gynecology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
2. Institute of Precision Medicine, Jining Medical University, Jining, China.
*These authors contributed equally to this work.
Received 2018-11-15; Accepted 2019-6-25; Published 2019-8-27
Abstract
Background: Endometrial cancer is the most common gynecologic malignancy in women in the developed countries. Despite recent progress in functional characterization of voltage-gated sodium channel (Nav) in multiple cancers, very little was known about the expression of Nav in human endometrial cancer. The present study sought to determine the role of Nav and molecular nature of this channel in the endometrial cancer.
Methods: PCR approach was introduced to determine expression level of Nav subunits in endometrial cancer specimens. Pharmacological agents were used to investigate Nav function in endometrial cancer cells. Flow cytometry were used to test cancer apoptosis, and invasion assays were applied to test tumor metastasis.
Results: Transcriptional levels of the all Nav α and β subunits were determined by real time-PCR in endometrial cancer with pair tissues of carcinoma and adjacent nonneoplastic tissue, Nav1.7 was the most highly expressed Nav subtype in endometrial cancer tissues. Nav1.7 level was closely associated with tumor size, local lymph node metastasis, and 5-year and 10-year survival ratio. Inhibition of this channel by Nav1.7 blocker PF-05089771, promoted cancer apoptosis and attenuated cancer cell invasion.
Conclusion: These results establish a relationship between voltage-gated sodium channel protein and endometrial cancer, and suggest that Nav1.7 is a potential prognostic biomarker and could serve as a novel therapeutic target for endometrial cancer.
Keywords: Endometrial Cancer, Nav1.7, Voltage-Gated Sodium Channel, Ion Channels.
Introduction
Endometrial cancer is a major cause of morbidity for women worldwide, and approximately 3% of women develop endometrial cancer at some point during their lifetimes [1]. The 5-year survival rate for women with stage I endometrial cancer is 90%, it drops to 57% in patients with stage III, and to 20% in patients with stage IV [2]. As the determinant of survival in endometrial cancer is the stage of disease at diagnosis, the early detection and effective therapy are of considerable importance. Recently, ion channels have emerged as new biomarkers for human cancers, and some have been shown to correlate with the main hallmarks of the cancer process and serve as pharmacological targets in the cancer chemotherapy [3].
The voltage-gated sodium channels (Navs) are responsible for the fast action potentials involved in nerve and cardiac conduction [4], they were recently found to play crucial roles in cancer development and progression [5]. The family of sodium channels has nine members named Nav1.1 through Nav1.9. Among them, the Nav1.5 has been shown to be associated with colon cancer and breast cancer metastasis [6, 7]; inhibition of Nav1.6 reduced invasiveness of cervical cancer primary culture cells [8]; and in prostate cancer, Nav1.8 expression was revealed to be closely correlated with pathologic stage of cancer specimens [9]. Despite recent progress in the functional characterization of sodium channel in multiple cancers, very little was known about the expression of Nav in human endometrial cancer; furthermore, the molecular basis of sodium channel in this type of cancer has not yet been identified.
In this study, we used primary cultures to investigate the potential role of Nav in endometrial cancer. The present study aimed to determine whether voltage-gated sodium channel protein functionally expressed in the endometrial cancer with metastatic potential, whether their expressions are associated with clinical outcome, and what molecular nature of sodium channels are in the endometrial cancer, whether their activities contribute cellular behaviors integral to metastasis.
Materials and Methods
Patients and tissue samples
A total of 80 surgical specimens of endometrial cancer tissues were collected from patients at the Department of Obstetrics and Gynecology, the First Affiliated Hospital, Sun Yat-Sen University from 2006 to 2016 without prior radiotherapy or chemotherapy. Twenty paired surgical tumor and normal adjacent tissues were obtained with the patients' consent from the patients registered at the First Affiliated Hospital. The normal adjacent tissue, defined as histologically benign-appearing tissue and judged by an experienced pathologist, is acquired from the margins of the tumor resection. A separate set of frozen tumor specimens for Kaplan-Meier analyses were obtained from sixty patients. The Nav1.7 expression determined by quantitative PCR was evaluated in those specimens, MRPL19 was used as the reference gene to normalize Nav1.7 expression, the group Nav1.7-High or Nav1.7-Low were defined as scores above or below the median. The study was approved by the Institutional Review Board of First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Patient studies were conducted in accordance with ethical guideline of Declaration of Helsinki.
Cell culture
Fresh endometrial cancer biopsies were digested with collagenase (1 mg/ml; Sigma-Aldrich) in Hanks Balanced Salt solution (HBSS) at 37°C for 30 min. The suspended cells were collected by centrifugation at 500 r.p.m for 5 min at 4°C, cells were transferred to a fresh tube containing HBSS, washed and centrifuged again. Then the primary cells were plated on coverslips in Falcon polystyrene microplates 6-well plates, and maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin and 100U/ml streptomycin (Invitrogen) in a 37°C incubator with 5% CO2 [10, 11].
RNA isolation and cDNA synthesis
Total RNA was extracted from endometrial cancer and nonneoplastic endometrial tissues using TRIzol RNA extraction agent (Invitrogen) according to the manufacturer's instructions [12]. Only RNA that resulted in an A260/280 ratio of 1.8-2.0 was reverse transcribed to generate cDNA. Synthesis of cDNA was carried out with SuperScript II RNase Reverse Transcriptase (Invitrogen) and primers (Invitrogen) at 42°C with 2µg of total RNA as template, in a final volume of 20µl. Negative controls for the reverse-transcription reaction were prepared by omitting the RT enzyme [13, 14]. For the reverse transcription-PCR, the relative intensity of Nav1.7 mRNA expression was measured by densitometry (ImageJ, Bethesda, USA). For conventional end-point PCR, 100 ng of cDNA was amplified following addition to a 30µl mastermix containing dNTPs, Platinum Taq DNA polymerase (Invitrogen) enzyme and appropriate forward and reverse primers for the desired Nav α subunits target gene. Amplicons were visualized under UV light following separation through a 1% agarose gel containing ethidium bromide.
Quantitative real time-PCR
Quantitation of Nav α and β subunits and MRPL19 mRNA was carried out by real time PCR using SYBR I green chemistry on an MJ Chromo 4 thermal cycler (BioRad, USA) [15-17]. Approximately 1 ng/µl of cDNA was added to Platinum SYBR Green qPCR Supermix-UDG (Invitrogen) and primers in a 25µl reaction. Standard curves were generated from serially diluted endometrial cDNA and the Nav subunits transcripts quantitated by normalizing expression relative to the reference gene MRPL19. The CT (threshold cycle) value was determined in each experimental group. The data normalization was performed by using the CT value from human MRPL19 (ΔCT=CTNav subunit -CTMRPL19), the ΔCT for EC samples was then normalized to NE samples (ΔΔCT=ΔCTEC-ΔCTNE), the ΔΔCT was converted to -2-ΔΔCT to calculate the relative expression levels of Nav α and β subunits [8]. The primer pairs used for polymerase chain reaction for all Nav α and β subunits and MRPL19 were shown in the Table 1.
Primers used in PCR determining expression of Nav subunits in endometrial cancer
| Protein | Gene | GenBank accession | Forward primer sequence (5´ to 3´) | Reverse primer sequence (5´ to 3´) |
|---|---|---|---|---|
| Nav1.1 | SCN1A | NM_001202435.2 | CCCGACTGTGACCCTAATAAAG | CAGAGGCTCTGCACTTTCTTC |
| Nav1.2 | SCN2A | NM_001040142.1 | GTGCTGGTCATTTTCTTGGGC | CTTGATTCAGCAGATGCGGC |
| Nav1.3 | SCN3A | NM_006922.3 | GGCAAAGGGAAGATCTGGTGG | CCATAAGCAACCCATTTGAGAAGC |
| Nav1.4 | SCN4A | NM_000334.4 | CTCGAGCTGGACCACCTTAAC | CGGACGAGTTCCCATCATAG |
| Nav1.5 | SCN5A | NM_198056.2 | CTTGGCCAAGATCAACCTGCTC | GATGACTCGGAAGAGCGTCG |
| Nav1.6 | SCN8A | NM_014191.3 | GCAGCCGGGAAAACATACATG | GCCTGTGCCTCTTCCTGTTGC |
| Nav1.7 | SCN9A | NM_001365536.1 | GCAAGGCGAAGCAGCAGAAC | GGCTTGGCTGATGTTACTGCTG |
| Nav1.8 | SCN10A | NM_006514.3 | CCTCTCTCCACTCCCACAATG | CACACTGCCATGACTAGCCC |
| Nav1.9 | SCN11A | NM_014139.2 | CTGACTGTGGTCCTGGTCATTG | CGATCCATTCCCCGCAGAGG |
| Navβ1 | SCN1B | BC112922.1 | GAGACCACCGCCGAGACCTTC | CGCCAGAGTGGTTGTAGGTG |
| Navβ2 | SCN2B | AY358945.1 | GTTCCTCCAGTTCCGCATGAAG | GACCTGCAGATGGATCTTGCC |
| Navβ3 | SCN3B | CH471065.1 | GGGTCAGTGTCTGCTTCCCTG | CCTCCTGGTGGCCATTCCG |
| Navβ4 | SCN4B | AY149967.1 | GGACCTGGAGTTCAGCGAC | GCAGGATGAGGATGAGGAG |
| MRPL19 | MRPL19 | NM_014763.3 | GCCAGTGGAAAAATCAGCCAG | GAATCTCCTGGACCCGAGG |
Flow cytometry analysis
The annexin V-fluorescence isothiocyanate (FITC)/PI apoptosis detection kit (BD Biosciences) was used to assess apoptosis. After 48 hours' drug incubation, the cells from each sample (1×105) were re-suspended in 200μl of staining buffer and mixed with 10μl of annexin V-FITC for 15 min. After adding 200μl staining buffer and 10μl PI, flow cytometry was performed to analyze the percentage of apoptotic cells [18, 19].
Invasion assays
The endometrial cancer cells (1×105) were seeded in the top well of a Matrigel-coated invasion chamber (BD Biosciences) in DMEM containing 0.1% FBS with or without pharmacological agents (Tetrodotoxin, veratridine or PF-05089771). The bottom well was filled with 750μl DMEM containing 10% FBS as a chemoattractant. After 6-48 hour, non-invading cells were scraped from the upper side of the insert using a cotton swab. Invading cells on the bottom of the insert were fixed and stained with Diff-Quick Stain (IMEB Inc., USA) according to manufacturer's instructions [20-22]. The total number of invading cells was counted for each insert under a light microscope (Nikon Corporation, Japan).
Data analysis
All data are presented as the means ± standard error of the mean. The n value denotes the number of independent experiments conducted. Significance between means was determined using either the two-tailed Student's paired t-test or one-way analysis of variance with Dunnett's multiple comparisons test. Kaplan-Meier and log rank tests were used to assess differences in overall survival or disease-specific survival by Nav1.7-High vs Nav1.7-Low. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of Nav α subunits in endometrial cancer and nonneoplastic endometrial samples
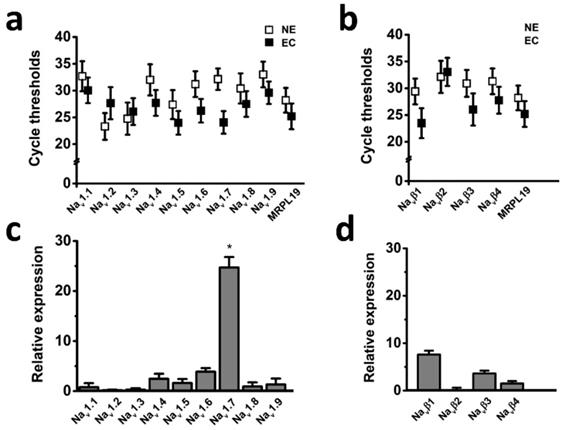
The voltage-gated sodium channel (Nav) has been shown to play important roles in cancer development and progression [5]; however, it has not been known whether Nav expression level has relationships with endometrial tumor malignancy. To test this possibility, we examined the mRNA expression level of Nav in endometrial cancer specimens. Transcriptional levels of the all Nav α and β subunits were determined by real time-PCR in the six cases of endometrial cancer with pair tissues of carcinoma (EC) and adjacent nonneoplastic tissue (NE), the mitochondrial ribosomal protein L19 (MRPL19) was introduced as the reference gene to normalize Nav subtype gene expression [23]. The cycle threshold values were plotted to compare gene expression, and the real time-PCR analyses revealed that Nav1.7 was the most highly expressed Nav subtype in the tissues (Fig. 1), and relative mRNA expression of Nav1.7 in EC biopsies were approximately 25-fold higher than in NE samples (Fig. 1c), indicating that overexpression of Nav1.7 was associated with endometrial tumorigenesis.
Nav1.7 expression was associated with endometrial cancer metastasis and clinical outcome
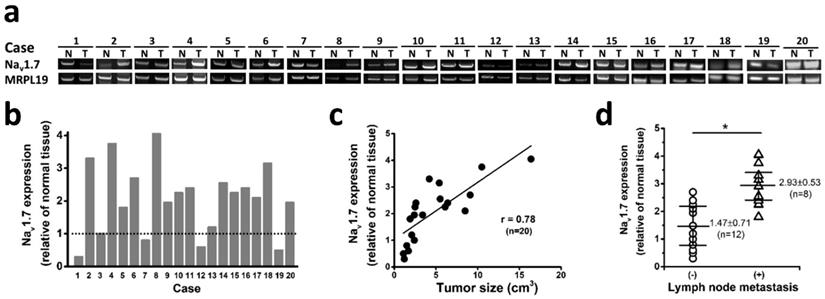
To investigate if Nav1.7 expression has clinical significance in tumor progression in endometrial cancer, we analyzed 20 sets of endometrial cancers with pair tissues of EC and adjacent NE tissue, and found that 75% cases (15 of 20 endometrial cancer) expressed significantly elevated level of Nav1.7 expression compared with paired adjacent normal tissue (Fig. 2a-b). Nav1.7 expression was downregulated in 4 cases, one possible reason is due to heterogeneity or individual difference in patients. More importantly, the Nav1.7 expression level was closely correlated with tumor size (Fig. 2c), a crucial indicator for the state of disease progression in human endometrial cancer [24]. In addition, the level of Nav1.7 expression in tumor tissues was significantly higher in the group of local lymph node metastasis (Fig. 2d).
Expression levels of Nav subunits in endometrial cancer with pair tissues of carcinoma (EC) and adjacent nonneoplastic tissue (NE). a-b Mean cycle threshold value of Nav α subunits (a), Nav β subunits (b), and housekeeping gene MRPL19 in primary EC biopsies (black boxes, n=6) and NE samples (white boxes, n=6). c-d Real-time quantitative PCR of Nav α (c) and β subunits (d) mRNA levels fold changes in EC and NE samples, bars showed the average fold-change ratios of Nav α or β subunits gene expression levels between EC and NE tissues for the indicated Nav subunits. MRPL19 was used as the reference gene to normalize Nav subunits gene expression (n=6, *P<0.05).
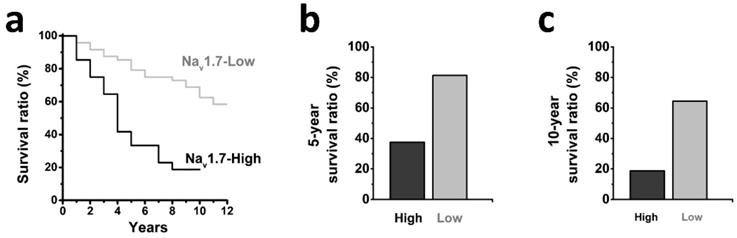
We further determined the association between tumor expression of Nav1.7 and clinical outcome of patients with endometrial cancer (Fig. 3a), and observed that patients with high-level tumor expression of Nav1.7 exhibited a shorter 5-year and 10-year survival ratio as compared with the Nav1.7-low group (38% vs 81% and 19% vs 62%, respectively) (Fig. 3b-c).
Nav1.7 involved in endometrial cancer apoptosis
We next asked whether Nav1.7 activities contribute to the development of endometrial cancer, we tested effects of veratridine and PF-05089771 on endometrial cancer cells. Veratridine is a Nav1.7 activator [25], it was able to induce persistent Nav1.7 currents [26], and inhibited channel inactivation and generated enhanced window currents. PF-05089771 was previously identified as a state-dependent Nav1.7 specific inhibitor interacting with Nav1.7 voltage-sensor domain of domain IV [27, 28]. We used flow cytometry analysis to investigate the consequences of veratridine and PF-05089771 on the cancer cell apoptosis. The cells were divided into three groups as shown in Suppl. Fig. 1a-c. The results showed that PF-05089771 were able to increase the number of early and late apoptotic cells, whereas veratridine reduced late apoptosis (Suppl. Fig. 1d), indicating that the Nav1.7 may have a critical role in endometrial cancer development.
Endometrial cancer invasion is mediated by Nav1.7
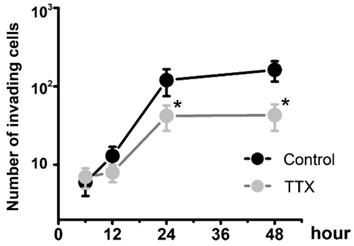
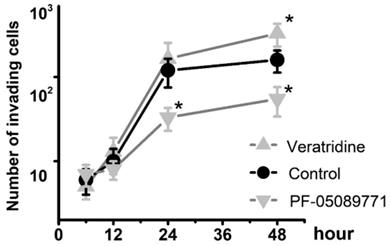
To determine whether Nav1.7 sodium channel participates in metastatic cell behaviors, the invasion assays were performed with endometrial cancer cells. The role of sodium channel in EC cells was assessed using the specific blocker Tetrodotoxin (TTX). The results revealed that TTX attenuated the relative invasiveness of EC cells. As shown in Fig. 4, the treatment with 10 µM TTX for 24 hours significantly decreased the number of invading cells. We then introduced veratridine and PF-05089771 to test roles of Nav1.7 in EC. The results revealed that 100µM veratridine increased invasion over 48-hour time period compared to control (Fig. 5). On the contrary, PF-05089771 significantly attenuated the relative invasiveness of EC cells, treatment with 100µM PF-05089771 remarkably reduced number of invading cells (Fig. 5). These results, together with the data that patients with local lymph node metastasis have higher level of Nav1.7 expressions (Fig. 2d), showed that the Nav1.7 plays a critical role in EC metastatic behaviors. And the effects of veratridine on cancer cell invasion were due to enhancement of Nav1.7 activities, whereas blockade of Nav1.7 by PF-05089771, attenuated endometrial cancer cell invasion.
Nav1.7 expressions associated with endometrial cancer metastasis and clinical outcome. a Nav1.7 expressions were determined in EC and NE tissues from 20 endometrial cancer patients. N and T, nonneoplastic endometrial tissues, and tumor areas of the same endometrial cancer patients, respectively. b Nav1.7 expression level in tumor biopsies. Nav1.7 level in adjacent normal tissues were used as control, housekeeping gene MRPL19 was used as the reference gene to normalize Nav1.7 expressions. c The association between Nav1.7 expression level and tumor size in the same surgical biopsies of endometrial cancer patients (r=0.78, n=20, P<0.05). d The tumor expression level of Nav1.7 was higher in the patients with local lymph node metastasis (2.93 ± 0.53, n=8) than those without lymph node metastasis (1.47 ± 0.71, n=12, *P<0.05).
a Kaplan-Meier analyses showing the correlation between the levels of Nav1.7 and the overall survival of patients with endometrial cancer (Nav1.7-High, n=38; Nav1.7-Low, n=21; P<0.05, log-rank test). b-c High Nav1.7 expression correlated with decreased survival in endometrial cancer, the 5-year survival ration (b) was decreased in Nav1.7-High group (38%) compared with Nav1.7-Low group (81%), and the 10-year survival ration (c) was decreased in Nav1.7-High group (19%) compared with Nav1.7-Low group (62%).
Endometrial cancer invasion is mediated by voltage-gated sodium channel. Total number of invading cancer cells was in the absence (Control) or presence of 10 µM TTX over the time period (from 6 to 48 hours). Data were from 6 independent experiments in each group, shown were means ± SEM. *P<0.05 versus Control.
Total number of invading cells was increased in the presence of 100µM veratridine and attenuated in the presence of 100µM PF-05089771 over the time period. Data were from 6 independent experiments in each group, shown were means ± SEM. *P<0.05 versus Control.
Discussion
This is the first study revealing the connection between voltage-gated sodium channels and endometrial cancer, examining the role of Nav1.7 sodium channel in this type of cancer.
Ion channels were well known to play significant roles in the growth and migration of cancer cells and contribute to multiple aspects and stages of cancer progress [3]. There were several ion channels implicated in endometrial cancer. The hERG K+ channels were found to be expressed with a higher frequency in primary human endometrial cancer compared to non-cancerous tissues [29]. The Ca2+ channel Cav1.3 required for estrogen-stimulated Ca2+ influx contributed broadly to the development of endometrial cancer [30]. A recent study reported that volume-activated Cl- channel play roles in endometrial tumor invasion and migration [31]. Compared to Ca2+, K+, and Cl- channel, however, the role of voltage-gated Na+ channel in the endometrial cancer remains unknown.
The voltage-gated Na+ channel has been established to be associated with metastatic cell behavior in cancer [5, 32, 33], several Na+ channel isoforms were identified to be expressed in different cancers, these included Nav1.5 in breast and colon cancers [6, 7], Nav1.6 in cervical cancer [8], and Nav1.8 in prostate cancer [9]. In this study, we characterized Nav isoform in human endometrial cancer. We used real-time-PCR to determine transcriptional levels of sodium channel α and β subunits, and discovered that Nav1.7 α subunit in EC samples were around 25-fold higher than in NE biopsies, Nav1.7 overexpression in tumor tissue was noted in 75% cases of endometrial cancer. More importantly, the level of Nav1.7 expression was significantly associated with tumor size and survival in tumor tissues. We showed that Nav1.7 associated with endometrial cancer development, the Nav1.7 activator veratridine reduced endometrial tumor cell apoptosis and promoted cancer invasion, and inhibition of Nav1.7 by PF-05089771 increase the number of apoptotic cells and attenuated invasive potential of cancer cells.
There are several theories regarding how Nav contribute to tumor progression. One explanation is that function upregulation of Nav, consequently activate the Na+/H+ exchanger (NHE) and enhance H+ efflux, thus leading to increased intracellular alkalinisation and decreased extracellular pH. In cancer cells, increased glycolytic metabolism gives rise to an excessive production of intracellular acidity; as a result, intracellular alkalinisation potentially facilitates cancer metabolism [34]. Another theory proposed that Nav could activate Na+/Ca2+ exchanger (NCX), leading to the entry of Ca2+ through the NCX, which induces Ca2+-dependent signaling to promote cancer cell proliferation and metastasis [35, 36]. In this study, Nav1.7 activator veratridine and inhibitor PF-05089771 affected endometrial cancer apoptosis and invasion, indicating that Nav1.7 have crucial roles for endometrial cancer progression. However, whether Nav1.7 activated NHE to increase H+ efflux to provide a favorable environment for endometrial tumor invasion, or induced Ca2+-dependent signaling by stimulating NCX activity to accelerate development of endometrial cancer, remain unclear; further studies are required to address these uncertainties.
In summary, the present study established a relationship between voltage-gated sodium channel protein and endometrial cancer. The Nav1.7, functionally expressed in the endometrial cancer, has strong links with clinical outcome. Its activity significantly contributes endometrial tumor progression. These findings highlight the importance of Nav1.7 in cancer development, and may provide novel insights into early detection and chemotherapeutics for endometrial cancer.
Abbreviations
Nav: voltage-gated sodium channel; Nav1.7: voltage-gated sodium channel α-subunit encoded by the SCN9A gene; EC: endometrial cancer tissues; NE: nonneoplastic endometrial samples; TTX: Tetrodotoxin.
Supplementary Material
Supplementary figure.
Acknowledgements
The authors would like to thank Dr M. He (Sun Yat-Sen University, Guangzhou, Guangdong, China) for pathological work, and other members of the laboratory for insightful comments on the manuscript.
Funding
The present study was supported by the National Natural Science Foundation (No. 81502226 and 81872128) and by the Natural Science Foundation of Shandong Province (ZR2019MH040) and grant for the Key Laboratory of Higher Education Institutes of Shandong Province, China.
Author Contributions
L.H. and S.Y. conceived the project and designed experiments. J.L., H.T., W.Y. performed research; J.L., H.T., W.Y., S.Y. and L.H. analyzed the data; L.H. and J.L. wrote the paper. All authors read and edited the manuscript. All authors approved the manuscript in its current form.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 2017;29:47-58
2. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094-108
3. Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015;1848:2685-702
4. Duclohier H. Structure-function studies on the voltage-gated sodium channel. Biochim Biophys Acta. 2009;1788:2374-9
5. Besson P, Driffort V, Bon E, Gradek F, Chevalier S, Roger S. How do voltage-gated sodium channels enhance migration and invasiveness in cancer cells? Biochim Biophys Acta. 2015;1848:2493-501
6. Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF. et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381-9
7. House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T. et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957-67
8. Hernandez-Plata E, Ortiz CS, Marquina-Castillo B, Medina-Martinez I, Alfaro A, Berumen J. et al. Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. Int J Cancer. 2012;130:2013-23
9. Suy S, Hansen TP, Auto HD, Kallakury BV, Dailey V, Danner M. et al. Expression of Voltage-Gated Sodium Channel Nav1.8 in Human Prostate Cancer is Associated with High Histological Grade. J Clin Exp Oncol. 2012;1:1-5
10. Qu M, Zhu Y, Jin M. MicroRNA-138 inhibits SOX12 expression and the proliferation, invasion and migration of ovarian cancer cells. Exp Ther Med. 2018;16:1629-38
11. Li W, Zhang H, Yang L, Wang Y. Cancerous inhibitor of protein phosphatase 2A regulates cisplatin resistance in ovarian cancer. Oncol Lett. 2019;17:1211-6
12. Bao Y, Zhang S, Guo Y, Wei X, Zhang Y, Yang Y. et al. Stromal expression of JNK1 and VDR is associated with the prognosis of esophageal squamous cell carcinoma. Clin Transl Oncol. 2018;20:1185-95
13. Fu JD, Yao JJ, Wang H, Cui WG, Leng J, Ding LY. et al. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1alpha and VEGF under a hypoxic state. Eur Rev Med Pharmacol Sci. 2019;23:155-61
14. Xu BB, Gu ZF, Ma M, Wang JY, Wang HN. MicroRNA-590-5p suppresses the proliferation and invasion of non-small cell lung cancer by regulating GAB1. Eur Rev Med Pharmacol Sci. 2018;22:5954-63
15. Liu Z, Jiang L, Zhang G, Li S, Jiang X. MiR-24 promotes migration and invasion of non-small cell lung cancer by targeting ZNF367. J BUON. 2018;23:1413-9
16. Wang W, Dong J, Wang M, Yao S, Tian X, Cui X. et al. miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncol Lett. 2018;15:6131-6
17. Bao Y, Guo Y, Yang Y, Wei X, Zhang S, Zhang Y. et al. PRSS8 suppresses colorectal carcinogenesis and metastasis. Oncogene. 2019;38:497-517
18. Zhang J, Geng H, Liu L, Zhang H. Synergistic cytotoxicity of homoharringtonine and etoposide in acute myeloid leukemia cells involves disrupted antioxidant defense. Cancer Manag Res. 2019;11:1023-32
19. Qi Y, Li J. Triptolide inhibits the growth and migration of colon carcinoma cells by down-regulation of miR-191. Exp Mol Pathol. 2019;107:23-31
20. Dong J, Wang M, Ni D, Zhang L, Wang W, Cui X. et al. MicroRNA-217 functions as a tumor suppressor in cervical cancer cells through targeting Rho-associated protein kinase 1. Oncol Lett. 2018;16:5535-42
21. Zhang W, Sun J, Chen J, Xu C, Zhang L. Downregulation of miR-95 in gastric cancer promotes EMT via regulation of Slug, thereby promoting migration and invasion. Oncol Rep. 2019;41:1395-403
22. Lin X, Liu X, Gong C. Expression of engrailed homeobox 2 regulates the proliferation, migration and invasion of non-small cell lung cancer cells. Oncol Lett. 2018;16:536-42
23. Ayakannu T, Taylor AH, Willets JM, Brown L, Lambert DG, McDonald J. et al. Validation of endogenous control reference genes for normalizing gene expression studies in endometrial carcinoma. Mol Hum Reprod. 2015;21:723-35
24. Todo Y, Watari H, Okamoto K, Hareyama H, Minobe S, Kato H. et al. Tumor volume successively reflects the state of disease progression in endometrial cancer. Gynecol Oncol. 2013;129:472-7
25. Vetter I, Mozar CA, Durek T, Wingerd JS, Alewood PF, Christie MJ. et al. Characterisation of Na(v) types endogenously expressed in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 2012;83:1562-71
26. Tsukamoto T, Chiba Y, Nakazaki A, Ishikawa Y, Nakane Y, Cho Y. et al. Inhibition of veratridine-induced delayed inactivation of the voltage-sensitive sodium channel by synthetic analogs of crambescin B. Bioorg Med Chem Lett. 2017;27:1247-51
27. Chernov-Rogan T, Li T, Lu G, Verschoof H, Khakh K, Jones SW. et al. Mechanism-specific assay design facilitates the discovery of Nav1.7-selective inhibitors. Proc Natl Acad Sci U S A. 2018;115:E792-E801
28. Theile JW, Fuller MD, Chapman ML. The Selective Nav1.7 Inhibitor, PF-05089771, Interacts Equivalently with Fast and Slow Inactivated Nav1.7 Channels. Mol Pharmacol. 2016;90:540-8
29. Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L. et al. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer. 2000;83:1722-9
30. Hao J, Bao X, Jin B, Wang X, Mao Z, Li X. et al. Ca2+ channel subunit alpha 1D promotes proliferation and migration of endometrial cancer cells mediated by 17beta-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015;29:2883-93
31. Li M, Wu DB, Wang J. Effects of volume-activated chloride channels on the invasion and migration of human endometrial cancer cells. Eur J Gynaecol Oncol. 2013;34:60-4
32. Patel F, Brackenbury WJ. Dual roles of voltage-gated sodium channels in development and cancer. Int J Dev Biol. 2015;59:357-66
33. Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin). 2012;6:352-61
34. Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E. et al. NaV1.5 Na(+) channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835-42
35. Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL. et al. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113-28
36. Guo Y, Bao Y, Guo D, Yang W. Pregnancy-associated plasma protein a in cancer: expression, oncogenic functions and regulation. Am J Cancer Res. 2018;8:955-63
Author contact
![]() Corresponding authors: Liang Hong, Institute of Precision Medicine, Jining Medical University, Jining, China, 272067. Tel.: +86-0537-361-6566, Fax: +86-0537-361-6566, E-mail: lianghongjnmc.edu.cn or Shuzhong Yao, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, 510080. Tel.: +86-20-8733-2686, Fax: +86-20-8733-2686, E-mail: yaoshuzhsysu.edu.cn
Corresponding authors: Liang Hong, Institute of Precision Medicine, Jining Medical University, Jining, China, 272067. Tel.: +86-0537-361-6566, Fax: +86-0537-361-6566, E-mail: lianghongjnmc.edu.cn or Shuzhong Yao, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, 510080. Tel.: +86-20-8733-2686, Fax: +86-20-8733-2686, E-mail: yaoshuzhsysu.edu.cn

 Global reach, higher impact
Global reach, higher impact