Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(26):6584-6593. doi:10.7150/jca.32407 This issue Cite
Research Paper
Prognostic value of ABO blood group in a Chinese population in Northwest China region with curatively resected rectal cancer
1. State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi'an, Shaanxi, China.
2. Department of radiotherapy, Xijing Hospital, Fourth Military Medical University, Xi'an, Shaanxi, China.
* These authors contributed equally to this work.
Received 2018-12-19; Accepted 2019-6-13; Published 2019-10-21
Abstract
A positive association between the ABO blood types and survival has been suggested in several malignancies. However, little is known about the relationship between ABO blood group and survival in rectal cancer patients. The aim of this study was to assess the role of the ABO blood types in predicting the prognosis of a Chinese population in Northwest China region with curatively resected rectal cancer. We retrospectively analyzed 1613 consecutive patients who underwent curative surgery for rectal cancer between June, 2011 and December, 2016. The relationship between the ABO blood types and overall survival (OS) was analyzed. The median follow-up period of the 1613 rectal cancer patients was 69.6 months with 1427 alive. There was a significance difference of survival among ABO blood groups (P=0.007). The mean overall survival (OS) of the blood type B patients was 70.8 months, O was 64.3, whereas the mean OS of the AB and A blood type patients was significantly lower, 58.4 months and 59.7 months respectively (P=0.007, log-rank test). Compared with patients with A and AB blood types, patients with blood type B and O were more likely to have better survival(P=0.001). A blood groups were associated with significantly decreased overall survival in rectal cancer patients (hazard ratio = 1.263; 95% confidence interval = 0.776-2.054, P =0.010). In order to confirm our above results, we performed the same investigation in an independent cohort from another hospital of 505 Chinese patients and get the similar results. Our study showed that ABO blood group is associated with survival in Northwest Chinese patients with rectal cancer and the blood type B and O were favourable prognostic factors for patients with rectal cancer.
Keywords: ABO blood group, rectal cancer, retrospective cohort, survival
Introduction
Colorectal cancer is the third-most commonly diagnosed cancer in males and the second-most common in female [1]. Each year, more than 1.2 million new cases of colorectal cancer are diagnosed worldwide [2]. Previously, colorectal cancer has its highest incidence in Western Europe and North America, but recently, the mortality and morbidity of colorectal cancer rapidly grows in the Chinese population in Northwest China region. According to the latest Cancer Statistics of China, colorectal cancer is the fifth newly diagnosed cancer among men, and the fourth among women in China [3]. Among those colorectal cancer patients, rectal cancer represents 40 percent of colorectal cancers. While curative surgery is the only option for long term survival, rectal cancer spreads more frequently to the thoracic organs, bone and nervous system and approximately 20% of rectal cancer patients lose opportunity for radical surgery on account of metastases [4, 5].
Although prognostic factors for rectal cancer have conducted intensive studies, including in the field of molecular biology, but for the prognosis of rectal cancer can be different despite similar stages and grades [6]. A better understanding of an ideal biomarker with readily available, inexpensive and reproducible of rectal cancer could improve the prognosis of patients and provide appropriate therapy strategies. Recently, the correlation between the ABO blood type and other malignancies, such as breast cancer, pancreatic cancer, lung cancer, esophageal squamous cell carcinoma, colon cancer, nasopharyngeal carcinoma, and obstetric cancers, has been continuously reported [7-14].
Previously Hamed et al. [15] enrolled 1025 colorectal cancer patients in two large prospective cohorts and observed the relationship between ABO blood group and risk of colorectal cancer, their results showed that the ABO blood group didn't have any association with risk of colorectal cancer. Well-known, many genetic and environmental risk factors were defined for cancer, including smoking, obesity, a higher-fat diet, rectal polyp and a family history. However, up to now, studies of the impact of ABO blood group on the survival of the Chinese population in Northwest China region with rectal cancer remained uncertain. Therefore, the aim of this retrospective analysis was to analyze the relationship between ABO blood type and the survival of rectal cancer patients in a Chinese population in Northwest China region as there is an interpopulation variation for this condition.
Material and Methods
Patient selection
The retrospective study included 1613 patients who were diagnosed with rectal adenocarcinoma and treated surgically between June, 2011 and December, 2016 at Division of Gastrointestinal Surgery, First Affiliated Hospital of Air Force Military Medical University. Enrolled patients were histologically confirmed and without distant metastasis. Patients with one of the following features, (stage IV) rectal cancer, with more than one primary cancer, with R1 or R2 resection, or death from postoperative complications, were excluded from our study. Other patients with missing data were also excluded. Patients were considered eligible only when the following data were available. Tumor differentiation grades were defined according to the World Health Organization criteria. Cancer staging was based on the American Joint Committee on Cancer Staging system (AJCC, 2002; Greene, American Joint Committee on Cancer, American Cancer Society, 2002). The study was approved by the ethics committee of First Affiliated Hospital of Air Force Military Medical University. All patients provided written consent for storage of their information in the hospital database, and for the research use of the information.
Follow-up and outcome
Each patient was followed up periodically until death or April 2017(every 3 mouth for the first 2 y, and every 6 mouth up to the fifth year) after surgery. The follow-up cycles varied from 3-6mo, with a median of 69.6 months. The follow-up visits consisted of a physical examination and laboratory studies at least every 6 months or when clinically indicated. The endpoint of the study was overall survival (OS). OS was calculated as the period from the date of diagnosis to the date of death from any cause or the date of last follow-up. Survival status was verified again using the best available methods, including checking clinical attendance records and direct telecommunication with the patients or their families.
A validation cohort
An independent cohort included 505 rectal adenocarcinoma patients who were diagnosed and treated surgically between December, 2012 and December, 2015 at Division of Gastrointestinal Surgery, Second Affiliated Hospital of Air Force Military Medical University, were used for confirm the above results. The admission and exclusion condition of the enrolled patients were the same as before. All patients provided written consent for storage of their information in the hospital database, and for the research use of the information. Each patient was followed up periodically until death or April 2017 (every 3 mouth for the first 2 y, and every 6 mouth up to the fifth year) after surgery. The follow-up cycles varied from 3-6mo, with a median of 59.6 months. The follow-up visits consisted of a physical examination and laboratory studies at least every 6 months or when clinically indicated. The endpoint of the study was overall survival (OS). OS was calculated as the period from the date of diagnosis to the date of death from any cause or the date of last follow-up. Survival status was verified again using the best available methods, including checking clinical attendance records and direct telecommunication with the patients or their families.
Statistical analysis
Chi-square test was used to compare categorical variables. ANOVA test was used to compare continuous variable, if they have no homogeneity of variance, then Kruskal Wallis Test will be used. The survival rates were evaluated by Kaplane-Meier survival analysis, and their significance was calculated by the log-rank test. Univariate and multivariate Cox regression analyses were performed. Multivariable analyses were performed for factors which were significantly associated with OS in univariate analyses. All the statistical analyses were conducted using IBM SPSS 20.0 software. A P value <0.05 was considered to be statistically significant.
Results
ABO blood group and clinicopathologic characteristics of the rectal cancer patients
A total of 1613 eligible patients with rectal cancer were analyzed at the gastrointestinal surgery in First Affiliated Hospital of Air Force Military Medical University from June, 2011 to December, 2016. Clinicopathologic characteristics of all subjects stratified by ABO blood group were displayed in Table 1. Based on the seventh edition of the TNM-UICC/AJCC classification system, the numbers of patients with stage I, II, and III disease were 439 (27.2%), 427 (26.5%), and 747(46.3%), respectively. The median age of the patients was 59 years (range, 19-86 years). Among the 1613 subjects, 411 (25.5%) were blood group A, 502 (31.1%) were blood group B, 545(33.8%) were blood group O, and the remaining 155 (9.6%) were blood group AB. No significant difference was found regarding gender, age, tumor size, tumor location, BMI, degree of differentiation, lymphatic/vascular invasion, perineural invasion, N stage, smoking history, drinking history, CEA level, CA199 level and CA125 level.
Association between the clinical prognosis of rectal cancer patients and ABO blood groups
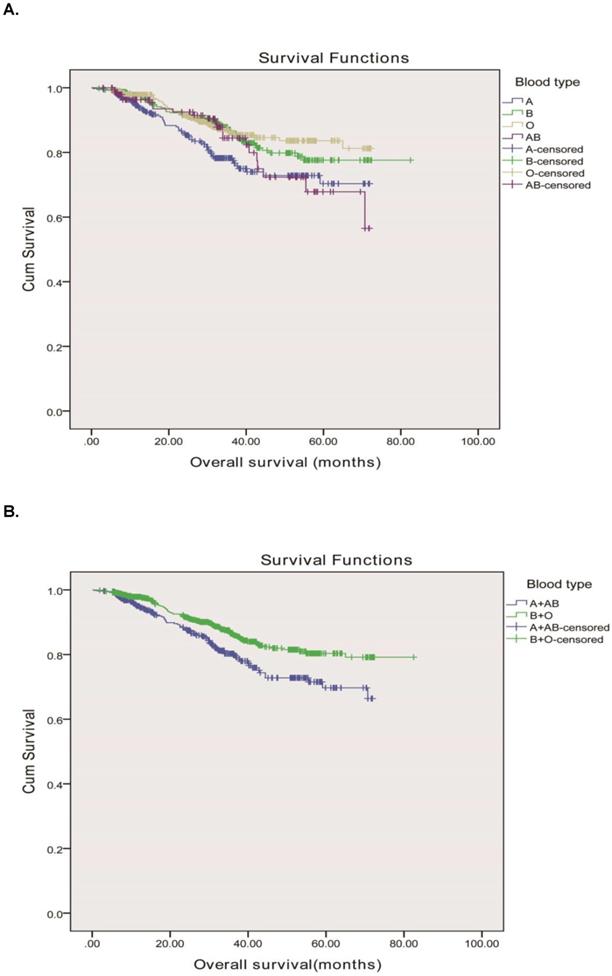
The median follow-up period of the 1613 rectal cancer patients was 69.6 months with 1427 (88.5%) alive and 186 (11.5%) dead from cancer-related diseases at the final clinical follow-up. The 5-year overall survival rates for rectal adenocarcinoma patients with the A, B, O and AB blood types were 84.9%, 89.2%, 91.2% and 85.8%, respectively. The ABO blood groups were closely associated with OS according to the Kaplane-Meier analysis (P=0.007) Figure 1A. OS was longer in rectal cancer patients with the group B (median, 70.8mo, 95% CI, 67.9-73.6 months) than in those with the group A (median, 58.4mo, 95% CI, 55.4-61.3 months), group O (median, 64.3mo, 95% CI, 62.2-66.5months), and group AB (median, 59.7mo, 95% CI, 55.2-64.2 months) (P = 0.007). Meanwhile, we found that the blood types B and O seemed to have a higher survival than blood types A and AB, therefore, we divided the whole group of patients into two subgroups. The subgroup analysis indicated that patients of blood group B and O had a longer OS compared to those of blood group A and AB (71.6 months vs. 58.8 months, P = 0.001) as shown in Figure 1B.
Then the relationship between ABO blood type and survival based on patients' clinicopathologic characteristics were examined. These analysis showed that ABO blood group could distinguish OS when stratified by gender (Female, P=0.014), age(<60, P=0.028), smoking history (no smoking, P=0.017) ,drinking history (no drinking, P=0.046), CEA (normal, P=0.007), CA199 (elevated, P=0.013), CA-125 (normal, P=0.004), tumor location (tumor height <6 cm, P=0.006),tumor size (≥3.4cm, P=0.009), pathology (well differentiated, P<0.001; poorly differentiated, P=0.014), perineural invasion (yes, P=0.023), vascular invasion (no, P=0.004), pT status (T1, P=0.026; T3, P=0.003), pN status (no, P=0.001).
To determine whether ABO blood type could serve as an independent prognostic factor, we examined OS using the Cox proportional hazards model. Univariate analysis was used to evaluated the influence of the patients' gender, age, body mass index(BMI),tumor location, pathology differentiation, lymphatic/vascular invasion, perineural invasion, T status, N status, TNM stage, smoking history, drinking history, ABO blood type, tumor size, serum CEA , CA199 and CA125 level on OS (Table 2). Our results showed that the poorly differentiated histology (P<0.001), lower BMI (P=0.007), lymphatic/vascular invasion (P<0.001), advanced pT stages (P <0.001), advanced pN stages (P<0.001), advanced tumor stages (P <0.001), ABO blood type (P=0.010) ,lower tumor height (P<0.001), bigger tumor size (P<0.001), serum CEA elevation (P = 0.011) , serum CA-199 elevation (P<0.001) and serum CA-125 elevation (P<0.001) were all significantly associated with shorter survival. Furthermore, the significant parameters in univariate analysis were also performed by multivariate analyses. The results revealed that lower BMI (P=0.031), lower tumor height (P=0.021), poorly differentiated histology (P=0.005), lymphatic/vascular invasion (P=0.017), advanced pN stages (P=0.029), ABO blood type (P=0.010), serum CA-199 elevation (P=0.022) and serum CA-125 elevation (P<0.001) were independent, significant predictors for OS (Table 2).
Overall survival, by ABO blood type, among patients in our study. A, Survival curve of the 1613 rectal adenocarcinoma patients according to the ABO blood groups. B, Survival curve of patients with blood types B+O and A + AB. We divided the whole group of patients into two subgroups, patients with B and O blood types and patients with A and AB blood groups. The two survival curve separated, and had significant difference. The 5-year overall survival was 90.3 vs. 85.2%, respectively, P = 0.001.
Validation in an independent cohort
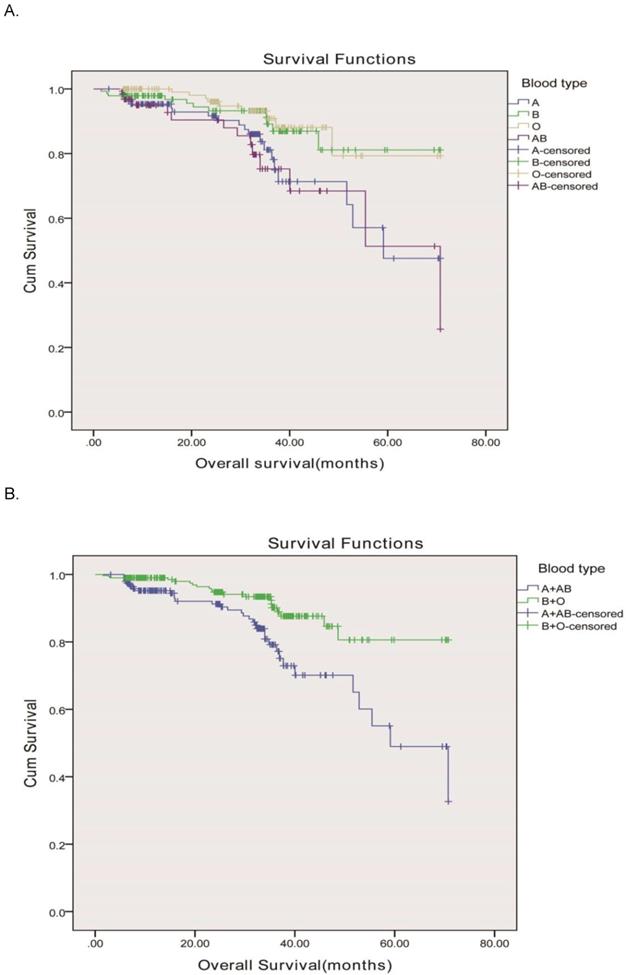
In order to validate our results, we used an independent cohort from another hospital for further confirm and the clinicopathologic characteristics of all subjects stratified by ABO blood group were displayed in Table 3. There were 505 enrolled rectal cancer patients, 287 (56.8%) male and 218 (43.2%) female, aged from 23-88 y (the median age was 69 y). Based on the seventh edition of the TNM-UICC/AJCC classification system, the numbers of patients with stage I, II, and III disease were 141 (27.9%), 124 (24.6%), and 240 (47.5%), respectively. The ABO blood groups were distributed as 140 patients with the blood group A, 142 patients with the blood group B, and 161 patients with the blood group O, and 62 patients with the blood group AB. Our results showed that the ABO blood groups were closely associated with OS according to the Kaplane-Meier analysis (P=0.003) Figure 2A. OS was longer in rectal cancer patients with the group O (median, 63.8mo, 95% CI, 59.1-68.7 months) than in those with the group B (median, 63.3mo, 95% CI, 58.9-67.7 months), group A (median, 54.7mo, 95% CI, 48.9-60.5months), and group AB (median, 54.3mo, 95% CI, 46.3-62.3 months) as shown in Figure 2A. Blood types B and O in this independent cohort also showed a higher survival than blood types A and AB. Therefore, we divided the whole group of patients into two subgroups. The subgroup analysis indicated that patients of blood group B and O also had a longer OS compared to those of blood group A and AB (63.7 months vs. 54.5months, P < 0.001) as shown in Figure 2B, similar to the results showed in our hospital.
Furthermore, this independent cohort analysis date also showed that the poorly differentiated histology (P=0.005), lymphatic/vascular invasion (P=0.003), advanced pT stages (P =0.042), advanced pN stages (P=0.006), advanced tumor stages (P =0.003), ABO blood type (P=0.006) ,lower tumor height (P=0.012), bigger tumor size (P=0.019), serum CA-199 elevation (P=0.038) and serum CA-125 elevation (P=0.023) were all significantly associated with shorter survival. Meanwhile, the significant parameters in univariate analysis were further analyzed in multivariate analyses. Our results showed that the ABO blood groups were independent predictors for OS (group A: hazard ratio [HR] = 0.832, 95% confidence interval [CI] = 0.416-1.666; group B: hazard ratio [HR] = 0.368, 95% confidence interval [CI] = 0.165-0.823; group O: hazard ratio [HR] = 0.299, 95% confidence interval [CI] = 0.128-0.699; group AB: hazard ratio [HR] = 1, 95% confidence interval [CI] = Reference). Our results also revealed that lower tumor height (P=0.004), poorly differentiated histology (P=0.002), lymphatic/vascular invasion (P=0.021), advanced pT stages (P =0.012), advanced pN stages (P=0.023), advanced tumor stages (P =0.015), ABO blood type (P=0.008) and serum CA-125 elevation (P=0.031) were independent, significant predictors for OS (Table 4). From this independent cohort, we got the similar conclusion, which further confirmed the ABO blood group is associated with survival in Chinese patients with rectal cancer and the blood type B and O were favourable prognostic factors for patients with rectal cancer.
Characteristics according to ABO blood type
| Patient characteristics | Total | ABO | P-values | |||
|---|---|---|---|---|---|---|
| A | B | O | AB | Pa | ||
| Total | 1613 | 411 | 502 | 545 | 155 | |
| Gender | 0.495 | |||||
| Male | 884 | 237 | 265 | 295 | 87 | |
| Female | 729 | 174 | 237 | 250 | 68 | |
| Age b(years) | 0.466 | |||||
| <60 | 807 | 225 | 250 | 266 | 66 | |
| ≥60 | 806 | 186 | 252 | 279 | 89 | |
| BMI b, kgm2 | 0.231 | |||||
| <18.5 | 134 | 39 | 41 | 46 | 8 | |
| 18.5-23.9 | 878 | 227 | 284 | 291 | 76 | |
| ≥24 | 601 | 145 | 177 | 208 | 71 | |
| Locationc | 0.970 | |||||
| tumour height >12 cm | 91 | 22 | 29 | 30 | 10 | |
| tumour height 6-12 cm | 667 | 174 | 201 | 224 | 68 | |
| tumour height <6 cm | 855 | 215 | 272 | 291 | 77 | |
| Pathology(adenocarcinoma) | 0.539 | |||||
| Well differentiated | 220 | 59 | 64 | 78 | 19 | |
| Moderately differentiated | 1200 | 292 | 383 | 406 | 119 | |
| Poorly differentiated | 193 | 60 | 55 | 61 | 17 | |
| Lymphatic/vascular invasion | 0.785 | |||||
| Yes | 547 | 139 | 168 | 192 | 48 | |
| No | 1066 | 272 | 334 | 353 | 107 | |
| PNI | 0.836 | |||||
| Yes | 1013 | 261 | 307 | 347 | 98 | |
| No | 600 | 150 | 195 | 198 | 57 | |
| pT status | 0.027 | |||||
| pT1 | 165 | 36 | 42 | 65 | 22 | |
| pT2 | 405 | 92 | 120 | 160 | 33 | |
| pT3 | 962 | 260 | 316 | 292 | 94 | |
| pT4 | 81 | 23 | 23 | 28 | 6 | |
| pN status | 0.057 | |||||
| pN0 | 829 | 198 | 259 | 295 | 77 | |
| pN1 | 517 | 141 | 171 | 148 | 57 | |
| pN2 | 267 | 72 | 72 | 102 | 21 | |
| pTNM stage | 0.043 | |||||
| Stage I | 439 | 102 | 122 | 174 | 41 | |
| Stage II | 427 | 104 | 152 | 130 | 41 | |
| Stage III | 747 | 205 | 228 | 241 | 73 | |
| Smoking history | 0.618 | |||||
| Yes | 461 | 112 | 138 | 167 | 44 | |
| No | 1152 | 299 | 364 | 378 | 111 | |
| Drinking history | 0.222 | |||||
| Yes | 331 | 80 | 91 | 125 | 35 | |
| No | 1282 | 331 | 411 | 420 | 120 | |
| Tumor size | A | B | O | AB | 0.253 | |
| ≥3.4 | 272 | 315 | 326 | 96 | ||
| <3.4 | 139 | 187 | 219 | 59 | ||
| CEA | 0.875 | |||||
| Normal | 1098 | 281 | 347 | 364 | 106 | |
| Elevated | 515 | 130 | 155 | 181 | 49 | |
| CA199 | 0.231 | |||||
| Normal | 1461 | 363 | 457 | 496 | 145 | |
| Elevated | 152 | 48 | 45 | 49 | 10 | |
| CA125 | 0.880 | |||||
| Normal | 1561 | 396 | 485 | 530 | 150 | |
| Elevated | 52 | 15 | 17 | 15 | 5 | |
Abbreviations: BMI=body mass index; pN status=pathological node status; pT statue=pathological tumour status; pTNM status=pathological tumour-node-metastasis stage.
a: χ2 test (A blood type vs B blood type vs O blood type vs AB blood type).
b: Chinese definition.
c: NCOTARGET. 2015;6(34):36884-9.
Univariate and multivariate Cox regression analysis for overall survival in patients with rectal cancer
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Pa | HR | 95% CI | Pa | |
| Gender | 0.188 | — | ||||
| Male | 1.00 | Reference | — | — | ||
| Female | 0.819 | 0.609-1.102 | — | — | ||
| Ageb (years) | 0.472 | — | ||||
| <60 | 1.111 | 0.833-1.482 | — | — | ||
| ≥60 | 1 | Reference | — | — | ||
| BMIb, kgm2 | 0.007 | 0.031 | ||||
| <18.5 | 2.168 | 1.369-3.432 | 1.808 | 1.127-2.899 | ||
| 18.5-23.9 | 1.197 | 0.864-1.657 | 1.045 | 0.751-1.454 | ||
| ≥24 | 1 | Reference | 1 | Reference | ||
| Locationc | 0.050 | 0.021 | ||||
| tumor height >12 cm | 0.888 | 0.465-1.695 | 0.826 | 0.429-1.594 | ||
| tumor height 6-12 cm | 0.685 | 0.504-0.932 | 0.639 | 0.465-0.877 | ||
| tumor height <6 cm | 1 | Reference | 1 | Reference | ||
| Pathology (adenocarcinoma) | <0.001 | 0.005 | ||||
| Well differentiated | 0.265 | 0.161-0.437 | 0.544 | 0.315-0.940 | ||
| Moderately differentiated | 0.346 | 0.243-0.492 | 0.537 | 0.369-0.782 | ||
| Poorly differentiated | 1 | Reference | 1 | Reference | ||
| lymphatic/vascular invasion | <0.001 | 0.017 | ||||
| Yes | 1 | Reference | 1 | Reference | ||
| No | 0.399 | 0.299-0.534 | 0.656 | 0.464-0.927 | ||
| PNI | 0.387 | — | ||||
| Yes | 1 | Reference | — | — | ||
| No | 0.879 | 0.655-1.179 | — | — | ||
| pT status | <0.001 | 0.092 | ||||
| pT1 | 0.196 | 0.085-0.452 | 0.380 | 0.25-1.159 | ||
| pT2 | 0.252 | 0.137-0.465 | 0.394 | 0.174-0.893 | ||
| pT3 | 0.662 | 0.405-1.083 | 0.927 | 0.551-1.561 | ||
| pT4 | 1 | Reference | 1 | Reference | ||
| pN status | <0.001 | 0.029 | ||||
| pN0 | 1.00 | Reference | 1.00 | Reference | ||
| pN1 | 2.093 | 1.468-2.985 | 0.369 | 0.125-1.087 | ||
| pN2 | 4.502 | 3.138-6.458 | 0.628 | 0.432-0.914 | ||
| pTNM stage | <0.001 | 0.651 | ||||
| Stage I | 0.253 | 0.162-0.395 | 1.624 | 0.463-5.703 | ||
| Stage II | 0.441 | 0.306-.0637 | 1.112 | 0.385-3.216 | ||
| Stage III | 1.00 | Reference | 1.00 | Reference | ||
| Smoking history | 0.930 | — | ||||
| Yes | 1.014 | 0.739-1.392 | — | — | — | |
| No | 1.00 | Reference | — | — | ||
| Drinking history | 0.479 | — | ||||
| Yes | 1.134 | 0.804-1.600 | — | — | ||
| No | 1.00 | Reference | — | — | ||
| ABO blood type | 0.010 | 0.013 | ||||
| A | 1.263 | 0.776-2.054 | 1.186 | 0.720-1.954 | ||
| B | 0.773 | 0.471-1.269 | 0.709 | 0.429-1.172 | ||
| O | 0.696 | 0.420-1.152 | 0.689 | 0.412-1.152 | ||
| AB | 1 | Reference | 1 | Reference | ||
| Tumor size(cm) | <0.001 | — | — | 0.060 | ||
| <3.4 | 0.525 | 0.377-0.731 | 0.714 | 0.503-1.014 | ||
| ≥3.4 | 1 | Reference | 1 | Reference | ||
| Serum CEA | 0.011 | 0.509 | ||||
| Normal | 0.680 | 0.508-0.912 | 0.902 | 0.902-0.663 | ||
| Elevated | 1 | Reference | 1 | Reference | ||
| CA19-9 | <0.001 | 0.022 | ||||
| Normal | 0.408 | 0.283-0.590 | 0.631 | 0.426-0.934 | ||
| Elevated | 1 | Reference | 1 | Reference | ||
| CA125 | <0.001 | <0.001 | ||||
| Normal | 0.299 | 0.184-0.486 | 0.287 | 0.174-0.474 | ||
| Elevated | 1 | Reference | 1 | Reference | ||
Abbreviations: BMI=body mass index; 95% CI=95% confidence interval; HR=hazard ratio; pTNM stage=pathological tumour-node-metastasis stage.
a: Cox proportional hazards model.
b: Chinese definition.
c: NCOTARGET. 2015;6(34):36884-9.
Characteristics according to ABO blood type
| Patient characteristics | Total | ABO | P-values | |||
|---|---|---|---|---|---|---|
| A | B | O | AB | Pa | ||
| Total | 505 | 140 | 142 | 161 | 62 | |
| Gender | 0.770 | |||||
| Male | 287 | 82 | 83 | 86 | 36 | |
| Female | 218 | 58 | 59 | 75 | 26 | |
| Age b(years) | 0.054 | |||||
| <60 | 233 | 71 | 59 | 82 | 21 | |
| ≥60 | 272 | 69 | 83 | 79 | 41 | |
| BMI b, kgm2 | 0.721 | |||||
| <18.5 | 36 | 9 | 13 | 10 | 4 | |
| 18.5-23.9 | 282 | 83 | 81 | 87 | 31 | |
| ≥24 | 187 | 48 | 48 | 64 | 27 | |
| Locationc | 0.339 | |||||
| tumour height >12 cm | 30 | 4 | 7 | 15 | 4 | |
| tumour height 6-12 cm | 209 | 62 | 62 | 60 | 25 | |
| tumour height <6 cm | 266 | 74 | 73 | 86 | 33 | |
| Pathology(adenocarcinoma) | 0.526 | |||||
| Well differentiated | 72 | 18 | 17 | 28 | 9 | |
| Moderately differentiated | 376 | 105 | 113 | 115 | 43 | |
| Poorly differentiated | 57 | 17 | 12 | 18 | 10 | |
| Lymphatic/vascular invasion | 0.868 | |||||
| Yes | 323 | 88 | 90 | 107 | 38 | |
| No | 182 | 52 | 52 | 54 | 24 | |
| PNI | 0.723 | |||||
| Yes | 169 | 51 | 49 | 49 | 20 | |
| No | 336 | 89 | 93 | 112 | 42 | |
| pT status | 0.024 | |||||
| pT1 | 60 | 15 | 12 | 25 | 8 | |
| pT2 | 119 | 29 | 34 | 50 | 6 | |
| pT3 | 289 | 85 | 86 | 74 | 44 | |
| pT4 | 37 | 11 | 10 | 12 | 4 | |
| pN status | 0.277 | |||||
| pN0 | 247 | 61 | 70 | 91 | 25 | |
| pN1 | 171 | 54 | 47 | 45 | 25 | |
| pN2 | 87 | 25 | 25 | 25 | 12 | |
| pTNM stage | 0.038 | |||||
| Stage I | 141 | 36 | 37 | 58 | 10 | |
| Stage II | 124 | 29 | 41 | 37 | 17 | |
| Stage III | 240 | 75 | 64 | 66 | 35 | |
| Smoking history | 0.390 | |||||
| Yes | 359 | 101 | 107 | 107 | 44 | |
| No | 146 | 39 | 35 | 54 | 18 | |
| Drinking history | 0.281 | |||||
| Yes | 400 | 114 | 118 | 122 | 46 | |
| No | 105 | 26 | 24 | 39 | 16 | |
| Tumor size | 0.155 | |||||
| ≥3.4 | 194 | 49 | 48 | 73 | 24 | |
| <3.4 | 311 | 91 | 94 | 88 | 38 | |
| CEA | 0.117 | |||||
| Normal | 333 | 90 | 105 | 99 | 39 | |
| Elevated | 172 | 50 | 37 | 62 | 23 | |
| CA199 | 0.889 | |||||
| Normal | 469 | 129 | 132 | 149 | 59 | |
| Elevated | 36 | 11 | 10 | 12 | 3 | |
| CA125 | 0.858 | |||||
| Normal | 488 | 134 | 137 | 157 | 60 | |
| Elevated | 17 | 6 | 5 | 4 | 2 | |
Abbreviations: BMI=body mass index; pN status=pathological node status; pT statue=pathological tumour status; pTNM status=pathological tumour-node-metastasis stage.
a:χ2 test (A blood type vs B blood type vs O blood type vs AB blood type).
b: Chinese definition.
c: NCOTARGET. 2015;6(34):36884-9.
Overall survival, by ABO blood type, among patients in our study. A, Survival curve of the 505 rectal adenocarcinoma patients according to the ABO blood groups. (P = 0.003)B, Survival curve of patients with blood types B+O and A + AB. We divided the whole group of patients into two subgroups, patients with B and O blood types and patients with A and AB blood groups. The two survival curve separated, and had significant difference. The 5-year overall survival was 86.8 vs. 80.6%, respectively, P <0.001.
Discussion
In the present study, we investigated the value of ABO blood group in predicting the prognosis of patients who underwent radical surgery for rectal cancer. We observed significantly better survival for participants with blood group B and O compared with A and AB blood group participants. Using multivariate analysis, significant associations with rectal cancer survival were found BMI, tumor location, poorly differentiated histology, lymphatic/vascular invasion, advanced pN stages, CA125, CA19-9. Our study is the first study that investigated this relationship in a Chinese population in Northwest China region and we found that blood type is an independent factor affecting the prognosis of patients with rectal cancer.
Univariate and multivariate Cox regression analysis for overall survival in patients with colon cancer
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Pa | HR | 95% CI | Pa | |
| Gender | 0.522 | — | ||||
| Male | 1.00 | Reference | — | — | ||
| Female | 0.29 | 0.468-1.470 | — | — | ||
| Ageb (years) | 0.929 | — | ||||
| <60 | 1 | Reference | — | — | ||
| ≥60 | 0.929 | 0.600-1.751 | — | — | ||
| BMIb, kgm2 | 0.971 | — | ||||
| <18.5 | 1 | Reference | — | — | ||
| 18.5-23.9 | 0.916 | 0.312-2.690 | — | — | ||
| ≥24 | 0.937 | 0.531-1.653 | — | — | ||
| Locationc | 0.012 | 0.004 | ||||
| tumor height >12 cm | 1 | Reference | 1 | Reference | ||
| tumor height 6-12 cm | 1.081 | 0.209-3.721 | 1.080 | 0.595-1.961 | ||
| tumor height <6 cm | 1.176 | 0.6812.031 | 1.126 | 0.236-5.365 | ||
| Pathology (adenocarcinoma) | 0.005 | 0.002 | ||||
| Well differentiated | 1 | Reference | 1 | Reference | ||
| Moderately differentiated | 1.144 | 0.295-4.436 | 2.338 | 0.539-10.135 | ||
| Poorly differentiated | 1.879 | 0.583-6.054 | 3.647 | 0.332-1.260 | ||
| lymphatic/vascular invasion | 0.003 | 0.021 | ||||
| Yes | 1 | Reference | 1 | Reference | ||
| No | 0.441 | 0.257-0.758 | 0.647 | 0.3324-1.260 | ||
| PNI | 0.522 | — | ||||
| Yes | 1 | Reference | — | — | ||
| No | 0.831 | 0.473-1.462 | — | — | ||
| pT status | 0.042 | 0.012 | ||||
| pT1 | 0.389 | 0.114-1.329 | 0.158 | 0.015-1.617 | ||
| pT2 | 0.282 | 0.095-0.841 | 0.111 | 0.015-0.834 | ||
| pT3 | 0.759 | 0.038-1.702 | 0.840 | 0.340-2.076 | ||
| pT4 | 1 | Reference | 1 | Reference | ||
| pN status | 0.006 | 0.023 | ||||
| pN0 | 0.322 | 0.161-0.646 | 1.00 | Reference | ||
| pN1 | 0.605 | 0.313-1.168 | 0.523 | 0.240-1.141 | ||
| pN2 | 1.00 | Reference | 0.889 | 0.263-1.914 | ||
| pTNM stage | 0.003 | 0.015 | ||||
| Stage I | 0.373 | 0.180-0.773 | 0.735 | 0.352-0.916 | ||
| Stage II | 0.373 | 0.174-0.801 | 0.816 | 0.485-2.572 | ||
| Stage III | 1.00 | Reference | 1.00 | Reference | ||
| Smoking history | 0.069 | — | ||||
| Yes | 1.852 | 0.954-3.596 | — | — | — | |
| No | 1.00 | Reference | — | — | ||
| Drinking history | 0. 112 | — | ||||
| Yes | 3.738 | 1.347-10.370 | — | — | ||
| No | 1.00 | Reference | — | — | ||
| ABO blood type | 0.006 | 0.008 | ||||
| A | 0.832 | 0.416-1.666 | 0.760 | 0.365-1.582 | ||
| B | 0.368 | 0.165-0.823 | 0.356 | 0.155-0.819 | ||
| O | 0.299 | 0.128-0.699 | 0.282 | 0.114-0.694 | ||
| AB | 1 | Reference | 1 | Reference | ||
| Tumor size(cm) | 0.019 | — | — | 0.241 | ||
| <3.4 | 0.622 | 0.342-1.130 | 0. 673 | 0.343-1.323 | ||
| ≥3.4 | 1 | Reference | 1 | Reference | ||
| Serum CEA | 0.196 | — | ||||
| Normal | 0.701 | 0.409-1.201 | — | — | ||
| Elevated | 1 | Reference | — | — | ||
| CA19-9 | 0.038 | 0.956 | ||||
| Normal | 0.854 | 0.340-2.149 | 0.434 | 0.226-1.154 | ||
| Elevated | 1 | Reference | 1 | Reference | ||
| CA125 | 0.023 | 0.031 | ||||
| Normal | 0. 475 | 0.161-0.809 | 0.854 | 0.199-3.668 | ||
| Elevated | 1 | Reference | 1 | Reference | ||
Abbreviations: BMI=body mass index; 95% CI=95% confidence interval; HR=hazard ratio; pTNM stage=pathological tumour-node-metastasis stage.
a:Cox proportional hazards model.
b:Chinese definition.
c: NCOTARGET. 2015;6(34):36884-9.
The ABO blood group is by far the most important among human blood group systems [16]. The ABO blood group system is based on expression of two antigens, A and/or B on the surface of the red blood cell; because expression of these antigens is codominant, patients may have type A, type B or type AB expression patterns. Lack of expression of either antigen results in the O phenotype [17]. For several decades, a role for ABO blood group antigens in the development of cancer has been suspected, but the results have been inconsistent. In a study conducted by Nozoe and Colleagues in 284 patients, non-O blood groups correlated with poorly differentiated grades of the tumour and AB blood group was associated with advanced stage and large tumour size, the significance of ABO blood group distribution might be associated with biological behavior of gastric adenocarcinoma patients, but no correlation was found between different ABO blood groups and overall survival [18]. By contrast, in another study the B/O group was found to independently correlate with unfavourable survival among patients who had ever smoked [11]. A large, prospective, population-based study has consistently documented an increased risk of gastric cancer in individuals with blood type A [19]. However, it was found not to be a prognosis factor for patients with gastric adenocarcinoma. In a recent study conducted in 1555 patients with surgically resected colon cancer, AB blood type were more likely to have a better survival than patients with non-AB blood types[12], but adverse consequences were found in our study. This might be caused by our small number of subjects as well as by interpopulation variation. The exact biological theories elaborating the link between ABO blood group and cancer are still insufficient. Researchers involved in blood group ABO antigens and their genes, developed by Hakomori have shown that human blood group antigens are expressed on the surface of red blood cells and other tissues, including cells of the gastrointestinal tract[20], broncho pulmonary, skin, urogenital epithelial and kidney[21]. These glycoconjugates may participate in modifying intercellular adhesion, membrane signaling, and immune surveillance, which could in turn influence tumorigenesis [20].
Although the mechanism for an association between blood group and rectal cancer risk is unknown, several plausible hypotheses exist. The normal ABO antigen is lost in cancer patients, and new tumor antigens are acquired. In colon carcinomas, 50% of tumors of the proximal colon demonstrated loss of antigen expression, although expression was retained in the adjacent tissues. Of note, in tumors of the distal colon, antigens, while undetectable in the adjacent tissues, were expressed in tumor cells [22, 23], thus, a structural change in the ABO antigen may occurs in rectal cancer. A likely mechanism for the alteration of ABH antigen expression in colorectal tissue is the loss or reduction of the glycosyltransferase enzymes required for the synthesis of ABH determinants [24]. Modified expression of blood group antigens on cancer cells may influence tumorigenesis by altering glycosyltransferase specificity [25] or increasing cell motility, resistance to apoptosis and immune escape [21]. It is therefore possible that a specific blood type may enhance disease progression or survival [21, 26]. A number of single nucleotide polymorphism studies have suggested that ABO gene may influence the systemic chronic inflammatory response that is closely related to carcinogenesis by regulating the serum levels of several circulating adhesion molecules and plasma inflammatory markers [27], such as tumor necrosis factor alpha [28, 29], soluble intercellular adhesion molecule (ICAM)-1 [30, 31], E-selectin [32, 33], and P-selectin [30]. All these adhesion molecules are important mediators of chronic inflammation and immune cell recruitment. They may, therefore, provide a biological basis for the postulated influence of ABO on cancer survival, by directly linking ABO blood group and tumour initiation and spread [34].
We acknowledge the limitations of our retrospective analysis. The retrospective nature of our study greatly increased the possibility that factors related to blood group influenced which cases were included in the analysis. Additional shortcoming of our analysis include the insufficient study population, the short follow-up percentage and the unavailability of detailed covariate data, which cannot allowed us to examine confounding and effect modification by several exposures of interest. Our study population was composed of all Chinese population in Northwest China region, which somewhat limits the generalizability of our results. Accordingly, the results of further investigations including more diverse populations (white/black/brown participants) from other institutes are needed to confirm our findings. In addition, even all patients had computed tomography scans of the chest and abdomen at the time of diagnosis, but not for brain, and it is possible that some patients had asymptomatic disease at the time of primary treatment. However, this would have had a negative influence on survival. Furthermore, hospital-based control populations may not be representative of the blood group distribution in the general population if the conditions leading to hospitalization are associated with blood group.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of China (No. 81472699, and No. 81272344).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin. 2016;66:7-30
2. Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E. et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576-2594
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer Statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132
4. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of Metastasis in Colon and Rectal Cancer. Sci Rep. 2016;6:29765
5. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide Trends in Incidence, Treatment and Survival of Colorectal Cancer Patients with Synchronous Metastases. Clin Exp Metastasis. 2015;32:457-465
6. Hua Y, Ma X, Liu X, Yuan X, Qin H, Zhang X. Abnormal Expression of mRNA, microRNA Alteration and Aberrant DNA Methylation Patterns in Rectal Adenocarcinoma. Plos One. 2017;12:e174461
7. Costantini M, Fassio T, Canobbio L, Landucci M, Resasco M, Boccardo F. Role of Blood Groups as Prognostic Factors in Primary Breast Cancer. Oncology. 1990;47:308-312
8. Ben Q, Wang K, Yuan Y, Li Z. Pancreatic Cancer Incidence and Outcome in Relation to ABO Blood Groups Among Han Chinese Patients: A Case-Control Study. Int J Cancer. 2011;128:1179-1186
9. Miyake M, Taki T, Hitomi S, Hakomori S. Correlation of Expression of H/Le(y)/Le(b) Antigens with Survival in Patients with Carcinoma of the Lung. N Engl J Med. 1992;327:14-18
10. Iodice S, Maisonneuve P, Botteri E, Sandri MT, Lowenfels AB. ABO Blood Group and Cancer. Eur J Cancer. 2010;46:3345-3350
11. Sun P, Chen C, Zhang F, An X, Li XY, Li YH. et al. The ABO Blood Group Predicts Survival in Esophageal Squamous Cell Carcinoma in Patients Who Ever Smoked: A Retrospective Study From China. Tumour Biol. 2014;35:7201-7208
12. Cao X, Wen ZS, Sun YJ, Li Y, Zhang L, Han YJ. Prognostic Value of ABO Blood Group in Patients with Surgically Resected Colon Cancer. Br J Cancer. 2014;111:174-180
13. Ouyang PY, Su Z, Mao YP, Liu Q, Xie FY. Prognostic Value of ABO Blood Group in Southern Chinese Patients with Established Nasopharyngeal Carcinoma. Br J Cancer. 2013;109:2462-2466
14. Yuzhalin AE, Kutikhin AG. ABO and Rh Blood Groups in Relation to Ovarian, Endometrial and Cervical Cancer Risk Among the Population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13:5091-5096
15. Khalili H, Wolpin BM, Huang ES, Giovannucci EL, Kraft P, Fuchs CS. et al. ABO Blood Group and Risk of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1017-1020
16. Storry JR, Olsson ML. The ABO Blood Group System Revisited: A Review and Update. Immunohematology. 2009;25:48-59
17. Rummel SK, Ellsworth RE. The Role of the Histoblood ABO Group in Cancer. Future Sci OA. 2016;2:O107
18. Nozoe T, Ezaki T, Baba H, Kakeji Y, Maehara Y. Correlation of ABO Blood Group with Clinicopathologic Characteristics of Patients with Esophageal Squamous Cell Carcinoma. Dis Esophagus. 2004;17:146-149
19. Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M. et al. Risk of Gastric Cancer and Peptic Ulcers in Relation to ABO Blood Type: A Cohort Study. Am J Epidemiol. 2010;172:1280-1285
20. Hakomori S. Antigen Structure and Genetic Basis of Histo-Blood Groups a, B and O: Their Changes Associated with Human Cancer. Biochim Biophys Acta. 1999;1473:247-266
21. Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis Histo-Blood Group Antigens in Cancer. Apmis. 2001;109:9-31
22. Schoentag R, Primus FJ, Kuhns W. ABH and Lewis Blood Group Expression in Colorectal Carcinoma. Cancer Res. 1987;47:1695-1700
23. Ernst C, Thurin J, Atkinson B, Wurzel H, Herlyn M, Stromberg N. et al. Monoclonal Antibody Localization of a and B Isoantigens in Normal and Malignant Fixed Human Tissues. Am J Pathol. 1984;117:451-461
24. Welshinger M, Finstad CL, Venkatraman E, Federici MG, Rubin SC, Lewis JJ. et al. Expression of a, B, and H Blood Group Antigens in Epithelial Ovarian Cancer: Relationship to Tumor Grade and Patient Survival. Gynecol Oncol. 1996;62:106-112
25. Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ. et al. ABO Blood Group and the Risk of Pancreatic Cancer. J Natl Cancer Inst. 2009;101:424-431
26. Marionneau S, Le Moullac-Vaidye B, Le Pendu J. Expression of Histo-Blood Group A Antigen Increases Resistance to Apoptosis and Facilitates Escape From Immune Control of Rat Colon Carcinoma Cells. Glycobiology. 2002;12:851-856
27. Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC. et al. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Res. 2008;68:323-328
28. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883-899
29. Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I. et al. A Genome-Wide Association Study Identifies Protein Quantitative Trait Loci (pQTLs). Plos Genet. 2008;4:e1000072
30. Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB. et al. Large-Scale Genomic Studies Reveal Central Role of ABO in sP-selectin and sICAM-1 Levels. Hum Mol Genet. 2010;19:1863-1872
31. Pare G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S. et al. Novel Association of ABO Histo-Blood Group Antigen with Soluble ICAM-1: Results of a Genome-Wide Association Study of 6,578 Women. Plos Genet. 2008;4:e1000118
32. Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q. et al. Genetic Variants in ABO Blood Group Region, Plasma Soluble E-selectin Levels and Risk of Type 2 Diabetes. Hum Mol Genet. 2010;19:1856-1862
33. Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE. et al. Genome-Wide Association Identifies the ABO Blood Group as a Major Locus Associated with Serum Levels of Soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958-1967
34. Franchini M, Favaloro EJ, Targher G, Lippi G. ABO Blood Group, Hypercoagulability, and Cardiovascular and Cancer Risk. Crit Rev Clin Lab Sci. 2012;49:137-149
Author contact
![]() Corresponding author: Xiaohua Li, MD, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, 127 Changle West Road, Xi'an, Shaanxi 710032, China. E-mail: lixiaohua1982edu.cn or xiaohualixhcom
Corresponding author: Xiaohua Li, MD, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, 127 Changle West Road, Xi'an, Shaanxi 710032, China. E-mail: lixiaohua1982edu.cn or xiaohualixhcom

 Global reach, higher impact
Global reach, higher impact