Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(8):2171-2180. doi:10.7150/jca.39615 This issue Cite
Research Paper
Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio in Hepatocellular Carcinoma Patients Treated with Liver Transplantation
1. Department of Hepatic Surgery and Liver Transplantation Center, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, 510630, China.
2. Department of Liver Surgery, Liver Transplantation Division, West China Hospital, Sichuan University, Chengdu, 610041, China.
3. Guangdong Key Laboratory of Liver Disease Research, Key Laboratory of Liver disease biotherapy and Translational Medicine of Guangdong Higher Education Institutes, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, 510630, China.
* These authors contributed equally to this study.
Received 2019-8-25; Accepted 2019-12-22; Published 2020-2-3
Abstract
Background: The albumin-to-alkaline phosphatase ratio (AAPR) is a newly developed index which was used to predict prognosis of HCC patients. However, its prognostic role in HCC patients undergoing liver transplantation (LT) remains unclear. This study aimed to investigate the correlation between AAPR and prognosis of these patients.
Methods: A total of 210 patients who underwent LT from January 2003 to January 2014 were retrospectively analyzed (149 for discovery and 61 for validation). Univariate and multivariate analyses were performed to determine the discriminative ability of the AAPR in predicting long-term survival. The area under the receiver operating characteristic (AUC) was calculated to compare the accuracy of different factors.
Results: Patients with high AAPR level were associated with less ascites rate (30.6% versus 53.2%, P=0.033) as well as more frequencies of Child-Pugh class A (73.6% versus 35.1%, P=0.001). Univariate and multivariate analyses suggested the AAPR was independent prognostic factor in predicting overall survival (HR: 0.585, 95% CI: 0.363-0.941, P=0.027). Validation cohort confirmed prognostic value of AAPR. Subgroup analysis demonstrated that reduced AAPR level was associated with worse prognosis in HCC patients categorized in Child-Pugh class A (P=0.029). The AUCs of the AAPR were 0.710 and 0.744 in predicting 3-year and 5-year survival outcomes, respectively.
Conclusions: The study showed in two independent cohorts of HCC patients treated by LT that elevated AAPR was associated with better OS. As a low-cost routine laboratory test, it should be considered as biomarker in the clinical management of HCC.
Keywords: hepatocellular carcinoma, liver transplantation, albumin-to-alkaline phosphatase ratio, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of malignancy-related mortality worldwide [1-4]. It gives rise to nearly 745500 deaths worldwide in 2012. Multiple treatment strategies are available for HCC, but surgical resection and liver transplantation (LT) are considered to be the most effective curative treatment modalities [5]. Hepatectomy removes solitary lesions in patients with preserved liver function, while LT provides both an oncologic resection as well as replacement of a diseased liver [6]. Moreover, in patients with underlying cirrhotic liver disease, LT is a more appropriate therapeutic strategy [7]. However, its application is restricted by the shortage of available donor livers [8]. Therefore, it is critical to select appropriate candidates for LT. The criteria of HCC candidates' selection varied in different transplantation centers all over the world. The Milan criteria, which were proposed by Mazzaferro et al in 1996, are considered as strict standard and restrict LT for HCC patients with a single tumor no more than five cm in diameter, or up to three tumors, none of which exceed three cm [7, 9] . Patients with HCC meeting Milan criteria were associated with favorable outcomes after LT. Recently, multiple expanded criteria have been introduced, such as the Hangzhou criteria and the University of California, San Francisco (UCSF) criteria [10, 11]. The Hangzhou criteria were proposed by Zheng et al in 2008 in consideration of the fact that the main etiology of HCC in China to encompass patients with and more numerous larger tumors [12]. The Hangzhou criteria restrict LT for HCC patients with a total tumor size no more than eight cm in diameter, or a total tumor diameter more than eight cm with a histopathologic grade of well or moderate differentiation as well as a preoperative alpha-fetoprotein (AFP) level no more than 400 ng/mL. HCC patients within the Hangzhou criteria were supposed to gain satisfactory survival after LT according to several centers reproduced the model [13, 14]. However, merely the criteria for HCC candidates' selection is not optimal. The combination selection criteria with staging systems or serum biomarkers will be more beneficial to predict prognosis of HCC patients undergoing LT.
Several scoring systems have been investigated to predict the clinical prognosis of HCC patients underwent LT and to guide therapeutic regimen. The American Joint Committee on Cancer (AJCC) TNM staging system, the Barcelona Clinic Liver Cancer (BCLC) system and the Japan Integrated Staging (JIS) are the most widely used ones in predicting survival outcomes of patients with HCC[15-17]. Moreover, serum parameters, such as circulating immune-inflammatory cells like neutrophils and lymphocytes, alpha-fetoprotein (AFP), albumin (ALB), bilirubin, and alkaline phosphatase (ALP), have also been investigated for their potential prognostic predicting value [18-21]. Increasing attention has been paid to the inflammation-based prognostic models in recent years, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and the systemic immune-inflammation index (SII) [9, 22, 23]. The prognosis of patients with HCC depends not only on tumor burden (tumor size, number, portal vein thrombosis and extrahepatic spread) but also underlying liver function [24]. The Child-Pugh (CP) classification and the Albumin-Bilirubin (ALBI) grade, introduced to assess liver function initially, have been verified to be prognostic predictors for HCC patients. Recently, a newly-presented biomarker named albumin-to-alkaline phosphatase ratio (AAPR) was used to predict survival of HCC patients undergoing curative resection and palliative therapy [25]. In advanced HCC patients without receiving any standard anti-cancer therapies, AAPR could also serve as a potentially valuable prognostic index [26]. Nevertheless, its potential prognostic value in patients with HCC undergoing liver transplantation is still unclear.
In the present study, we analyzed a cohort of patients with HCC meeting Hangzhou criteria to explore the correlation between AAPR and clinicopathological characteristics, and its value in predicting survival outcomes.
Material and methods
Study population
We retrospectively enrolled patients who underwent LT from January 2003 to May 2013 as the discovery cohort. The validation cohort included patients treated by LT from June 2013 to January 2014. All the included patients were newly diagnosed as HCC meeting Hangzhou criteria and admitted in the Third Affiliated Hospital of Sun Yat-sen University. Patients were excluded if they received liver resection, radiofrequency ablation, transarterial chemoembolization, chemotherapy or other anti-cancer therapies before LT [27]. Patients were also excluded if gastrointestinal hemorrhage happened prior to LT. All eligible patients or their relative freely gave written informed consent. This study was approved by the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University, in accordance with the guidelines of the 1975 Declaration of Helsinki [28].
Data collection and follow-up
The following data of pretreatment clinical information were reviewed and collected from the hospital handwritten or electronic medical records: patients' basic information, medical history and laboratory examination including serum alanine transaminase (ALT), aspartate transaminase (AST), ALB, total bilirubin (TBIL) and ALP were collected at the time of preoperative, then were analyzed for baseline evaluation. Tumor-related clinicopathological characteristics including preoperative AFP level, differentiation, the number of tumor nodules, maximum tumor diameter and metastasis were also acquired. The definition of microvascular invasion (MVI) is based on the guideline of the HCC standardized pathological diagnosis [29]. The AAPR was calculated from dividing the ALB level by serum ALP level, where ALB was in g/L and ALP in U/L [25]. Patients were followed up according to National Comprehensive Cancer Network (NCCN), regularly contrast-enhanced ultrasonography per month at first year, then every 3 months for 2 years, and then every 6 months thereafter. Besides, we contact those who determined not to go back to the hospital to reexamination through telephone follow-up survey. All the patients were followed up for 5 years at the endpoint.
Statistical analysis
The software of SPSS (version 22.0), MedCalc (version 15.2.2.0) and Graphpad Prism (version 5.0) were used to perform statistical analyses. We used Pearson's chi square test and student's t-test to investigate the correlation of categorical and continuous variables to AAPR level respectively. The primary endpoint was overall survival (OS), defined as the duration from surgery to HCC-associated mortality. The secondary endpoint was recurrence-free survival (RFS), defined as the duration from surgery to recurrence. Receiver-operating characteristic (ROC) curves were applied to determine the optimal cut-offs as Youden index attained maximum value at 5 years posttransplant. Patients were categorized by AAPR level. Kaplan-Meier method was used to perform survival analysis for the groups with different cut-off values, and their differences were tested with log-rank test. Those clinicopathological parameters with P<0.05 in the univariable Cox proportional hazards regression were considered for generating multivariable Cox regression (enter method) to identify potential independent prognostic factors for OS of HCC patients underwent LT. A two-tailed P value less than 0.05 was considered statistically significant. Subgroup analysis was conducted in patients with liver function of CP class A. Kaplan-Meier curves were introduced to analyze the correlation between AAPR level and OS as well as RFS in HCC patients with proper liver function.
Results
Patient characteristics
The baseline demographic and clinical characteristics of discovery and validation cohort were showed in Table 1. The entire cohort contained a total of 210 HCC patients treated by LT with mean age of 51.47 (198 male). There was no statistical difference between discovery and validation cohort in baseline characteristics.
In the discovery cohort, a total of 149 patients with newly diagnosed HCC were included with a mean age of 51.26. Most of the included patients were male (141, 94.6%). Additionally, 138 patients were associated with HBV infection. Among the total sample set, 80 patients with liver function of Child A grade, 63 patients were associated with pretreatment ascites. As for laboratory test, ALT, AST, TBIL, ALB and ALP were 52.42±39.02, 65.79±43.74, 65.73±137.42, 37.54±5.46, and 102.87±25.25, respectively. 56 patients were associated with tumor size over 5 cm, 27 patients with tumors of well-differentiation, 24 with MVI and 56 patients with tumor-node-metastasis (TNM) stage III. 3-year survival rate and 5-year survival rate for total sample size were 61.7% and 45.6%.
In the validation cohort, 61 patients with a mean age of 52.15 (57 male). 55 patients were associated with HBV infection. The CP classification were: A (n=30, 49.5%), B&C (n=31, 50.5%). 26 patients were associated with pretreatment ascites. As for laboratory test, ALT, AST, TBIL, ALB and ALP were 51.49±33.24, 69.84±51.31, 83.48±161.17, 37.59±5.19, and 114.97±34.92, respectively. 21 patients were associated with tumor size over 5 cm, 17 patients with tumors of well-differentiation, 14 with MVI and 30 patients with tumor-node-metastasis (TNM) stage III. 3-year survival rate and 5-year survival rate for total sample size were 61.7% and 45.6%.
Characteristics of entire cohort of HCC patients underwent liver transplantation.
| Variables | All patients (n=210) | Discovery (n=149) | Validation (n=61) | P Value |
|---|---|---|---|---|
| Age (year) | 51.47±9.76 | 51.26 ±9.96 | 52.15±9.28 | 0.524 |
| Gender (M/F) | 198/12 | 141/8 | 57/4 | 0.729 |
| HBsAg (+/-) | 193/17 | 138/11 | 55/6 | 0.547 |
| Child-Pugh class | ||||
| A | 110 | 80 | 30 | 0.524 |
| B&C | 100 | 69 | 31 | |
| Ascites (+/-) | 89/121 | 63/86 | 26/35 | 0.513 |
| ALT (IU/L) | 52.21±37.28 | 52.42±39.02 | 51.49±33.24 | 0.859 |
| AST (IU/L) | 67.53±46.59 | 65.79±43.74 | 69.84±51.31 | 0.648 |
| TBIL (μmol/L) | 70.75±144.22 | 65.73±137.42 | 83.48±161.17 | 0.415 |
| ALB (g/L) | 37.55±5.37 | 37.54±5.46 | 37.59±5.19 | 0.981 |
| ALP (U/L) | 106.38±28.84 | 102.87±25.25 | 114.97±34.92 | 0.783 |
| AFP (≥400/<400)(ng/dL) | 45/165 | 29/120 | 16/45 | 0.583 |
| Tumor size (>5cm/≤5cm) | 77/133 | 56/93 | 21/40 | 0.628 |
| Differentiation (Well/Moderate) | 44/166 | 27/122 | 17/44 | 0.135 |
| MVI (+/-) | 38/172 | 24/125 | 14/47 | 0.070 |
| TNM stages (Ⅰ-Ⅱ/Ⅲ) | 134/76 | 93/56 | 41/20 | 0.503 |
| MELD score | 9.29±7.05 | 8.94±7.16 | 10.14±6.79 | 0.469 |
| 1-year survival | 189/21 | 138/11 | 51/10 | 0.059 |
| 3-year survival | 131/79 | 92/57 | 39/22 | 0.726 |
| 5-year survival | 102/108 | 68/81 | 34/27 | 0.060 |
AAPR, albumin-to-alkaline phosphatase ratio; M, male; F, female; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; MVI, microvascular invasion; TNM, tumor-node-metastasis; MELD score, model for end-stage liver diseases score. Data were expressed as numbers of patients or mean ± SD.
Correlation between patient clinicopathological characteristics with AAPR level
The optimal cut-off values with the maximum Youden index value were 0.38 and 112 for AAPR and ALP, respectively. Consequently, in the discovery cohort, 72 patients with AAPR value more than 0.38 were categorized into high AAPR group and 77 patients in low group. In validation cohort, 26 patients were classified into AAPR high group while 35 patients in low group. The correlation between AAPR level with clinicopathological characteristics were shown in Table 2. Higher 3-year survival rate (72.2% vs 51.9% in discovery cohort and 80.8% vs 51.4% in validation cohort, respectively) and 5-year survival rate (61.1% vs 31.2% in discovery cohort and 73% vs 42.9% in validation cohort, respectively) were observed in high AAPR group.
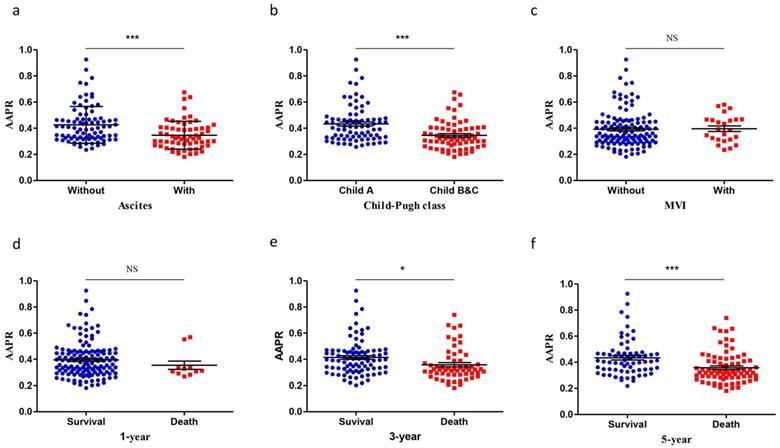
In the discovery cohort, a higher AAPR level was observed in patients without ascites (0.426±0.015 vs 0.347±0.013, P<0.001, Figure 1a). Patients with Child A grade liver function were associated with higher AAPR value (0.433±0.015 vs 0.346±0.013, P<0.001, Figure 1b). No significant difference was found between patients with MVI and those without (Figure 1c). Patients stratified in 3-year survival group showed higher AAPR value (0.414±0.014) than death group (0.358±0.016) (P=0.011, Figure 1e). Patients were associated with lower AAPR level in 5-year death group (0.358±0.013) than survival group (0.433±0.017) (P<0.001, Figure 1f).
Survival analysis
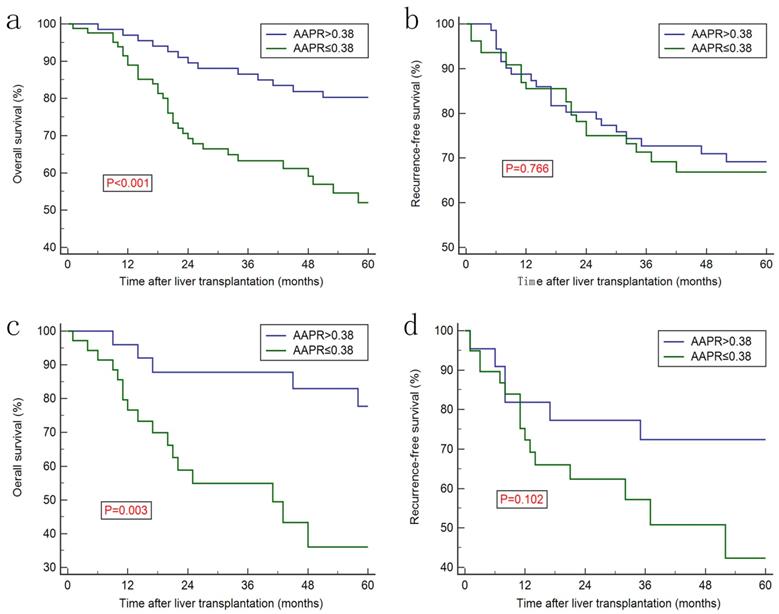
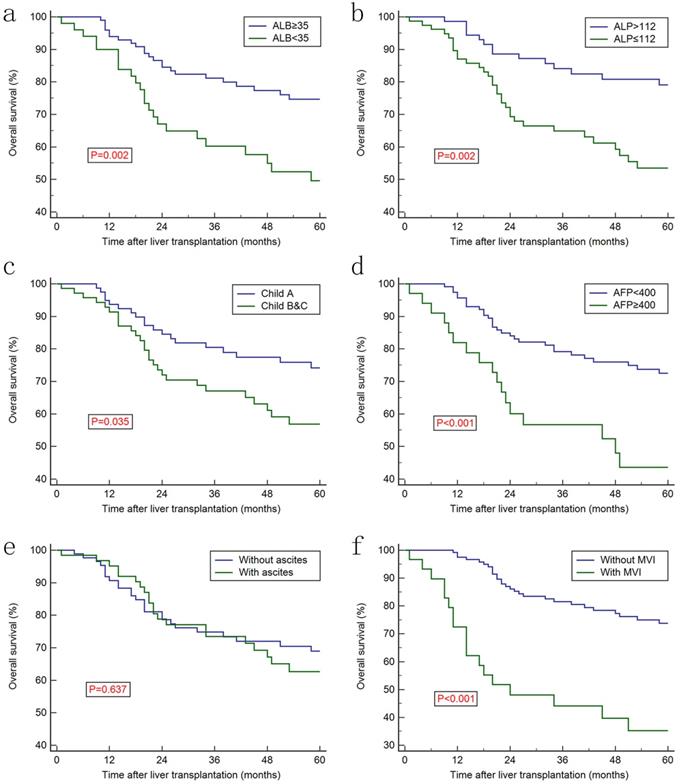
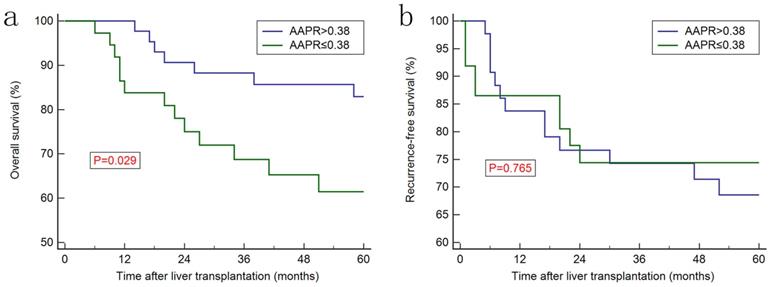
Increased AAPR level was associated with better prognosis in discovery cohort (Figure 2a) and in the validation cohort (Figure 2c), whereas no significant difference was observed regarding recurrence-free survival (RFS) between two groups (Figure 2b and 2d). Furthermore, Kaplan-Meier curves showed that the ALB, ALP, AFP, CP classification and MVI were prognostic factors for OS (Figure 3). Univariate analysis revealed that CP class [hazard ration (HR): 1.858, 95% confidence interval (CI): 1.037-3.329, P=0.035], ALB (HR: 0.419, 95% CI: 0.223-0.790, P=0.002), ALP (HR: 2.625, 95% CI: 1.471-4.686, P<0.001), AFP (HR: 2.626, 95% CI: 1.243-5.551, P<0.001), TNM stage (HR: 2.370, 95% CI: 1.496-3.754, P<0.001) and AAPR (HR: 0.505, 95% CI: 0.325-0.784, P<0.001) were associated with significant difference. However, after conducting multivariate analysis, only AAPR was identified to be independent prognostic factor for HCC patients underwent LT (HR: 0.585, 95% CI: 0.363-0.941, P=0.027) (Table 3). In subgroup analyses, Kaplan-Meier confirmed the overall outcomes that patients with high AAPR level were associated longer OS compared to those with low level (P=0.029, Figure 4a) in patients with CP class A. The RFS remained comparable between two groups (P=0.765, Figure 4b).
Scatter plots showing the AAPR values in different subgroups categorized by: (a) pretreatment ascites; (b) Child-Pugh class; (c) pretreatment MVI; (d) 1-year survival state; (e) 3-year state and (f) 5-year state. The means and standard deviations for each group were signified by the black lines within the scatter plots. AAPR: albumin-to-alkaline phosphatase ratio; MVI: microvascular invasion. *: P<0.05; **: P<0.01; ***: P<0.001.
Correlation between AAPR and clinicopathological characteristics in discovery and validation cohorts.
| Variables | Discovery | Validation | ||||
|---|---|---|---|---|---|---|
| AAPR>0.38 (n=72) | AAPR≤0.38 (n=77) | P Value | AAPR>0.38 (n=26) | AAPR≤0.38 (n=35) | P Value | |
| Age (year) | 49.89±9.90 | 52.55±9.92 | 0.104 | 50.35±8.46 | 53.49±9.75 | 0.194 |
| Gender (M/F) | 70/2 | 71/6 | 0.278 | 24/2 | 33/2 | 0.762 |
| HBsAg (+/-) | 69/3 | 69/8 | 0.212 | 26/0 | 29/6 | 0.026 |
| Child-Pugh class | ||||||
| A | 53 | 27 | 0.001 | 15 | 16 | 0.363 |
| B&C | 19 | 50 | 11 | 19 | ||
| Ascites (+/-) | 22/50 | 41/36 | 0.033 | 10/16 | 16/19 | 0.579 |
| ALT (IU/L) | 51.64±36.41 | 53.14±41.55 | 0.815 | 46.88±24.74 | 54.91±38.36 | 0.355 |
| AST (IU/L) | 63.08±35.15 | 68.32±50.59 | 0.467 | 61.04±30.35 | 76.37±62.17 | 0.252 |
| TBIL (μmol/L) | 53.76±109.58 | 75.61±155.96 | 0.337 | 73.23±151.79 | 92.19±170.56 | 0.113 |
| ALB (g/L) | 41.60±3.80 | 34.23±4.23 | <0.001 | 39.61±4.98 | 36.09±4.88 | 0.007 |
| ALP (U/L) | 83.85±17.68 | 120.65±16.92 | <0.001 | 79.24±10.92 | 141.52±19.04 | <0.001 |
| AFP (≥400/<400)(ng/dL) | 12/60 | 17/60 | 0.418 | 6/20 | 10/25 | 0.636 |
| Tumor size (>5cm/≤5cm) | 27/45 | 29/48 | 1.000 | 7/19 | 14/21 | 0.296 |
| Differentiation (Well/Moderate) | 17/55 | 10/67 | 0.135 | 11/15 | 6/29 | 0.03 |
| MVI (+/-) | 13/59 | 11/66 | 0.657 | 8/18 | 6/29 | 0.217 |
| TNM stages (Ⅰ-Ⅱ/Ⅲ) | 47/25 | 46/31 | 0.503 | 18/8 | 23/12 | 0.777 |
| MELD score | 8.50±5.39 | 9.35±8.51 | 0.469 | 9.50±6.20 | 10.63±7.26 | 0.526 |
| 1-year survival | 70/2 | 68/9 | 0.057 | 25/1 | 26/9 | 0.022 |
| 3-year survival | 52/20 | 40/37 | 0.012 | 21/5 | 18/17 | 0.018 |
| 5-year survival | 44/28 | 24/53 | <0.001 | 19/7 | 15/20 | 0.018 |
AAPR, albumin-to-alkaline phosphatase ratio; M, male; F, female; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; MVI, microvascular invasion; TNM, tumor-node-metastasis; MELD score, model for end-stage liver diseases score. Data were expressed as numbers of patients or mean ± SD.
Kaplan-Meier curves for overall survival and recurrence-free survival stratified by AAPR level in discovery cohort (a & b) and validation cohort (c &d).
Kaplan-Meier survival curves for overall survival stratified by: ALB of 35 g/dl (a), (d) the optimal cut-off value of ALP at 112 (b), Child-Pugh class(c), AFP (d), pretreatment ascites status (e) and MVI status (f). AAPR: albumin-to-alkaline phosphatase ratio; ALB: albumin; ALP: alkaline phosphatase; MVI: microvascular invasion.
Kaplan-Meier curves for overall survival (a) and recurrence-free survival (b) in subgroup of patients with liver function of Child-Pugh class A.
Independent prognostic factors for overall survival by the univariate and multivariate Cox proportional hazards regression model for discovery cohort.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.007 | 0.978-1.037 | 0.652 | |||
| Gender (F/M) | 0.731 | 0.177-3.018 | 0.665 | |||
| HBsAg (+/-) | 0.557 | 0.268-1.156 | 0.116 | |||
| Child-Pugh class | 1.858 | 1.037-3.329 | 0.035 | 1.418 | 0.884-2.277 | 0.148 |
| Ascites (+/-) | 1.149 | 0.640-2.063 | 0.637 | |||
| ALT | 0.999 | 0.993-1.005 | 0.651 | |||
| AST | 1.001 | 0.995-1.006 | 0.842 | |||
| TBIL | 1.001 | 0.999-1.002 | 0.250 | |||
| ALB | 0.419 | 0.223-0.790 | 0.002 | |||
| ALP | 2.625 | 1.471-4.686 | <0.001 | |||
| AFP (≥400/<400) | 2.626 | 1.243-5.551 | <0.001 | 1.574 | 0.949-2.613 | 0.079 |
| Differentiation (Moderate/Well) | 1.263 | 0.698-2.287 | 0.440 | |||
| Tumor size (>5/≤5) | 1.355 | 0.872-2.103 | 0.176 | |||
| MVI (+/-) | 4.029 | 1.742-9.325 | <0.001 | 1.934 | 0.668-5.594 | 0.224 |
| TNM stages (Ⅲ/Ⅰ-Ⅱ) | 2.370 | 1.496-3.754 | <0.001 | 1.387 | 0.762-2.524 | 0.285 |
| MELD score | 1.023 | 0.994-1.052 | 0.118 | |||
| AAPR | 0.505 | 0.325-0.784 | <0.001 | 0.585 | 0.363-0.941 | 0.027 |
AAPR, albumin-to-alkaline phosphatase ratio; M, male; F, female; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; ALP, alkaline phosphatase; AFP, alpha-fetoprotein; MVI, microvascular invasion; TNM, tumor-node-metastasis; MELD score, model for end-stage liver diseases score. HR, hazard ratio; CI, confidence interval.
Accuracy for AAPR in predicting OS
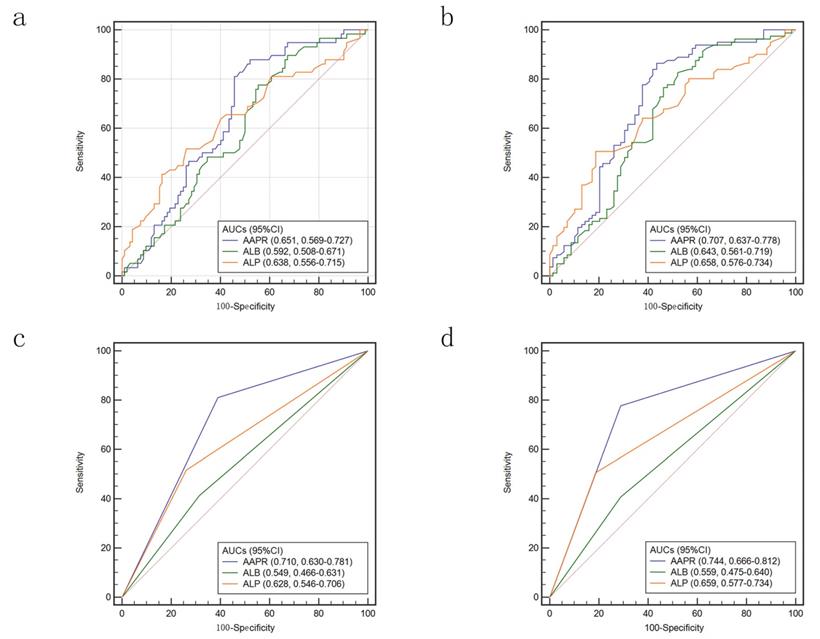
ROC curves were used to compare the accuracy of AAPR, ALB and ALP in predicting prognosis of HCC patients underwent LT. When we used continuous variable to conduct ROC curves, the area under the receiver operating characteristic (AUC) of AAPR was 0.651 for 3-year survival and 0.707 for 5-year survival (Figure 5a & 5b). The AUCs were higher than that of ALB and ALP. While the variables were treated as categorical variables, both AUCs were larger. For predicting 3-year survival, the AUCs were 0.710, 0.549 and 0.628 for AAPR, ALB and ALP, respectively (Figure 5c). When 5-year survival was set as endpoint, the AUCs were 0.744, 0.559 and 0.659 for AAPR, ALB and ALP, respectively (Figure 5d).
Comparison of AUCs for AAPR, ALB and ALP: calculated as continuous variables in predicting 3-year survival (a) and 5-year-survival (b); calculated as categorized variables in predicting 3-year survival (c) and 5-year-survival (d). AUC: area under the receiver operating characteristic; AAPR: albumin-to-alkaline phosphatase ratio; ALB: albumin; ALP: alkaline phosphatase.
Discussion
HCC remains one of the most complicated abdominal malignancies, and its therapeutic strategy remains a challenge [30]. Various scoring systems were developed to evaluate the prognosis and guide clinical management of HCC patients. However, there is still lack of a universally accept scoring system for classification HCC [31, 32]. Liver function is an easily accessible laboratory parameter, which is a necessarily detected index for patients with HCC. Child-Pugh classification and the ALBI score, concerning liver function, were suggested to be prognostic systems for HCC patients underwent liver resection and other standard anti-cancer therapies [33-35]. Serum ALB, a kind of protein synthesized in liver, is an indictor reflects the protein synthetic capability. Moreover, it remains a modulator for inflammatory response, which is essential in retardation of HCC [36]. Recent studies have demonstrated the ability of ALB in stabilizing cell proliferation and exerting antioxidant reaction for anti-carcinogenesis [37, 38]. Therefore, it was considered to be an independent prognostic factor for HCC patients and a variable integrated into several scoring systems like the ALBI score and the Chinese University Prognostic Index (CUPI) system [39]. The serum ALP, another one of the most routinely detected parameters in laboratory test, is a hydrolase enzyme and exists throughout the body, especially concentrates in the liver, bones, kidney and placenta with multiple isoforms. Several studies have reported that the ALP level increases during childhood and other diseases such as hepatic diseases, osteomalacia, and bone tumor [40]. Several evidences indicated that ALP played important roles in strengthening cancer cell proliferation, vascular invasion and distant metastasis [41]. In addition, ALP has also been reported as an independent factor for HCC patients which was correlated with cirrhosis and prognosis [42]. The AAPR was introduced by Chan et al initially to predict prognosis of HCC patients who underwent surgical resection and palliative therapy [25]. Cai et al investigated its prognostic value in advanced HCC patients who did not receive any standard anti-cancer treatments [26]. Nonetheless, it remained unclear whether AAPR could accurate predict the prognosis of HCC patients underwent LT.
The present study showed that pre-transplantation AAPR was an independent prognostic index for HCC patients within Hangzhou criteria. However, there was no significant correlation between the AAPR and RFS of HCC patients underwent LT. The estimated AAPR level was higher in patients with liver function of CP class A and patients without pretreatment ascites to those with CP class B & C and those with ascites. It confirmed the results that patients with high AAPR value were associated with higher frequencies of CP class A as well as ascites compared to those with lower AAPR level. After calculating Youden index, a 0.38 was supposed to be optimal cut-off value. Kaplan-Meier curves indicated that patients with AAPR value of more than 0.38 were associated with longer OS. Univariate and multivariate analyses confirmed that the AAPR was independent prognostic index for HCC patients underwent LT. In subgroup of patients with liver function of CP class A, the AAPR remained its prognostic role in predicting OS.
This was the first study investigated the potential prognostic value of AAPR in HCC patients underwent LT. ALB and ALP are both simple but different and objective variables, which are more easily applied in clinical practice. However, ALB and ALP have never been put together to evaluate their combined prognostic significance. Hence, we introduced a novel and simple index, AAPR. The AAPR is a powerful prognostic indicator with the highest c-index and χ2 (by LR test) among other liver biochemical parameters [25]. In our study, comparison of ROC curves implied that AAPR preceded ALB or ALP alone as a more accurate prognostic index for OS in HCC patients underwent LT. Unlike other serodiagnosis or iconographical detections, it was a novel index readily derived from a simple low-cost routine blood test which would not increase the total medical costs. Furthermore, our study analyzed the correlation between AAPR level with clinicopathological characteristics of patients. Further researches were in need to confirm our primary outcomes and elucidate the potential molecular mechanisms.
However, there were several limitations that warrant consideration when interpreting our findings. Firstly, the present study was retrospectively designed, which would be associated with potential selection bias though strict criteria were developed in population enrollment. Secondly, all the patients were Chinese from single center and most of them were associated with chronic HBV infection. Furthermore, the sample size was not large enough to perform subgroup analysis. Additionally, a validation cohort was in need to confirm our main results. Finally, the underlying molecular mechanism of the correlation between the AAPR with prognosis of HCC should be further investigated.
Conclusions
In summary, the present study showed in two independent cohorts of HCC patients treated with LT suggested that increased AAPR was associated with better prognosis. Multivariable analysis identified AAPR as a potential prognostic factor for OS. Subgroup analysis of patients categorized in CP class A revealed the AAPR played a prognostic role in predicting OS. As a low-cost routine laboratory test, it provided additional prognostic information for tumor scoring systems and could be viewed as biomarker in the clinical management of HCC.
Abbreviations
AAPR: albumin-to-alkaline phosphatase ratio; LT : liver transplantation; AAPR: albumin-to-alkaline phosphatase ratio; AUC: receiver operating characteristic; UCSF: University of California, San Francisco; AFP: alpha-fetoprotein; AJCC: American Joint Committee on Cancer; BCLC: Barcelona Clinic Liver Cancer; JIS: Japan Integrated Staging; ALB: albumin; ALP: alkaline phosphatase; NLR: neutrophil-to-lymphocyte ratio; SII: systemic immune-inflammation index; CP: Child-Pugh; ALBI: Albumin-Bilirubin; MVI: microvascular invasion; NCCN: National Comprehensive Cancer Network; OS: overall survival; RFS: recurrence-free survival; CUPI: Chinese University Prognostic Index.
Acknowledgements
This work was supported by the grants from National key research and development program (2017YFA0104304); National Natural Science Foundation of China (81570593, 81770648, 81802402, 81870447); Science and Technology Planning Project of Guangzhou (201508020262, 2014J4100128, 201400000001-3); Key Scientific and Technological Projects of Guangdong Province (2014B020228003, 2014B030301041); Natural Science Foundation of Guangdong Province (2015A030312013); Sun Yat-Sen University Clinical Research 5010 Program (2014006); Sci-tech Research Development Program of Guangdong province (2017A020215178, 2017A020215023); The PhD Start-up Fund of Natural Science Foundation of Guangdong Province (2018A030310323); Medical Scientific Research Foundation of Guangdong Province (A2018130).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA: a cancer journal for clinicians. 2012;62:394-9
2. Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nature reviews Gastroenterology & hepatology. 2010;7:448-58
3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
4. Chu H, Williams B, Schnabl B. Gut microbiota, fatty liver disease, and hepatocellular carcinoma. Liver research. 2018;2:43-51
5. EASL-EORTC clinical practice guidelines. management of hepatocellular carcinoma. Journal of hepatology. 2012;56:908-43
6. Gao F, Li X, Geng M, Ye X, Liu H, Liu Y. et al. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine. 2015;94:e639
7. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. The New England journal of medicine. 1996;334:693-9
8. Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J. et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. Journal of hepatology. 2017;67:708-15
9. Fu H, Zheng J, Cai J, Zeng K, Yao J, Chen L. et al. Systemic Immune-Inflammation Index (SII) is Useful to Predict Survival Outcomes in Patients After Liver Transplantation for Hepatocellular Carcinoma within Hangzhou Criteria. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;47:293-301
10. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology (Baltimore, Md). 2001;33:1394-403
11. Menon KV, Hakeem AR, Heaton ND. Review article: liver transplantation for hepatocellular carcinoma - a critical appraisal of the current worldwide listing criteria. Alimentary pharmacology & therapeutics. 2014;40:893-902
12. Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M. et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-32
13. Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L. et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-41
14. Gao T, Xia Q, Qiu DK, Feng YY, Chi JC, Wang SY. et al. Comparison of survival and tumor recurrence rates in patients undergoing liver transplantation for hepatitis B-related hepatocellular carcinoma using Milan, Shanghai Fudan and Hangzhou criteria. Journal of digestive diseases. 2013;14:552-8
15. Chun YH, Kim SU, Park JY, Kim DY, Han KH, Chon CY. et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. European journal of cancer (Oxford, England: 1990). 2011;47:2568-75
16. Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T. et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology (Baltimore, Md). 2004;40:1396-405
17. Reig M, Darnell A, Forner A, Rimola J, Ayuso C, Bruix J. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Seminars in liver disease. 2014;34:444-55
18. Cescon M, Bertuzzo VR, Ercolani G, Ravaioli M, Odaldi F, Pinna AD. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunological state on recurrence and prognosis. World journal of gastroenterology. 2013;19:9174-82
19. Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G. et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. Journal of hepatology. 2017;66:552-9
20. Hu L, Xue F, Li Y, Shao M, Sun Y, Wei G. A long-term follow-up and comprehensive observation of risk and prognosis factors of recurrence and survival after resection of hepatocellular carcinoma. Cell biochemistry and biophysics. 2014;69:421-31
21. Carr BI, Guerra V. Hepatocellular Carcinoma Extrahepatic Metastasis in Relation to Tumor Size and Alkaline Phosphatase Levels. Oncology. 2016;90:136-42
22. Galun D, Bogdanovic A, Djokic Kovac J, Bulajic P, Loncar Z, Zuvela M. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing country. Cancer management and research. 2018;10:977-88
23. Zheng J, Cai J, Li H, Zeng K, He L, Fu H. et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;44:967-81
24. Chan AW, Chong CC, Mo FK, Wong J, Yeo W, Johnson PJ. et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2016;31:1766-72
25. Chan AW, Chan SL. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. 2015. 2015:564057
26. Cai X, Chen Z, Chen J, Ma X, Bai M, Wang T. et al. Albumin-to-Alkaline Phosphatase Ratio as an Independent Prognostic Factor for Overall Survival of Advanced Hepatocellular Carcinoma Patients without Receiving Standard Anti-Cancer Therapies. Journal of Cancer. 2018;9:189-97
27. Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ. et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2014;12:1454-87
28. Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. British journal of clinical pharmacology. 2004;57:695-713
29. Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH. et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-87
30. Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH. et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. Journal of hepatology. 2016;64:601-8
31. Maida M, Orlando E, Camma C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World journal of gastroenterology. 2014;20:4141-50
32. Chen J, Chen C-Y, Nguyen C, Chen L, Lee K, Stiles BL. Emerging signals regulating liver tumor initiating cells. Liver research. 2018;2:73-80
33. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng MJ, Skowronska A. et al. Assessment of liver dysfunction in hepatocellular carcinoma (HCC): An international collaborative study. Journal of Clinical Oncology. 2014:32
34. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD. et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. The British journal of surgery. 2016;103:725-34
35. Edeline J, Blanc JF, Johnson P, Campillo-Gimenez B, Ross P, Ma YT. et al. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. International. 2016;36:1821-8
36. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-99
37. Nojiri S, Joh T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int J Mol Sci. 2014;15:5163-74
38. Reebye V, Saetrom P, Mintz PJ, Huang KW, Swiderski P, Peng L. et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology. 2014;59:216-27
39. Chan SL, Johnson PJ, Mo F, Berhane S, Teng M, Chan AW. et al. International validation of the Chinese university prognostic index for staging of hepatocellular carcinoma: a joint United Kingdom and Hong Kong study. Chinese journal of cancer. 2014;33:481-91
40. Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging clinical and experimental research. 2015;27:413-8
41. Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS. et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440-9
42. Xu XS, Wan Y, Song SD, Chen W, Miao RC, Zhou YY. et al. Model based on gamma-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World journal of gastroenterology. 2014;20:10944-52
Author contact
![]() Corresponding authors: Genshu Wang; Yang Yang; Jun Zheng; MD, PhD, Department of Hepatic Surgery and Liver Transplantation Center, Guangdong Key Laboratory of Liver Disease Research, Key Laboratory of Liver disease biotherapy and Translational Medicine of Guangdong Higher Education Institutes, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, 510630, China. Tel: +86-20-85252177; Fax: +86-20-85252276; E-mail: wgsh168com (GS Wang); yysysucom (Y Yang); 575511384com (J Zheng).
Corresponding authors: Genshu Wang; Yang Yang; Jun Zheng; MD, PhD, Department of Hepatic Surgery and Liver Transplantation Center, Guangdong Key Laboratory of Liver Disease Research, Key Laboratory of Liver disease biotherapy and Translational Medicine of Guangdong Higher Education Institutes, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, 510630, China. Tel: +86-20-85252177; Fax: +86-20-85252276; E-mail: wgsh168com (GS Wang); yysysucom (Y Yang); 575511384com (J Zheng).

 Global reach, higher impact
Global reach, higher impact